Aging-associated Increase of GATA4 levels in Articular Cartilage is Linked to Impaired Regenerative Capacity of Chondrocytes and Osteoarthritis
Curation statements for this article:-
Curated by eLife
eLife Assessment
This study presents an important finding on the role of GATA4 in aging- and OA-associated cartilage pathology. The conclusions are well supported by compelling in vitro and in vivo evidence. This work will be of broad interest to both cell biologists and orthopedic/skeletal health clinicians.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Abstract
Although the causal association between aging and osteoarthritis (OA) has been documented, our understanding of the underlying mechanism remains incomplete. To define the regulatory molecules governing chondrocyte aging, we performed transcriptomic analysis of young and old human chondrocytes from healthy donors. The data predicted that GATA binding protein 4 (GATA4) may play a key role in mediating the difference between young and old chondrocytes. Results from immunostaining and western blot showed significantly higher GATA4 levels in old human or mouse chondrocytes when compared to young cells. Moreover, overexpressing GATA4 in young chondrocytes remarkably reduced their cartilage-forming capacity in vitro and induced the upregulation of proinflammatory cytokines. Conversely, suppressing GATA4 expression in old chondrocytes, through either siRNA or a small-molecule inhibitor NSC140905, increased the production of aggrecan and collagen type II, and also decreased levels of matrix-degrading enzymes. In OA mice induced by surgical destabilization of the medial meniscus, intraarticular injection of lentiviral vectors carrying mouse Gata4 resulted in a higher OA severity, synovial inflammation, and pain level when compared to control vectors. Mechanistically, we found that overexpressing GATA4 significantly increased the phosphorylation of SMAD1/5. Our work demonstrates that the aging-associated increase of GATA4 in chondrocytes plays a vital role in OA progression, which may also serve as a target to reduce osteoarthritis in the older population.
Article activity feed
-

-

-

eLife Assessment
This study presents an important finding on the role of GATA4 in aging- and OA-associated cartilage pathology. The conclusions are well supported by compelling in vitro and in vivo evidence. This work will be of broad interest to both cell biologists and orthopedic/skeletal health clinicians.
-

Reviewer #1 (Public review):
Summary:
This manuscript assesses the differences between young and aged chondrocytes. Through transcriptomic analysis and further assessments in chondrocytes, GATA4 was found to be increased in aged chondrocyte donors compared to young. Subsequent mechanistic analysis with lentiviral vectors, siRNAs, and a small molecule were used to study the role of GATA4 in young and old chondrocytes. Lastly, an in vivo study was used to assess the effect of GATA4 expression on osteoarthritis progression in a DMM mouse model.
Strengths:
This work linked the over expression of GATA4 to NF-kB signaling pathway activation, alterations to the TGF-b signaling pathway, and found that GATA4 increased the progression of OA compared to the DMM control group. Indicating that GATA4 contributes to the onset and progression of OA in …
Reviewer #1 (Public review):
Summary:
This manuscript assesses the differences between young and aged chondrocytes. Through transcriptomic analysis and further assessments in chondrocytes, GATA4 was found to be increased in aged chondrocyte donors compared to young. Subsequent mechanistic analysis with lentiviral vectors, siRNAs, and a small molecule were used to study the role of GATA4 in young and old chondrocytes. Lastly, an in vivo study was used to assess the effect of GATA4 expression on osteoarthritis progression in a DMM mouse model.
Strengths:
This work linked the over expression of GATA4 to NF-kB signaling pathway activation, alterations to the TGF-b signaling pathway, and found that GATA4 increased the progression of OA compared to the DMM control group. Indicating that GATA4 contributes to the onset and progression of OA in aged individuals.
Comments on revised version:
Great work! All my concerns have been well addressed.
-

Reviewer #2 (Public review):
Summary:
This study elucidated the impact of GATA4 on aging- and injury-induced cartilage degradation and osteoarthritis (OA) progression, based on the team's finding that GATA expression is positively correlated with aging in human chondrocytes. By integrating cell culture of human chondrocytes, gene manipulation tools (siRNA, lentivirus), biological/biochemical analyses and murine models of post-traumatic OA, the team found that increasing GATA4 levels reduced anabolism and increased catabolism of chondrocytes from young donors, likely through upregulation of the BMP pathway, and that this impact is not correlated with TGF-β stimulation. Conversely, silencing GATA4 by siRNA attenuated catabolism and elevated aggrecan/collagen II biosynthesis of chondrocytes from old donors. The physiological relevance of …
Reviewer #2 (Public review):
Summary:
This study elucidated the impact of GATA4 on aging- and injury-induced cartilage degradation and osteoarthritis (OA) progression, based on the team's finding that GATA expression is positively correlated with aging in human chondrocytes. By integrating cell culture of human chondrocytes, gene manipulation tools (siRNA, lentivirus), biological/biochemical analyses and murine models of post-traumatic OA, the team found that increasing GATA4 levels reduced anabolism and increased catabolism of chondrocytes from young donors, likely through upregulation of the BMP pathway, and that this impact is not correlated with TGF-β stimulation. Conversely, silencing GATA4 by siRNA attenuated catabolism and elevated aggrecan/collagen II biosynthesis of chondrocytes from old donors. The physiological relevance of GATA4 was further validated by the accelerated OA progression observed in lentivirus-infected mice in the DMM model.
Strengths:
This is a highly significant and innovative study that provides new molecular insights into cartilage homeostasis and pathology in the context of aging and disease. The experiments were performed in a comprehensive and rigorous manner. The data were interpreted thoroughly in the context of the current literature.
Weaknesses:
The only aspect that would benefit from further clarification is a more detailed discussion of aging-associated ECM changes in the context of prior literature.
-

