Branched actin polymerization drives invasive protrusion formation to promote myoblast fusion during mouse skeletal muscle regeneration
Curation statements for this article:-
Curated by eLife
eLife Assessment
This study presents a valuable finding regarding the role of Arp2/3 and the actin nucleators N-WASP and WAVE complexes in myoblast fusion. The data presented is convincing, and the work will be of interest to biologists studying skeletal muscle stem cell biology in the context of skeletal muscle regeneration.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Skeletal muscle regeneration is a multistep process involving the activation, proliferation, differentiation, and fusion of muscle stem cells, known as satellite cells. Fusion of satellite cell-derived myoblasts (SCMs) is indispensable for generating the multinucleated, contractile myofibers during muscle repair. However, the molecular and cellular mechanisms underlying SCM fusion during muscle regeneration remain incompletely understood. Here, we reveal a critical role for branched actin polymerization in SCM fusion during mouse skeletal muscle regeneration. Using conditional knockouts of the Arp2/3 complex and its actin nucleation-promoting factors N-WASP and WAVE, we demonstrate that branched actin polymerization is specifically required for SCM fusion but dispensable for satellite cell proliferation, differentiation, and migration. We show that the N-WASP and WAVE complexes have partially redundant functions in regulating SCM fusion and that branched actin polymerization is essential for generating invasive protrusions at fusogenic synapses in SCMs. Together, our study identifies branched-actin regulators as key components of the myoblast fusion machinery and establishes invasive protrusion formation as a critical mechanism enabling myoblast fusion during skeletal muscle regeneration.
Article activity feed
-

-

-

eLife Assessment
This study presents a valuable finding regarding the role of Arp2/3 and the actin nucleators N-WASP and WAVE complexes in myoblast fusion. The data presented is convincing, and the work will be of interest to biologists studying skeletal muscle stem cell biology in the context of skeletal muscle regeneration.
-

Reviewer #1 (Public review):
Overall, the manuscript reveals the role for actin polymerization to drive fusion of myoblasts during adult muscle regeneration. This pathway regulates fusion in many contexts, but whether it was conserved in adult muscle regeneration remained unknown. Robust genetic tools and histological analyses were used to convincingly support the claims.
-

Reviewer #2 (Public review):
To fuse, differentiated muscle cells must rearrange their cytoskeleton and assemble actin-enriched cytoskeletal structures. These actin foci are proposed to generate mechanical forces necessary to drive close membrane apposition and the fusion pore formation. While the study of these actin-rich structures has been conducted mainly in drosophila and in vertebrate embryonic development, the present manuscript present clear evidence this mechanism is necessary for fusion of adult muscle stem cells in vivo, in mice. The data presented here clearly demonstrate that ARP2/3 and SCAR/WAVE complexes are required for differentiating satellite cells fusion into multinucleated myotubes, during skeletal muscle regeneration.
-

Reviewer #3 (Public review):
This manuscript addresses an important biological question regarding the mechanisms of muscle cell fusion during regeneration. The primary strength of this work lies in the clean and convincing experiments, with the major conclusions being well-supported by the data provided.
The authors have satisfactorily addressed my inquiries.
-

Author response:
The following is the authors’ response to the previous reviews.
Reviewer #3 (Public review):
The authors have satisfactorily addressed my inquiries. However, I had to look quite hard to find where they responded to my final comment regarding the potential role of Arpc2 post-fusion during myofiber growth and/or maintenance, which I eventually located on page 7. I would appreciate it if the authors could state this point more explicitly, perhaps by adding a sentence such as "However, we cannot rule out the possibility that Arpc2 may also play a role in....." to improve clarity of communication.
While I understood from the original version that this issue falls beyond the immediate scope of the study, I believe it is important to adopt a more cautious and rigorous interpretative framework, especially given the widespread …
Author response:
The following is the authors’ response to the previous reviews.
Reviewer #3 (Public review):
The authors have satisfactorily addressed my inquiries. However, I had to look quite hard to find where they responded to my final comment regarding the potential role of Arpc2 post-fusion during myofiber growth and/or maintenance, which I eventually located on page 7. I would appreciate it if the authors could state this point more explicitly, perhaps by adding a sentence such as "However, we cannot rule out the possibility that Arpc2 may also play a role in....." to improve clarity of communication.
While I understood from the original version that this issue falls beyond the immediate scope of the study, I believe it is important to adopt a more cautious and rigorous interpretative framework, especially given the widespread use of this experimental approach. In particular, when a gene could potentially have additional roles in myofibers, it may be helpful to explicitly acknowledge that possibility. Even if Arpc2 may not necessarily be one of them, such roles cannot be fully excluded without direct testing.
We appreciate the reviewer’s comments and have included several sentences at the end of the “Branched actin polymerization is required for SCM fusion” section to address this question:
“The severe myoblast fusion defects observed in early stages of regeneration (e.g. dpi 4.5) provide a good explanation for the presence of thin muscle fibers in ArpC2 cKO mice at dpi 14 (Fig. 2B and 2C) and dpi 28 (Fig. S4A and S4B). These thin muscle fibers could be either elongated mononucleated muscle cells or multinucleated myofibers each containing a small number of nuclei due to occasional fusion events (comparable to those in Myomixer cKO muscles) (Fig. 2B and 2C; Fig. S4A and S4B). Whether Arp2/3 and branched actin polymerization play a role in the growth and/or maintenance of post-fusion multinucleated myofibers requires future loss-of-function studies in which ArpC2 cKO is generated using a myofiber-specific cre driver.”
-