Reviewer #3 (Public review):
Summary:
This is an exciting, comprehensive paper that demonstrates the role of GATA4 on OA-like changes in chondrocytes. The authors present elegant reverse translational experiments that justify this mechanism and demonstrate the sufficiency of GATA4 in a mouse model of osteoarthritis (DMM), where GATA4 drove cartilage degeneration and pain in a manner that was significantly worse than DMM alone. This could pave the way for new therapies for OA that account for both structural changes and pain.
Strengths:
(1) GATA4 was identified from human chondrocytes.
(2) IHC and sequencing confirmed GATA4 presence.
(3) Activation of SMADs is clearly shown in vitro with GATA4 overexpression.
(4) The role of GATA4 was functionally assessed in vivo using the mouse DMM model, where the authors uncovered that GATA4 worsens …
Reviewer #3 (Public review):
Summary:
This is an exciting, comprehensive paper that demonstrates the role of GATA4 on OA-like changes in chondrocytes. The authors present elegant reverse translational experiments that justify this mechanism and demonstrate the sufficiency of GATA4 in a mouse model of osteoarthritis (DMM), where GATA4 drove cartilage degeneration and pain in a manner that was significantly worse than DMM alone. This could pave the way for new therapies for OA that account for both structural changes and pain.
Strengths:
(1) GATA4 was identified from human chondrocytes.
(2) IHC and sequencing confirmed GATA4 presence.
(3) Activation of SMADs is clearly shown in vitro with GATA4 overexpression.
(4) The role of GATA4 was functionally assessed in vivo using the mouse DMM model, where the authors uncovered that GATA4 worsens OA structure and hyperalgesia in male mice.
(5) It is interesting that GATA4 is largely known to be found in cardiac cells and to have a role in cardiac repair, metabolism, and inflammation, among other things listed by the authors in the discussion (in liver, lung, pancreas). What could this new knowledge of GATA4 mean for OA as a potentially systemically mediated disease, where cardiac disease and metabolic syndrome are often co-morbid?
Weaknesses:
I do not have further comments. Thank you for addressing the previously mentioned concerns.
-

Author response:
The following is the authors’ response to the previous reviews
Reviewer #2 (Public review):
The only aspect that would benefit from further clarification is a more detailed discussion of aging-associated ECM changes in the context of prior literature.
Thank you. Please refer to the new section (Lines 604-617)
Reviewer #3 (Public review):
(1) It would be useful to explain why GATA4 was chosen over HIF1a, which was the most differentially expressed.
Thank you. Please refer to Lines 530-537.
“Of note, Hypoxia-Inducible Factor 1α (HIF1 α) was the most differentially expressed gene predicted to regulate chondrocyte aging. The connection between HIF1 α and aging has been previously reported.[32] Furthermore, additional studies have investigated HIF1 in association with OA and assessed its use as a therapeutic …
Author response:
The following is the authors’ response to the previous reviews
Reviewer #2 (Public review):
The only aspect that would benefit from further clarification is a more detailed discussion of aging-associated ECM changes in the context of prior literature.
Thank you. Please refer to the new section (Lines 604-617)
Reviewer #3 (Public review):
(1) It would be useful to explain why GATA4 was chosen over HIF1a, which was the most differentially expressed.
Thank you. Please refer to Lines 530-537.
“Of note, Hypoxia-Inducible Factor 1α (HIF1 α) was the most differentially expressed gene predicted to regulate chondrocyte aging. The connection between HIF1 α and aging has been previously reported.[32] Furthermore, additional studies have investigated HIF1 in association with OA and assessed its use as a therapeutic target.[33,34] Therefore, we decided to focus on GATA4, which was less studied in chondrocytes but highly associated with cellular senescence, an aging hallmark. However, our selection did not dampen the importance of HIF1α and other molecules listed in Figure 1D in chondrocyte aging. They can be further studied in the future using the same strategy employed in the current work.”
(2) In Figure 5, it would be useful to demonstrate the non-surgical or naive limbs to help contextualize OARSI scores and knee hyperalgesia changes.
In the current study, we focused on the DMM control and DMM Gata4 virus groups so we did not include a sham control group. We recognized this was a limitation of this study.
(3) While there appear to be GATA4 small-molecule inhibitors in various stages of development that could be used to assess the effects in age-related OA, those experiments are out of scope for the current study.
We agree with this comment that the results are still preliminary, which was the reason that we put it in the supplementary materials. However, we felt like the result is informative, which will support the potential of GATA4 as a therapeutic target and inspire the development of more specific inhibitors. Therefore, we would still keep the results in the current study.
-

-

eLife Assessment
This study presents an important finding on the role of GATA4 in aging- and OA-associated cartilage pathology. The conclusions are well supported by compelling in vitro and in vivo evidence. This work will be of broad interest to both cell biologists and orthopedic clinicians.
-

Reviewer #1 (Public review):
Summary:
This manuscript assesses the differences between young and aged chondrocytes. Through transcriptomic analysis and further assessments in chondrocytes, GATA4 was found to be increased in aged chondrocyte donors compared to young. Subsequent mechanistic analysis with lentiviral vectors, siRNAs, and a small molecule were used to study the role of GATA4 in young and old chondrocytes. Lastly, an in vivo study was used to assess the effect of GATA4 expression on osteoarthritis progression in a DMM mouse model.
Strengths:
This work linked the over expression of GATA4 to NF-kB signaling pathway activation, alterations to the TGF-b signaling pathway, and found that GATA4 increased the progression of OA compared to the DMM control group. Indicating that GATA4 contributes to the onset and progression of OA in …
Reviewer #1 (Public review):
Summary:
This manuscript assesses the differences between young and aged chondrocytes. Through transcriptomic analysis and further assessments in chondrocytes, GATA4 was found to be increased in aged chondrocyte donors compared to young. Subsequent mechanistic analysis with lentiviral vectors, siRNAs, and a small molecule were used to study the role of GATA4 in young and old chondrocytes. Lastly, an in vivo study was used to assess the effect of GATA4 expression on osteoarthritis progression in a DMM mouse model.
Strengths:
This work linked the over expression of GATA4 to NF-kB signaling pathway activation, alterations to the TGF-b signaling pathway, and found that GATA4 increased the progression of OA compared to the DMM control group. Indicating that GATA4 contributes to the onset and progression of OA in aged individuals.
Comments on revised version:
Great work! All my concerns have been well addressed.
-

Reviewer #2 (Public review):
Summary:
This study elucidated the impact of GATA4 on aging- and injury-induced cartilage degradation and osteoarthritis (OA) progression, based on the team's finding that GATA expression is positively correlated with aging in human chondrocytes. By integrating cell culture of human chondrocytes, gene manipulation tools (siRNA, lentivirus), biological/biochemical analyses and murine models of post-traumatic OA, the team found that increasing GATA4 levels reduced anabolism and increased catabolism of chondrocytes from young donors, likely through upregulation of the BMP pathway, and that this impact is not correlated with TGF-β stimulation. Conversely, silencing GATA4 by siRNA attenuated catabolism and elevated aggrecan/collagen II biosynthesis of chondrocytes from old donors. The physiological relevance of …
Reviewer #2 (Public review):
Summary:
This study elucidated the impact of GATA4 on aging- and injury-induced cartilage degradation and osteoarthritis (OA) progression, based on the team's finding that GATA expression is positively correlated with aging in human chondrocytes. By integrating cell culture of human chondrocytes, gene manipulation tools (siRNA, lentivirus), biological/biochemical analyses and murine models of post-traumatic OA, the team found that increasing GATA4 levels reduced anabolism and increased catabolism of chondrocytes from young donors, likely through upregulation of the BMP pathway, and that this impact is not correlated with TGF-β stimulation. Conversely, silencing GATA4 by siRNA attenuated catabolism and elevated aggrecan/collagen II biosynthesis of chondrocytes from old donors. The physiological relevance of GATA4 was further validated by the accelerated OA progression observed in lentivirus-infected mice in the DMM model.
Strengths:
This is a highly significant and innovative study that provides new molecular insights into cartilage homeostasis and pathology in the context of aging and disease. The experiments were performed in a comprehensive and rigorous manner. The data were interpreted thoroughly in the context of the current literature.
Weaknesses:
The only aspect that would benefit from further clarification is a more detailed discussion of aging-associated ECM changes in the context of prior literature.
-

Reviewer #3 (Public review):
Summary:
This is an exciting, comprehensive paper that demonstrates the role of GATA4 on OA-like changes in chondrocytes. The authors present elegant reverse translational experiments that justify this mechanism and demonstrate the sufficiency of GATA4 in a mouse model of osteoarthritis (DMM), where GATA4 drove cartilage degeneration and pain in a manner that was significantly worse than DMM alone. This could pave the way for new therapies for OA that account for both structural changes and pain.
Strengths:
(1) GATA4 was identified from human chondrocytes.
(2) IHC and sequencing confirmed GATA4 presence.
(3) Activation of SMADs is clearly shown in vitro with GATA4 overexpression.
(4) The role of GATA4 was functionally assessed in vivo using the mouse DMM model, where the authors uncovered that GATA4 worsens …
Reviewer #3 (Public review):
Summary:
This is an exciting, comprehensive paper that demonstrates the role of GATA4 on OA-like changes in chondrocytes. The authors present elegant reverse translational experiments that justify this mechanism and demonstrate the sufficiency of GATA4 in a mouse model of osteoarthritis (DMM), where GATA4 drove cartilage degeneration and pain in a manner that was significantly worse than DMM alone. This could pave the way for new therapies for OA that account for both structural changes and pain.
Strengths:
(1) GATA4 was identified from human chondrocytes.
(2) IHC and sequencing confirmed GATA4 presence.
(3) Activation of SMADs is clearly shown in vitro with GATA4 overexpression.
(4) The role of GATA4 was functionally assessed in vivo using the mouse DMM model, where the authors uncovered that GATA4 worsens OA structure and hyperalgesia in male mice.
(5) It is interesting that GATA4 is largely known to be found in cardiac cells and to have a role in cardiac repair, metabolism, and inflammation, among other things listed by the authors in the discussion (in liver, lung, pancreas). What could this new knowledge of GATA4 mean for OA as a potentially systemically mediated disease, where cardiac disease and metabolic syndrome are often co-morbid?
Weaknesses:
(1) It would be useful to explain why GATA4 was chosen over HIF1a, which was the most differentially expressed.
(2) In Figure 5, it would be useful to demonstrate the non-surgical or naive limbs to help contextualize OARSI scores and knee hyperalgesia changes.
(3) While there appear to be GATA4 small molecule inhibitors in various stages of development that could be used to assess the effects in age-related OA, those experiments are out of scope for the current study.
Comments on revised version:
I do not have further comments. Thank you for addressing the previously mentioned concerns.
-