-

-

eLife Assessment
This study presents a valuable finding regarding the role of Arp2/3 and the actin nucleators N-WASP and WAVE complexes in myoblast fusion. The data presented is convincing, and the work will be of interest to biologists studying skeletal muscle stem cell biology in the context of skeletal muscle regeneration.
-

Reviewer #1 (Public review):
Overall, the manuscript reveals the role for actin polymerization to drive fusion of myoblasts during adult muscle regeneration. This pathway regulates fusion in many contexts, but whether it was conserved in adult muscle regeneration remained unknown. Robust genetic tools and histological analyses were used to convincingly support the claims.
-

Reviewer #2 (Public review):
To fuse, differentiated muscle cells must rearrange their cytoskeleton and assemble actin-enriched cytoskeletal structures. These actin foci are proposed to generate mechanical forces necessary to drive close membrane apposition and the fusion pore formation. While the study of these actin-rich structures has been conducted mainly in drosophila and in vertebrate embryonic development, the present manuscript present clear evidence this mechanism is necessary for fusion of adult muscle stem cells in vivo, in mice. The data presented here clearly demonstrate that ARP2/3 and SCAR/WAVE complexes are required for differentiating satellite cells fusion into multinucleated myotubes, during skeletal muscle regeneration.
-

Reviewer #3 (Public review):
The authors have satisfactorily addressed my inquiries. However, I had to look quite hard to find where they responded to my final comment regarding the potential role of Arpc2 post-fusion during myofiber growth and/or maintenance, which I eventually located on page 7. I would appreciate it if the authors could state this point more explicitly, perhaps by adding a sentence such as "However, we cannot rule out the possibility that Arpc2 may also play a role in....." to improve clarity of communication.
While I understood from the original version that this issue falls beyond the immediate scope of the study, I believe it is important to adopt a more cautious and rigorous interpretative framework, especially given the widespread use of this experimental approach. In particular, when a gene could potentially …
Reviewer #3 (Public review):
The authors have satisfactorily addressed my inquiries. However, I had to look quite hard to find where they responded to my final comment regarding the potential role of Arpc2 post-fusion during myofiber growth and/or maintenance, which I eventually located on page 7. I would appreciate it if the authors could state this point more explicitly, perhaps by adding a sentence such as "However, we cannot rule out the possibility that Arpc2 may also play a role in....." to improve clarity of communication.
While I understood from the original version that this issue falls beyond the immediate scope of the study, I believe it is important to adopt a more cautious and rigorous interpretative framework, especially given the widespread use of this experimental approach. In particular, when a gene could potentially have additional roles in myofibers, it may be helpful to explicitly acknowledge that possibility. Even if Arpc2 may not necessarily be one of them, such roles cannot be fully excluded without direct testing.
-