Author response:
The following is the authors’ response to the original reviews
Reviewer #1 (Public review):
This manuscript assesses the differences between young and aged chondrocytes. Through transcriptomic analysis and further assessments in chondrocytes, GATA4 was found to be increased in aged chondrocyte donors compared to young donors. Subsequent mechanistic analysis with lentiviral vectors, siRNAs, and a small molecule was used to study the role of GATA4 in young and old chondrocytes. Lastly, an in vivo study was used to assess the effect of GATA4 expression on osteoarthritis progression in a DMM mouse model.
Strengths:
This work linked the overexpression of GATA4 to NF-kB signaling pathway activation, alterations to the TGF-b signaling pathway, and found that GATA4 increased the progression of OA compared to the DMM control …
Author response:
The following is the authors’ response to the original reviews
Reviewer #1 (Public review):
This manuscript assesses the differences between young and aged chondrocytes. Through transcriptomic analysis and further assessments in chondrocytes, GATA4 was found to be increased in aged chondrocyte donors compared to young donors. Subsequent mechanistic analysis with lentiviral vectors, siRNAs, and a small molecule was used to study the role of GATA4 in young and old chondrocytes. Lastly, an in vivo study was used to assess the effect of GATA4 expression on osteoarthritis progression in a DMM mouse model.
Strengths:
This work linked the overexpression of GATA4 to NF-kB signaling pathway activation, alterations to the TGF-b signaling pathway, and found that GATA4 increased the progression of OA compared to the DMM control group. This indicates that GATA4 contributes to the onset and progression of OA in aged individuals.
The authors thank the reviewer for reviewing our manuscript and providing insightful comments.
Weaknesses:
(1) A couple of sentences should be added to the introduction, to emphasize the role GATA4 plays, such as the alterations to the TGF-b signaling pathway and the increased activation of the NF-kB pathway.
As suggested, we have expanded on these signaling pathways in the Introduction to highlight the known functions of GATA4. Importantly, there was no previous study reporting the roles of GATA4 in regulating TGF-β pathway.
“Many growth factors contribute to the chondro-supportive environment in the knee joint. Particularly, transforming growth factor-b (TGF-b) plays a key role in maintaining chondrocytes and replenishing ECM loss. However, during OA, TGF-b can induce catabolic processes in chondrocytes, resulting in matrix stiffening, osteophytes, and chondrocyte hypertrophy.[10-12]” (Lines 80-84)
“Mechanistically, upregulation of GATA4 was shown to increase nuclear factor-kB (NF-kB) pathway activation.[14,15] NF-κB is thought to amplify and potentially propagate cellular senescence during the aging process through the senescence-associated secretory phenotype (SASP), which could contribute to a low-grade state of chronic inflammation.[16]” (Lines 99-102)
“When GATA4 was over expressed, we found that there were alterations to the TGF-b signaling pathway and activation of the NF-kB signaling pathway.” (Lines 106-108)
(2) Figure 1F, the GATA4 histology image should be bigger.
We have now increased the size of the image in revised Figure 1F.
(3) Further discussion should be conducted regarding the reasoning as to why GATA4 increases the phosphorylation of SMAD1/5.
Thank you. The underlying mechanism of GATA4 activating SMAD1/5 has not been previously investigated. We have now elaborated on this in the discussion and have added more relevant publications.
“Our study indicated that there was an observed decrease in chondrogenesis and an increase in hypertrophy-related genes following GATA4 overexpression (Figure 2G).” (Lines 572-574)
“These previous studies and literature review inspired us to explore the potential association between GATA4 levels and the activation of SMAD1/5.” (Lines 587-588)
“In this study, it was shown that GATA4 was necessary for bone morphogenic protein-6 (BMP-6) mediated IL-6 induction, in which there are multiple GATA binding domains on the IL-6 promoter. This work further showed that GATA4 interacts with SMAD 2,3 and 4.[55] Studies have suggested that BMP pathways and GATA4 work synergistically to regulate SMAD signaling.56 This information indicates that the involvement of GATA4 in the TGF-b signaling pathway is complex and further studies should be conducted to better assess this relationship.” (Lines 594-599)
(4) More information should be included to clarify why GATA4 is thought to be linked to DNA damage and the pathway that is associated with that.
We have now included further information in the discussion to clarify the association between DNA damage and GATA4 upregulation.
“The study by Kang et al. demonstrated that the suppression of p62 following DNA damage leads to GATA4 accumulation due to the lack of autophagy.13 DNA damage is known to increase with age.71 Therefore, we believe that DNA damage due to aging is a key driver of the upregulation of GATA4 in old chondrocytes.” (Lines 642-646)
(5) Please add further information regarding the limitations of the animal study conducted in this work and future plans to assess this.
We have included more limitations of the animal study that was conducted in this work and have expanded on the future plans to use inducible GATA4 expression in transgenic mouse lines to study the role of GATA4 overexpression in OA onset and progression.
“Third, during our in vivo work, the intraarticular injection of GATA4 lentivirus was not chondrocyte-specific. Therefore, the injection also allowed for other cell types to overexpress GATA4. Future work should be conducted using transgenic mouse lines for cartilage-specific inducible overexpression or depletion of Gata4 to further investigate the role of GATA4 in chondrocytes.” (666-670)
(6) In Figure 5, GATA4 should be changed to Gata4 in the graphed portions for consistency.
Thanks. We have made the necessary adjustments throughout the manuscript.
Reviewer #2 (Public review):
(1) While it is convincing that GATA4 expression is elevated in elderly individuals, and that it has a detrimental impact on cartilage health, the authors might want to add further discussion on the variability among individual human donors, especially given the finding that the elevation of GATA4 was not observed in chondrocytes from donor O1 (Figure 1G).
The authors thank the reviewer for reviewing our manuscript and providing insightful comments.
As suggested, we have included more discussion on the variability among donors.
“Although we found that GATA4 was generally increased with aging, some young donors also exhibited increased levels of GATA4, which may be associated with increased DNA damage, as discussed above, or other stressors. Therefore, GATA4 should be used together in conjunction with other aging biomarkers, such as the epigenetic clock [72] to precisely define chondrocyte aging. Future work should examine biological versus chronological aging and epigenetic clock-based assessments to explain the variabilities in GATA4 expression among donors.” (Lines 658-663)
(2) It might also be worth adding additional discussion on the interplay between senescent chondrocytes and the dysfunctional ECM during aging. As noted by the authors, aging is associated with decreased sGAG content and likely degenerative changes in the collagen II network, so the microniche of chondrocytes, and thus cell-matrix crosstalk through the pericellular matrix, is also altered or impaired.
Thank you for this comment. We have included more discussion on the interplay of chondrocyte senescence and dysfunctional ECM during aging, with a specific focus on the microniche of chondrocytes.
“Additionally, a common hallmark of chondrocyte aging is the alternation of ECM, including composition change [2] and stiffening.[57] ECM stiffness can directly affect chondrocyte phenotype and proliferation, and contribute to OA.[58] A recent study by Fu et al. associated matrix stiffening with the promotion of chondrocyte senescence.[59] Furthermore, matrix stiffening has been associated with modulating the TGF-b signaling pathway.[60-62] Future studies should investigate the potential of matrix stiffening and the effect of GATA4 on pericellular matrix proteins such as decorin[63,64], biglycan, collagen VI and XV, as these proteins assist with the regulation of biochemical interactions and assist with the maintenance of the chondrocyte microenvironment.[65] Herein, the TGF-b signaling pathway can further alter the extracellular microenvironment[62], which could promote cellular senescence and subsequently NF-kB pathway activation.” (Lines 600-610)
(2) If applicable, please also add Y3 and O3 to Figure S1 for visual comparison across individual donors.
As suggested, we added Y3 and O3 to the revised Figure S1 for more visual comparisons across individual donors.
(3) Figure 3C, the molecular weight labels are off.
Thanks. We corrected this mistake.
(4) Line 438 - Please clarify in text that the highest efficiency of siRNA chosen was siRNA2.
As suggested, we added the reason for selecting siRNA2.
“Several GATA4 siRNAs were tested, and the one with the highest efficiency was selected based off RT-qPCR results, which indicated that siRNA2 treatment induced lowest expression of GATA4. (Supplementary Figure S6).” (Lines 448-450)
(5) Did the authors test the timeline of sustained knockdown of GATA4 by siRNA?
We used a 7-day timepoint of chondrogenesis, and RT-qPCR results demonstrated that there was a downregulation of GATA4 expression at this timepoint (Figure 4). In the current in vitro study, we did not examine the efficacy of GATA4 siRNA for longer than 7 days.
Reviewer #3( Public review):
(1) It would be useful to explain why GATA4 was chosen over HIF1a, which was the most differentially expressed.
The authors thank the reviewer for reviewing our manuscript and providing insightful comments.
When we first saw the results, we did consider studying the role of HIF1a in aging because it was the most differentially expressed. When we reviewed the relevant literature, we found that HIF1a was commonly upregulated in aged individuals which was thought to be linked to hypoxia and increased oxidated stress (PMID: 12470896, PMID: 12573436). Further investigation found studies that investigated HIF1a in chondrocytes and the use of in vivo work to investigate its role in osteoarthritis (PMID: 32214220). Indicating that HIF1a plays a protective role during OA by suppressing the activation of NF-kB pathway. Moreover, there is work that has been conducted assessing the stabilization of HIF1a by regulating mitophagy and using HIF1a as a potential therapeutic target for OA (PMID: 32587244). Since there have been many studies investigating the correlation of HIF1a expression and OA, we felt that it would be more innovative to look at other molecules, such as GATA4. Moreoever, as we highlighted in the Introducion and Disucussion, through testing in cell types other than chondrocytes, GATA4 was shown to be associated with DNA damage and senescence, which are both aging hallmarks. Given the fact that roles of GATA4 in chodnrocytes had not been previous studies, we thus chose GATA4 in this study.
“Of note, Hypoxia-Inducible Factor 1a (HIF1a) was the most differentially expressed gene predicted to regulate chondrocyte aging. The connection between HIF1a and aging has been previously reported.32 Furthermore, additional studies have investigated HIF1a in association with OA and assessed its use as a therapeutic target.[33,34] Therefore, we decided to focus on GATA4, which was less studied in chondrocytes but highly associated with cellular senescence, an aging hallmark. However, our selection did not dampen the importance of HIF1α and other molecules listed in Figure 1D in chondrocyte aging. They can be further studied in the future using the same strategy employed in the current work.” (Lines 526-533)
(2) In Figure 5, it would be useful to demonstrate the non-surgical or naive limbs to help contextualize OARSI scores and knee hyperalgesia changes.
Thank you for your comment. Based on prior experience, the OARSI score of mice in the sham group had an OARSI score ranging from 0-0.5. In the current study, we focused on the DMM control and DMM Gata4 virus groups so we did not include a sham control group. We recognized this was a limitation of this study.
“We measured the naive limbs for knee hyperalgesia before DMM surgery, and found the average threshold was 507g. We have highlighted the threshold measurement in the figure legend.507 g was the threshold baseline for non-surgery mice (dashed line).” (Lines 499-500)
(3) While there appear to be GATA4 small-molecule inhibitors in various stages of development that could be used to assess the effects in age-related OA, those experiments are out of scope for the current study.
We agree with this comment that the results are still preliminary, which was the reason that we put it in the supplementary materials. However, we felt like the result is informative, which will support the potential of GATA4 as a therapeutic target and inspire the development of more specific inhibitors. Therefore, if the reviewer agrees, we want to keep the results in the current study.