Author response:
The following is the authors’ response to the original reviews
Reviewer #1 (Public review):
Overall, the manuscript reveals the role of actin polymerization to drive the fusion of myoblasts during adult muscle regeneration. This pathway regulates fusion in many contexts, but whether it was conserved in adult muscle regeneration remained unknown. Robust genetic tools and histological analyses were used to support the claims convincingly.
We very much appreciate the positive comments from this Reviewer.
There are a few interpretations that could be adjusted.
The beginning of the results about macrophages traversing ghost fibers after regeneration was a surprise given the context in the abstract and introduction. These results also lead to new questions about this biology that would need to be answered to substantiate …
Author response:
The following is the authors’ response to the original reviews
Reviewer #1 (Public review):
Overall, the manuscript reveals the role of actin polymerization to drive the fusion of myoblasts during adult muscle regeneration. This pathway regulates fusion in many contexts, but whether it was conserved in adult muscle regeneration remained unknown. Robust genetic tools and histological analyses were used to support the claims convincingly.
We very much appreciate the positive comments from this Reviewer.
There are a few interpretations that could be adjusted.
The beginning of the results about macrophages traversing ghost fibers after regeneration was a surprise given the context in the abstract and introduction. These results also lead to new questions about this biology that would need to be answered to substantiate the claims in this section. Also, it is unclear the precise new information learned here because it seems obvious that macrophages would need to extravasate the basement membrane to enter ghost fibers and macrophages are known to have this ability. Moreover, the model in Figure 4D has macrophages and BM but there is not even mention of this in the legend. The authors may wish to consider removing this topic from the manuscript.
We appreciate this comment and acknowledge that the precise behavior of macrophages when they infiltrate and/or exit the ghost fibers during muscle regeneration is not the major focus of this study. However, we think that visualizing macrophages squeezing through tiny openings on the basement membrane to infiltrate and/or exit from the ghost fibers is valuable. Thus, we have moved the data from the original main Figure 2 to the new Figure S1.
Regarding the model in Figure 4D, we have removed the macrophages because the depicted model represents a stage after the macrophages’ exit from the ghost fiber.
Which Pax7CreER line was used? In the methods, the Jax number provided is the Gaka line but in the results, Lepper et al 2009 are cited, which is not the citation for the Gaka line.
The Pax7CreER line used in this study is the one generated in Lepper et al. 2009. We corrected this information in “Material and Methods” of the revised manuscript.
Did the authors assess regeneration in the floxed mice that do not contain Cre as a control? Or is it known these alleles do not perturb the function of the targeted gene?
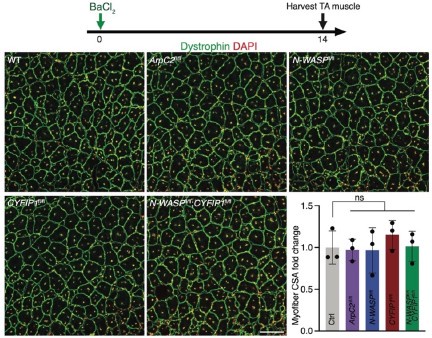
We examined muscle regeneration in the floxed mice without Cre. As shown in Figure 1 below, none of the homozygous ArpC2fl/fl, N-WASPfl/fl, CYFIP1fl/fl or N-WASPfl/fl;CYFIP1fl/fl alleles affected muscle regeneration, indicating that these alleles do not perturb the function of the targeted gene.
Author response image 1.
The muscle regeneration was normal in mice with only floxed target gene(s). Cross sections of TA muscles were stained with anti-Dystrophin and DAPI at dpi 14. n = 3 mice of each genotype, and > 80 ghost fibers in each mouse were examined. Mean ± s.d. values are shown in the dot-bar plot, and significance was determined by two-tailed student’s t-test. ns: not significant. Scale bar: 100 μm.
The authors comment: 'Interestingly, expression of the fusogenic proteins, MymK and MymX, was up-regulated in the TA muscle of these mice (Figure S4F), suggesting that fusogen overexpression is not able to rescue the SCM fusion defect resulted from defective branched actin polymerization.' It is unclear if fusogens are truly overexpressed because the analysis is performed at dpi 4 when the expression of fusogens may be decreased in control mice because they have already fused. Also, only two animals were analyzed and it is unclear if MymX is definitively increased. The authors should consider adjusting the interpretation to SCM fusion defect resulting from defective branched actin polymerization is unlikely to be caused by a lack of fusogen expression.
We agree with the Reviewer that fusogen expression may simply persist till later time points in fusion mutants without being up-regulated. We have modified our interpretation according to the Reviewer’s suggestion.
Regarding the western blots in the original Figure S4F, we now show one experiment from each genotype, and include the quantification of MymK and MymX protein levels from 3 animals in the revised manuscript (new Figure S5F-S5H).
Reviewer #1 (Recommendations for the authors):
(1) The ArpC2 cKO data could be presented in a clearer fashion. In the text, ArpC2 is discussed but in the figure, there are many other KOs presented and ArpC2 is the fourth one shown in the figure. The other KOs are discussed later. It may be worthwhile for the authors to rearrange the figures to make it easier for readers.
Thank you for this suggestion. We have rearranged the genotypes in the figures accordingly and placed ArpC2 cKO first.
The authors comment: 'Since SCM fusion is mostly completed at dpi 4.5 (Figure 1B) (Collins et al. 2024)'. This is not an accurate statement of the cited paper. While myofibers are formed by dpi 4.5 with centralized nuclei, there are additional fusion events through at least 21dpi. The authors should adjust their statement to better reflect the data in Collins et al 2024, which could include mentioning that primary fusions could be completed at dpi 4.5 and this is the process they are studying.