In particular, our in vitro study demonstrated the potential of using small-molecule GATA4 to enhance the quality of cartilage created by old chondrocytes. We can validate the findings in vivo, as well as develop other GATA4 inhibitors. (Lines 673-675)
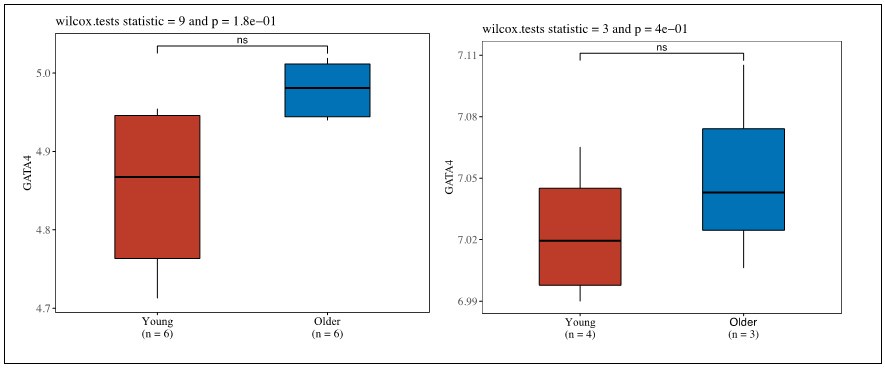
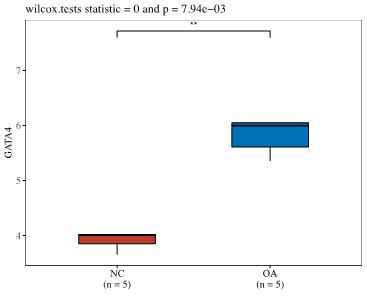
(4) Is GATA4 upregulated in chondrocytes in publicly available databases?
Thank you for this question. We have examined the public databases and have found that there is data showing the trend that GATA4 is upregulated in aged or OA chondrocytes in work conducted by Ungethuem et al (PMID: 20858714). In one study by Ramos et al. (PMID: 25054223), we noticed that GATA4 expression levels were the same in both young and old groups, which may be due to the relatively smaller sample size in the young group compared to old group (4 vs 26).
Work Conducted by Grogan et al. (Unpublished https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE39795)
Author response image 1.
Author response image 2.
Work conducted by Ramos et al. (PMID: 25054223).
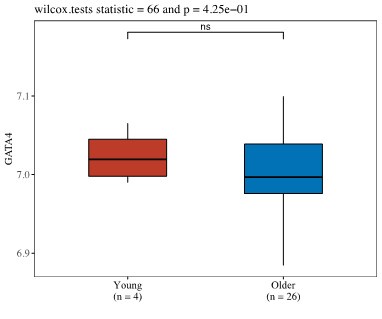
Author response image 3.
Work conducted by Ungethuem et al (PMID: 20858714).
(5) In many cases, the figure captions describe the experiment vs. the outcome. It may be more compelling to state the main finding in the figure title, and you might consider changing it from what is stated at present. For example, Figure 2: instead of the impact of overexpression, you may say GATA4 overexpression impairs cartilage formation (as stated in the results).
Thanks for the suggestion. We have made the following changes to the figure captions as suggested.
Figure 1: GATA4 is upregulated in aged chondrocytes (Line 373)
Figure 2: Overexpressing GATA4 impairs the hyaline cartilage formation capacity of young chondrocytes (Lines 408-409)
Figure 3: GATA4 overexpression activates SMAD1/5 (Line 436)
Figure 4: Suppressing GATA4 in old chondrocytes promotes cartilage formation and lowers expression of proinflammatory cytokines (Line 467)
Figure 5: Gata4 overexpression in the knee joints accelerates OA progression in mice. (Line 593)
(6) It would be useful to provide a little more information about the human tissue donors, if that is available.
We have provided more information about the tissue donors in the revised Supplementary Table S1.
(7) While aging-like changes were observed in young chondrocytes with GATA4 overexpression, it would be interesting to directly evaluate if there is a change in biological versus chronological age in these tissues. Companies like Zymo can provide this biological v chronological age epigenetic clock-based assessments if that is of interest, to say the young chondrocytes are looking "older".
Thank you for this information. We agree that it will be important to assess epigenetic changes in GATA-overexpressing cells. We are contacting the company to learn more about their technology. Meanwhile, we added this to the future work section of the manuscript.
“Although we found that GATA4 was generally increased with aging, some young donors also exhibited increased levels of GATA4, which may be associated with increased DNA damage, as discussed above, or other stressors. Therefore, GATA4 should be used together in conjunction with other aging biomarkers, such as the epigenetic clock [72] to precisely define chondrocyte aging. Future work should examine biological versus chronological aging and epigenetic clock-based assessments to explain the variabilities in GATA4 expression among donors.” (Lines 658-663)
(8) It is not clear the age at which the mice received DMM in the methods, but it is shown in Figure 5.
We have added the age at which the mice received the DMM surgery to the methods section.
“Intraarticular injections were administered to mice between 10-12 weeks of age under general anesthesia to safeguard the well-being of the animals and to minimize procedural discomfort.” (Line 300)
“One week after viral vector injection, DMM surgery was performed to induce the OA model on mice 11-13 weeks of age.” (Line 312-313)
(9) It is not clear which factors were assayed using Luminex, and it would be great to add.
Thank you for this comment, we have added a comprehensive list of proteins assessed using Luminex into a new supplementary table 6 (S6).
(10) Also interesting, loss of GATA4 seems to prevent diet-induced obesity in mice and promote insulin sensitivity (potentially via GLP-1 secretion). I wonder if there may be a metabolic axis here too? PMID: 21177287. I may have missed parts of the discussion of the role of GATA4 in metabolism, but it might be an interesting addition to the discussion.
In the current study, we have not investigated the role of GATA4 in obesity. As suggested, we have included a discussion of GATA4 in metabolism.
“Furthermore, GATA4 might be associated with metabolic regulation. A study conducted by Patankar et al. investigated how GATA4 regulates obesity. Specifically, they used intestine-specific Gata4 knockout mice to study diet-induced obesity, showing that the knockout mice were resistant to the high-fat diet, and that glucagon-like peptide-1 (GLP-1) release was increased. These findings indicated a decreased risk for the development for insulin resistance in knockout mice.[44] This work was taken a step further in a subsequent publication, in which the same team investigated the dietary lipid-dependent and independent effects on the development of steatosis and fibrosis in Gata4 knockout mice. The results from this work suggested that the knockdown of Gata4 increases GLP-1 release, in turn suppressing the development of hepatic steatosis and fibrosis, ultimately blocking hepatic de novo lipogenesis.[45] These studies are especially interesting with the rise of GLP-1 based therapy for the treatment of OA.46,47 Thus, the coupling of GATA4-related metabolic dysfunction and OA should be further investigated.” (Lines 542-553)
(11) Another potential citation: GATA4 regulates angiogenesis and persistence of inflammation in rheumatoid arthritis PMID: 29717129 - around the inflammatory axis potential in OA? since GATA4 was reported in FLS from OA- PMC11183113.
Thank you. We have included this work/citation in the discussion section.\
“Further studies have shown that GATA4 regulates angiogenesis and inflammation in fibroblast-like synoviocytes in rheumatoid arthritis, indicating that GATA4 is required for the inflammation induced by IL-1b. This study also demonstrated that GATA4 binds to promoter regions on Vascular Endothelial Growth Factor (VEGF)-A and VEGFC to enhance transcription and regulate angiogenesis.[15]” (Lines 558-562)
-