We have adjusted our statement accordingly in the revised manuscript.
The authors comment: 'Consistent with this, the frequency distribution of SCM number per ghost fiber displayed a dramatic shift toward higher numbers in the ArpC2cKO mice (Figure S5C). These results indicate that the actin cytoskeleton plays an essential role in SCM fusion as the fusogenic proteins. Should it read 'These results indicate that the actin cytoskeleton plays AS an essential role in SCM fusion as the fusogenic proteins'?
Yes, and we adjusted this statement accordingly in the revised manuscript.
Minor comments
(1) In the results the authors state 'To induce genetic deletion of ArpC2 in satellites....'; 'satellites' is a term not typically used for satellite cells.
Thanks for catching this. We changed “satellites” to satellite cells.
(2) In the next sentence, the satellite should be capitalized.
Done.
(3) The cross-section area should be a 'cross-sectional area'.
Changed.
Reviewer #2 (Public review):
To fuse, differentiated muscle cells must rearrange their cytoskeleton and assemble actinenriched cytoskeletal structures. These actin foci are proposed to generate mechanical forces necessary to drive close membrane apposition and fusion pore formation.
While the study of these actin-rich structures has been conducted mainly in drosophila, the present manuscript presents clear evidence this mechanism is necessary for the fusion of adult muscle stem cells in vivo, in mice.
We thank this Reviewer for the positive comment.
However, the authors need to tone down their interpretation of their findings and remember that genetic proof for cytoskeletal actin remodeling to allow muscle fusion in mice has already been provided by different labs (Vasyutina E, et al. 2009 PMID: 19443691; Gruenbaum-Cohen Y, et al., 2012 PMID: 22736793; Hamoud et al., 2014 PMID: 24567399). In the same line of thought, the authors write they "demonstrated a critical function of branched actin-propelled invasive protrusions in skeletal muscle regeneration". I believe this is not a premiere, since Randrianarison-Huetz V, et al., previously reported the existence of finger-like actin-based protrusions at fusion sites in mice myoblasts (PMID: 2926942) and Eigler T, et al., live-recorded said "fusogenic synapse" in mice myoblasts (PMID: 34932950). Hence, while the data presented here clearly demonstrate that ARP2/3 and SCAR/WAVE complexes are required for differentiating satellite cell fusion into multinucleated myotubes, this is an incremental story, and the authors should put their results in the context of previous literature.
In this study, we focused on elucidating the mechanisms of myoblast fusion during skeletal muscle regeneration, which remained largely unknown. Thus, we respectfully disagree with this Reviewer that “this is an incremental story” for the following reasons –
First, while we agree with this Reviewer that “genetic proof for cytoskeletal actin remodeling to allow muscle fusion in mice has already been provided by different labs”, most of the previous genetic studies, including ours (Lu et al. 2024), characterizing the roles of actin regulators (Elmo, Dock180, Rac, Cdc42, WASP, WIP, WAVE, Arp2/3) in mouse myoblast fusion were conducted during embryogenesis (Laurin et al. 2008; Vasyutina et al. 2009; Gruenbaum-Cohen et al. 2012; Tran et al. 2022; Lu et al. 2024), instead of during adult muscle regeneration, the latter of which is the focus of this study.
Second, prior to this study, several groups tested the roles of SRF, CaMKII theta and gemma, Myo10, and Elmo, which affect actin cytoskeletal dynamics, in muscle regeneration. These studies have shown that knocking out SRF, CaMKII, Myo10, or Elmo caused defects in mouse muscle regeneration, based on measuring the cross-sectional diameters of regenerated myofibers only (Randrianarison-Huetz et al. 2018; Eigler et al. 2021; Hammers et al. 2021; Tran et al. 2022). However, none of these studies visualized myoblast fusion at the cellular and subcellular levels during muscle regeneration in vivo. For this reason, it remained unclear whether the muscle regeneration defects in these mutants were indeed due to defects in myoblast fusion, in particular, defects in the formation of invasive protrusions at the fusogenic synapse. Thus, the previous studies did not demonstrate a direct role for the actin cytoskeleton, as well as the underlying mechanisms, in myoblast fusion during muscle regeneration in vivo.
Third, regarding actin-propelled invasive protrusions at the fusogenic synapse, our previous study (Lu et al. 2024) revealed these structures by fluorescent live cell imaging and electron microscopy (EM) in cultured muscle cells, as well as EM studies in mouse embryonic limb muscle, firmly establishing a direct role for invasive protrusions in mouse myoblast fusion in cultured muscle cells and during embryonic development. Randrianarison-Huetz et al. (2018) reported the existence of finger-like actin-based protrusions at cell contact sites of cultured mouse myoblasts. It was unclear from their study, however, if these protrusions were at the actual fusion sites and if they were invasive (Randrianarison-Huetz et al. 2018). Eigler et al. (2021) reported protrusions at fusogenic synapse in cultured mouse myoblasts. It was unclear from their study, however, if the protrusions were actin-based and if they were invasive (Eigler et al. 2021). Neither Randrianarison-Huetz et al. (2018) nor Eigler et al. (2021) characterized protrusions in developing mouse embryos or regenerating adult muscle.
Taken together, to our knowledge, this is the first study to characterize myoblast fusion at the cellular and subcellular level during mouse muscle regeneration. We demonstrate that branched actin polymerization promotes invasive protrusion formation and myoblast fusion during the regeneration process. We believe that this work has laid the foundation for additional mechanistic studies of myoblast fusion during skeletal muscle regeneration.
The citations in the original manuscript were primarily focused on previous in vivo studies of Arp2/3 and the actin nucleation-promoting factors (NPFs), N-WASP and WAVE (Richardson et al. 2007; Gruenbaum-Cohen et al. 2012), and of invasive protrusions mediating myoblast fusion in intact animals (Drosophila, zebrafish and mice) (Sens et al. 2010; Luo et al. 2022; Lu et al. 2024). We agree with this reviewer, however, that it would be beneficial to the readers if we provide a more comprehensive summary of previous literature, including studies of both intact animals and cultured cells, as well as studies of additional actin regulators upstream of the NPFs, such as small GTPases and their GEFs. Thus, we have significantly expanded our Introduction to include these studies and cited the corresponding literature in the revised manuscript.
Reviewer #2 (Recommendations for the authors):
(1) I am concerned that the authors did not evaluate the efficiency of the target allele deletion efficiency following Pax7-CreER activation. The majority, if not all, of the published work focusing on this genetic strategy presents the knock-down efficiency using either genotyping PCR, immunolocalization, western-blot; etc...
(2) Can the authors provide evidence that the N-WASP, CYFIP1, and ARPC2 proteins are depleted in TAM-treated tissue? Alternatively, can the author perform RT-qPCR on freshly isolated MuSCs to validate the absence of N-WASP, CYFIP1, and ARPC2 mRNA expression?
Thank you for these comments. We have assessed the target allele deletion efficiency with isolated satellite cells from TAM-injected mice in which Pax7-CreER is activated. Western blot analyses showed that the protein levels of N-WASP, CYFIP1, and ArpC2 significantly decreased in the satellite cells of knockout mice. Please see the new Figure S2.
Reviewer #3 (Public review):
The manuscript by Lu et al. explores the role of the Arp2/3 complex and the actin nucleators NWASP and WAVE in myoblast fusion during muscle regeneration. The results are clear and compelling, effectively supporting the main claims of the study. However, the manuscript could benefit from a more detailed molecular and cellular analysis of the fusion synapse. Additionally, while the description of macrophage extravasation from ghost fibers is intriguing, it seems somewhat disconnected from the primary focus of the work.
Despite this, the data are robust, and the major conclusions are well supported. Understanding muscle fusion mechanism is still a widely unexplored topic in the field and the authors make important progress in this domain.
We appreciate the positive comments from this Reviewer.
We agree with this Reviewer and Reviewer #1 that the macrophage study is not the primary focus of the work. However, we think that visualizing macrophages squeezing through tiny openings on the basement membrane to infiltrate and/or exit from the ghost fibers is valuable. Thus, we have moved the data from the original main Figure 2 to the new Figure S1.
I have a few suggestions that might strengthen the manuscript as outlined below.
(1) Could the authors provide more detail on how they defined cells with "invasive protrusions" in Figure 4C? Membrane blebs are commonly observed in contacting cells, so it would be important to clarify the criteria used for counting this specific event.
Thanks for this suggestion. We define invasive protrusions as finger-like protrusions projected by a cell into its fusion partner. Based on our previous studies (Sens et al. 2010; Luo et al. 2022; Lu et al. 2024), these invasive protrusions are narrow (with 100-250 nm diameters) and propelled by mechanically stiff actin bundles. In contrast, membrane blebs are spherical protrusions formed by the detachment of the plasma membrane from the underlying actin cytoskeleton. In general, the blebs are not as mechanically stiff as invasive protrusions and would not be able to project into neighboring cells. Thus, we do not think that the protrusions in Figure 4B are membrane blebs. We clarified the criteria in the text and figure legends of the revised manuscript.
(2) Along the same line, please clarify what each individual dot represents in Figure 4C. The authors mention quantifying approximately 83 SCMs from 20 fibers. I assume each dot corresponds to data from individual fibers, but if that's the case, does this imply that only around four SCMs were quantified per fiber? A more detailed explanation would be helpful.
To quantitatively assess invasive protrusions in Ctrl and mutant mice, we analyzed 20 randomly selected ghost fibers per genotype. Within each ghost fiber, we examined randomly selected SCMs in a single cross section (a total of 83, 147 and 93 SCMs in Ctrl, ArpC2cKO and MymXcKO mice were examined, respectively).
In Figure 4C, each dot was intended to represent the percentage of SCMs with invasive protrusions in a single cross section of a ghost fiber. However, we mistakenly inserted a wrong graph in the original Figure 4C. We sincerely apologize for this error and have replaced it with the correct graph in the new Figure 4C.
(3) Localizing ArpC2 at the invasive protrusions would be a strong addition to this study. Furthermore, have the authors examined the localization of Myomaker and Myomixer in ArpC2 mutant cells? This could provide insights into potential disruptions in the fusion machinery.