-

eLife Assessment
This study presents an important finding on the role of GATA4 in aging and OA-associated cartilage pathology. The evidence supporting the conclusions is compelling, with rigorous in vitro and in vivo data. The work will be of broad interest to cell biologists and orthopedic clinicians.
-

Reviewer #1 (Public review):
Summary:
This manuscript assesses the differences between young and aged chondrocytes. Through transcriptomic analysis and further assessments in chondrocytes, GATA4 was found to be increased in aged chondrocyte donors compared to young donors. Subsequent mechanistic analysis with lentiviral vectors, siRNAs, and a small molecule was used to study the role of GATA4 in young and old chondrocytes. Lastly, an in vivo study was used to assess the effect of GATA4 expression on osteoarthritis progression in a DMM mouse model.
Strengths:
This work linked the overexpression of GATA4 to NF-kB signaling pathway activation, alterations to the TGF-b signaling pathway, and found that GATA4 increased the progression of OA compared to the DMM control group. This indicates that GATA4 contributes to the onset and progression …
Reviewer #1 (Public review):
Summary:
This manuscript assesses the differences between young and aged chondrocytes. Through transcriptomic analysis and further assessments in chondrocytes, GATA4 was found to be increased in aged chondrocyte donors compared to young donors. Subsequent mechanistic analysis with lentiviral vectors, siRNAs, and a small molecule was used to study the role of GATA4 in young and old chondrocytes. Lastly, an in vivo study was used to assess the effect of GATA4 expression on osteoarthritis progression in a DMM mouse model.
Strengths:
This work linked the overexpression of GATA4 to NF-kB signaling pathway activation, alterations to the TGF-b signaling pathway, and found that GATA4 increased the progression of OA compared to the DMM control group. This indicates that GATA4 contributes to the onset and progression of OA in aged individuals.
Weaknesses:
(1) A couple of sentences should be added to the introduction, to emphasize the role GATA4 plays, such as the alterations to the TGF-b signaling pathway and the increased activation of the NF-kB pathway.
(2) Figure 1F, the GATA4 histology image should be bigger.
(3) Further discussion should be conducted regarding the reasoning as to why GATA4 increases the phosphorylation of SMAD1/5.
(4) More information should be included to clarify why GATA4 is thought to be linked to DNA damage and the pathway that is associated with that.
(5) Please add further information regarding the limitations of the animal study conducted in this work and future plans to assess this.
(6) In Figure 5, GATA4 should be changed to Gata4 in the graphed portions for consistency.
-