We have examined the localization of the Arp2/3 complex on the invasive protrusions in cultured SCMs and included the data in Figure 4A of the original manuscript. Specifically, we showed enrichment of mNeongreen-tagged Arp2, a subunit of the Arp2/3 complex, on the invasive protrusions at the fusogenic synapse of cultured SCMs (see the enlarged panels on the right; also see supplemental video 4). The small size of the invasive protrusions on SCMs prevented a detailed analysis of the precise Arp2 localization along the protrusions. Please see our recently published paper (Lu et al. 2024) for the detailed localization and function of the Arp2/3 complex during invasive protrusion formation in cultured C2C12 cells.
We have also attempted to localize the Arp2/3 complex in the regenerating muscle in vivo using an anti-ArpC2 antibody (Millipore, 07-227-I), which was used in many studies to visualize the Arp2/3 complex in cultured cells. Unfortunately, the antibody detected non-specific signals in the regenerating TA muscle of the ArpC2cKO animals. Thus, it cannot be used to detect specific ArpC2 signals in muscle tissues. Besides the specificity issue of the antibody, it is technically challenging to visualize invasive protrusions with an F-actin probe at the fusogenic synapses of regenerating muscle by light microscopy, due to the high background of F-actin signaling within the muscle cells.
Regarding the fusogens, we show that both are present in the TA muscle of the ArpC2cKO animals by western blot (Figure S5F-S5H). Thus, the fusion defect in these animals is not due to the lack of fusogen expression. Since the focus of this study is on the role of the actin cytoskeleton in muscle regeneration, the subcellular localization of the fusogens was not investigated in the current study.
(4) As a minor curiosity, can ArpC2 WT and mutant cells fuse with each other?
Our previous work in Drosophila embryos showed that Arp2/3-mediated branched actin polymerization is required in both the invading and receiving fusion partners (Sens et al. 2010). To address this question in mouse muscle cells, we co-cultured GFP+ WT cells with mScarleti+ WT (or mScarleti+ ArpC2cKO cells) in vitro and assessed their ability to fuse with one another. We found that ArpC2cKO cells could barely fuse with WT cells (new Figure 3F and 3G), indicating that the Arp2/3-mediated branched actin polymerization is required in both fusion partners. This result is consistent with our findings in Drosophila embryos.
(5) The authors report a strong reduction in CSA at 14 dpi and 28 dpi, attributing this defect primarily to failed myoblast fusion. Although this claim is supported by observations at early time points, I wonder whether the Arp2/3 complex might also play roles in myofibers after fusion. For instance, Arp2/3 could be required for the growth or maintenance of healthy myofibers, which could also contribute to the reduced CSA observed, since regenerated myofibers inherit the ArpC2 knockout from the stem cells. Could the authors address or exclude this possibility? This is rather a broader criticism of how things are being interpreted in general beyond this paper.
This is an interesting question. It is possible that Arp2/3 may play a role in the growth or maintenance of healthy myofibers. However, the muscle injury and regeneration process may not be the best system to address this question because of the indispensable early step of myoblast fusion. Ideally, one may want to knockout Arp2/3 in myofibers of young healthy mice and observe fiber growth in the absence of muscle injury and compare that to the wild-type littermates. Since these experiments are out of the scope of this study, we revised our conclusion that the fusion defect in ArpC2cKO mice should account, at least in part, for the strong reduction in CSA at 14 dpi and 28 dpi, without excluding additional possibilities such as Arp2/3’s potential role in the growth or maintenance of healthy myofibers.
References:
Eigler T, Zarfati G, Amzallag E, Sinha S, Segev N, Zabary Y, Zaritsky A, Shakked A, Umansky KB, Schejter ED et al. 2021. ERK1/2 inhibition promotes robust myotube growth via CaMKII activation resulting in myoblast-to-myotube fusion. Dev Cell 56: 3349-3363 e3346.
Gruenbaum-Cohen Y, Harel I, Umansky KB, Tzahor E, Snapper SB, Shilo BZ, Schejter ED. 2012. The actin regulator N-WASp is required for muscle-cell fusion in mice. Proc Natl Acad Sci U S A 109: 11211-11216.
Hammers DW, Hart CC, Matheny MK, Heimsath EG, Lee YI, Hammer JA, 3rd, Cheney RE, Sweeney HL. 2021. Filopodia powered by class x myosin promote fusion of mammalian myoblasts. Elife 10.
Laurin M, Fradet N, Blangy A, Hall A, Vuori K, Cote JF. 2008. The atypical Rac activator Dock180 (Dock1) regulates myoblast fusion in vivo. Proc Natl Acad Sci U S A 105: 15446-15451.
Lu Y, Walji T, Ravaux B, Pandey P, Yang C, Li B, Luvsanjav D, Lam KH, Zhang R, Luo Z et al. 2024. Spatiotemporal coordination of actin regulators generates invasive protrusions in cell-cell fusion. Nat Cell Biol 26: 1860-1877.
Luo Z, Shi J, Pandey P, Ruan ZR, Sevdali M, Bu Y, Lu Y, Du S, Chen EH. 2022. The cellular architecture and molecular determinants of the zebrafish fusogenic synapse. Dev Cell 57: 1582-1597 e1586.
Randrianarison-Huetz V, Papaefthymiou A, Herledan G, Noviello C, Faradova U, Collard L, Pincini A, Schol E, Decaux JF, Maire P et al. 2018. Srf controls satellite cell fusion through the maintenance of actin architecture. J Cell Biol 217: 685-700.
Richardson BE, Beckett K, Nowak SJ, Baylies MK. 2007. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development 134: 4357-4367.
Sens KL, Zhang S, Jin P, Duan R, Zhang G, Luo F, Parachini L, Chen EH. 2010. An invasive podosome-like structure promotes fusion pore formation during myoblast fusion. J Cell Biol 191: 1013-1027.
Tran V, Nahle S, Robert A, Desanlis I, Killoran R, Ehresmann S, Thibault MP, Barford D, Ravichandran KS, Sauvageau M et al. 2022. Biasing the conformation of ELMO2 reveals that myoblast fusion can be exploited to improve muscle regeneration. Nat Commun 13: 7077.
Vasyutina E, Martarelli B, Brakebusch C, Wende H, Birchmeier C. 2009. The small G-proteins Rac1 and Cdc42 are essential for myoblast fusion in the mouse. Proc Natl Acad Sci U S A 106: 8935-8940.
-

-

eLife Assessment
This study presents a valuable finding regarding the role of Arp2/3 and the actin nucleators N-WASP and WAVE complexes in myoblast fusion. The data presented is convincing, but it is suggested to perform validation of the knock-down efficiency of the mouse model and making adjustments to some of the data interpretation. The work will be of interest to biologists studying skeletal muscle stem cell biology in the context of skeletal muscle regeneration.
-

Reviewer #1 (Public review):
Overall, the manuscript reveals the role of actin polymerization to drive the fusion of myoblasts during adult muscle regeneration. This pathway regulates fusion in many contexts, but whether it was conserved in adult muscle regeneration remained unknown. Robust genetic tools and histological analyses were used to support the claims convincingly.
There are a few interpretations that could be adjusted.
The beginning of the results about macrophages traversing ghost fibers after regeneration was a surprise given the context in the abstract and introduction. These results also lead to new questions about this biology that would need to be answered to substantiate the claims in this section. Also, it is unclear the precise new information learned here because it seems obvious that macrophages would need to …
Reviewer #1 (Public review):
Overall, the manuscript reveals the role of actin polymerization to drive the fusion of myoblasts during adult muscle regeneration. This pathway regulates fusion in many contexts, but whether it was conserved in adult muscle regeneration remained unknown. Robust genetic tools and histological analyses were used to support the claims convincingly.
There are a few interpretations that could be adjusted.
The beginning of the results about macrophages traversing ghost fibers after regeneration was a surprise given the context in the abstract and introduction. These results also lead to new questions about this biology that would need to be answered to substantiate the claims in this section. Also, it is unclear the precise new information learned here because it seems obvious that macrophages would need to extravasate the basement membrane to enter ghost fibers and macrophages are known to have this ability. Moreover, the model in Figure 4D has macrophages and BM but there is not even mention of this in the legend. The authors may wish to consider removing this topic from the manuscript.
Which Pax7CreER line was used? In the methods, the Jax number provided is the Gaka line but in the results, Lepper et al 2009 are cited, which is not the citation for the Gaka line.
Did the authors assess regeneration in the floxed mice that do not contain Cre as a control? Or is it known these alleles do not perturb the function of the targeted gene?
The authors comment: 'Interestingly, expression of the fusogenic proteins, MymK and MymX, was up-regulated in the TA muscle of these mice (Fig. S4F), suggesting that fusogen overexpression is not able to rescue the SCM fusion defect resulted from defective branched actin polymerization.' It is unclear if fusogens are truly overexpressed because the analysis is performed at dpi 4 when the expression of fusogens may be decreased in control mice because they have already fused. Also, only two animals were analyzed and it is unclear if MymX is definitively increased. The authors should consider adjusting the interpretation to SCM fusion defect resulting from defective branched actin polymerization is unlikely to be caused by a lack of fusogen expression.
-