Reviewer #2 (Public review):
Summary:
This study elucidated the impact of GATA4 on aging- and injury-induced cartilage degradation and osteoarthritis (OA) progression, based on the team's finding that GATA expression is positively correlated with aging in human chondrocytes. By integrating cell culture of human chondrocytes, gene manipulation tools (siRNA, lentivirus), biological/biochemical analyses and murine models of post-traumatic OA, the team found that increasing GATA4 levels reduced anabolism and increased catabolism of chondrocytes from young donors, likely through upregulation of the BMP pathway, and that this impact is not correlated with TGF-β stimulation. Conversely, silencing GATA4 by siRNA attenuated catabolism and elevated aggrecan/collagen II biosynthesis of chondrocytes from old donors. The physiological relevance of …
Reviewer #2 (Public review):
Summary:
This study elucidated the impact of GATA4 on aging- and injury-induced cartilage degradation and osteoarthritis (OA) progression, based on the team's finding that GATA expression is positively correlated with aging in human chondrocytes. By integrating cell culture of human chondrocytes, gene manipulation tools (siRNA, lentivirus), biological/biochemical analyses and murine models of post-traumatic OA, the team found that increasing GATA4 levels reduced anabolism and increased catabolism of chondrocytes from young donors, likely through upregulation of the BMP pathway, and that this impact is not correlated with TGF-β stimulation. Conversely, silencing GATA4 by siRNA attenuated catabolism and elevated aggrecan/collagen II biosynthesis of chondrocytes from old donors. The physiological relevance of GATA4 was further validated by the accelerated OA progression observed in lentivirus-infected mice in the DMM model.
Strengths:
This is a highly significant and innovative study that provides new molecular insights into cartilage homeostasis and pathology in the context of aging and disease. The experiments were performed in a comprehensive and rigorous manner. The data were interpreted thoroughly in the context of the current literature.
Weaknesses:
(1) While it is convincing that GATA4 expression is elevated in elderly individuals, and that it has a detrimental impact on cartilage health, the authors might want to add further discussion on the variability among individual human donors, especially given the finding that the elevation of GATA4 was not observed in chondrocytes from donor O1 (Figure 1G).
(2) It might also be worth adding additional discussion on the interplay between senescent chondrocytes and the dysfunctional ECM during aging. As noted by the authors, aging is associated with decreased sGAG content and likely degenerative changes in the collagen II network, so the microniche of chondrocytes, and thus cell-matrix crosstalk through the pericellular matrix, is also altered or impaired.
-

Reviewer #3 (Public review):
Summary:
This is an exciting, comprehensive paper that demonstrates the role of GATA4 on OA-like changes in chondrocytes. The authors present elegant reverse translational experiments that justify this mechanism and demonstrate the sufficiency of GATA4 in a mouse model of osteoarthritis (DMM), where GATA4 drove cartilage degeneration and pain in a manner that was significantly worse than DMM alone. This could pave the way for new therapies for OA that account for both structural changes and pain.
Strengths:
(1) GATA4 was identified in human chondrocytes.
(2) IHC and sequencing confirmed GATA4 presence.
(3) Activation of SMADs is clearly shown in vitro with GATA4 overexpression.
(4) The role of GATA4 was functionally assessed in vivo using the mouse DMM model, where the authors uncovered that GATA4 worsens OA …
Reviewer #3 (Public review):
Summary:
This is an exciting, comprehensive paper that demonstrates the role of GATA4 on OA-like changes in chondrocytes. The authors present elegant reverse translational experiments that justify this mechanism and demonstrate the sufficiency of GATA4 in a mouse model of osteoarthritis (DMM), where GATA4 drove cartilage degeneration and pain in a manner that was significantly worse than DMM alone. This could pave the way for new therapies for OA that account for both structural changes and pain.
Strengths:
(1) GATA4 was identified in human chondrocytes.
(2) IHC and sequencing confirmed GATA4 presence.
(3) Activation of SMADs is clearly shown in vitro with GATA4 overexpression.
(4) The role of GATA4 was functionally assessed in vivo using the mouse DMM model, where the authors uncovered that GATA4 worsens OA structure and hyperalgesia in male mice.
(5) It is interesting that GATA4 is largely known to be found in cardiac cells and to have a role in cardiac repair, metabolism, and inflammation, among other things listed by the authors in the discussion (in liver, lung, pancreas). What could this new knowledge of GATA4 mean for OA as a potentially systemically mediated disease, where cardiac disease and metabolic syndrome are often co-morbid?
Weaknesses:
(1) It would be useful to explain why GATA4 was chosen over HIF1a, which was the most differentially expressed.
(2) In Figure 5, it would be useful to demonstrate the non-surgical or naive limbs to help contextualize OARSI scores and knee hyperalgesia changes.
(3) While there appear to be GATA4 small-molecule inhibitors in various stages of development that could be used to assess the effects in age-related OA, those experiments are out of scope for the current study.
-