Reviewer #2 (Public review):
To fuse, differentiated muscle cells must rearrange their cytoskeletaon and assemble actin-enriched cytoskeletal structures. These actin foci are proposed to generate mechanical forces necessary to drive close membrane apposition and fusion pore formation.
While the study of these actin-rich structures has been conducted mainly in drosophila, the present manuscript presents clear evidence this mechanism is necessary for the fusion of adult muscle stem cells in vivo, in mice.
However, the authors need to tone down their interpretation of their findings and remember that genetic proof for cytoskeletal actin remodeling to allow muscle fusion in mice has already been provided by different labs (Vasyutina E, et al. 2009 PMID: 19443691; Gruenbaum-Cohen Y, et al., 2012 PMID: 22736793; Hamoud et al., 2014 PMID: …
Reviewer #2 (Public review):
To fuse, differentiated muscle cells must rearrange their cytoskeletaon and assemble actin-enriched cytoskeletal structures. These actin foci are proposed to generate mechanical forces necessary to drive close membrane apposition and fusion pore formation.
While the study of these actin-rich structures has been conducted mainly in drosophila, the present manuscript presents clear evidence this mechanism is necessary for the fusion of adult muscle stem cells in vivo, in mice.
However, the authors need to tone down their interpretation of their findings and remember that genetic proof for cytoskeletal actin remodeling to allow muscle fusion in mice has already been provided by different labs (Vasyutina E, et al. 2009 PMID: 19443691; Gruenbaum-Cohen Y, et al., 2012 PMID: 22736793; Hamoud et al., 2014 PMID: 24567399). In the same line of thought, the authors write they "demonstrated a critical function of branched actin-propelled invasive protrusions in skeletal muscle regeneration". I believe this is not a premiere, since Randrianarison-Huetz V, et al., previously reported the existence of finger-like actin-based protrusions at fusion sites in mice myoblasts (PMID: 2926942) and Eigler T, et al., live-recorded said "fusogenic synapse" in mice myoblasts (PMID: 34932950).
Hence, while the data presented here clearly demonstrate that ARP2/3 and SCAR/WAVE complexes are required for differentiating satellite cell fusion into multinucleated myotubes, this is an incremental story, and the authors should put their results in the context of previous literature.
-

Reviewer #3 (Public review):
The manuscript by Lu et al. explores the role of the Arp2/3 complex and the actin nucleators N-WASP and WAVE in myoblast fusion during muscle regeneration. The results are clear and compelling, effectively supporting the main claims of the study. However, the manuscript could benefit from a more detailed molecular and cellular analysis of the fusion synapse. Additionally, while the description of macrophage extravasation from ghost fibers is intriguing, it seems somewhat disconnected from the primary focus of the work.
Despite this, the data are robust, and the major conclusions are well supported. Understanding muscle fusion mechanism is still a widely unexplored topic in the field and the authors make important progress in this domain.
I have a few suggestions that might strengthen the manuscript as …
Reviewer #3 (Public review):
The manuscript by Lu et al. explores the role of the Arp2/3 complex and the actin nucleators N-WASP and WAVE in myoblast fusion during muscle regeneration. The results are clear and compelling, effectively supporting the main claims of the study. However, the manuscript could benefit from a more detailed molecular and cellular analysis of the fusion synapse. Additionally, while the description of macrophage extravasation from ghost fibers is intriguing, it seems somewhat disconnected from the primary focus of the work.
Despite this, the data are robust, and the major conclusions are well supported. Understanding muscle fusion mechanism is still a widely unexplored topic in the field and the authors make important progress in this domain.
I have a few suggestions that might strengthen the manuscript as outlined below.
(1) Could the authors provide more detail on how they defined cells with "invasive protrusions" in Figure 4C? Membrane blebs are commonly observed in contacting cells, so it would be important to clarify the criteria used for counting this specific event.
(2) Along the same line, please clarify what each individual dot represents in Figure 4C. The authors mention quantifying approximately 83 SCMs from 20 fibers. I assume each dot corresponds to data from individual fibers, but if that's the case, does this imply that only around four SCMs were quantified per fiber? A more detailed explanation would be helpful.
(3) Localizing ArpC2 at the invasive protrusions would be a strong addition to this study. Furthermore, have the authors examined the localization of Myomaker and Myomixer in ArpC2 mutant cells? This could provide insights into potential disruptions in the fusion machinery.
(4) As a minor curiosity, can ArpC2 WT and mutant cells fuse with each other?
(5) The authors report a strong reduction in CSA at 14 dpi and 28 dpi, attributing this defect primarily to failed myoblast fusion. Although this claim is supported by observations at early time points, I wonder whether the Arp2/3 complex might also play roles in myofibers after fusion. For instance, Arp2/3 could be required for the growth or maintenance of healthy myofibers, which could also contribute to the reduced CSA observed, since regenerated myofibers inherit the ArpC2 knockout from the stem cells. Could the authors address or exclude this possibility? This is rather a broader criticism of how things are being interpreted in general beyond this paper.
-