Cardiac fibroblasts regulate myocardium and coronary vasculature development in the murine heart via the collagen signaling pathway
Curation statements for this article:-
Curated by eLife
eLife Assessment
This study provides a comprehensive analysis of gene expression and bioinformatics data, offering important insights into the roles of fibroblasts in cardiac development. The large and well-analyzed single-cell RNA sequencing (scRNA-seq) dataset is compelling and a significant contribution to the field, and will be of broad interest to the scientific community.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The fibroblast (FB), cardiomyocyte (CM), and vascular endothelial cell (Vas_EC) are the three major cell types in the heart, yet their relationships during development are largely unexplored. To address this gap, we employed RNA staining of the FB marker gene Col1a1 together with the CM marker gene Actn2 and the Vas_EC marker gene Cdh5 at various stages of mouse heart development. This approach enabled us to discern the anatomical pattern of cardiac FBs and identify approximately one EC and four CMs directly interacting with each FB. Molecularly, through the analysis of single-cell mRNA sequencing (scRNA-seq) data, we unveiled collagen as the top signaling molecule derived from FBs influencing CM and Vas_EC development. Subsequently, we used a Pdgfra-CreER controlled diphtheria toxin A (DTA) system to ablate the FBs at different stages. We found that the ablation of FBs disrupted myocardium and vasculature development and led to embryonic heart defects. Using scRNA-seq, we further profiled the ablated hearts and identified molecular defects in their ventricular CMs and Vas_ECs compared to control hearts. Moreover, we identified a reduction of collagen in the ablated hearts and predicted collagen as the major signaling pathway regulating the differentially expressed genes in the ablated ventricular CMs. Finally, we performed both short-term and long-term FB ablation at the neonatal stage. We found that short-term ablation caused a reduction in collagen and Vas_EC density, while long-term ablation may induce compensatory collagen expression without causing heart function reduction. In summary, our study has identified the function of FBs in regulating myocardium and vasculature development in the mouse heart and implicated an important role for the collagen pathway in this process.
Article activity feed
-

-

-

-

eLife Assessment
This study provides a comprehensive analysis of gene expression and bioinformatics data, offering important insights into the roles of fibroblasts in cardiac development. The large and well-analyzed single-cell RNA sequencing (scRNA-seq) dataset is compelling and a significant contribution to the field, and will be of broad interest to the scientific community.
-

Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. The work includes extensive gene expression profiling and bioinformatic analysis. The prenatal fibroblast ablation studies show new information on the requirement of these cells on heart maturation before birth.
The strengths of the manuscript are the new single cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice. Extensive data are presented on mouse embryo fibroblast diversity and morphology in response to fibroblast ablation. Histological data support localization of major cardiac cell types and effects of fibroblast ablation on cardiac gene expression at different times …
Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. The work includes extensive gene expression profiling and bioinformatic analysis. The prenatal fibroblast ablation studies show new information on the requirement of these cells on heart maturation before birth.
The strengths of the manuscript are the new single cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice. Extensive data are presented on mouse embryo fibroblast diversity and morphology in response to fibroblast ablation. Histological data support localization of major cardiac cell types and effects of fibroblast ablation on cardiac gene expression at different times of development.
A weakness of the study is that the major conclusions regarding collagen signaling and heart maturation are based on gene expression patterns and are not functionally validated.
Comments on Revised Version (from BRE):
Most of my comments have been adequately addressed. Additional comments on new data in the revised manuscript are below.
(1) In the new figure S11, it is not really possible to draw major conclusions on mitral valve morphology and maturation since the planes of sections to not seem comparable. Observations regarding attachment to the papillary muscle might be dependent on the particular section being evaluated. However, it is useful to see that the valves are not severely affected in the ablated animals.
(2) In the last supplemental figure S19, it is not possible to determine if results are or are not statistically significant for n=2 as shown for FS and EF for the ablated animals and controls. The text says that there is a trend of improved heart function, but evaluation of additional animals is needed to support this conclusion.
-

Reviewer #2 (Public review):
This study aims to elucidate the role of fibroblasts in regulating myocardium and vascular development through signaling to cardiomyocytes and endothelial cells. This focus is significant, given that fibroblasts, cardiomyocytes, and vascular endothelial cells are the three primary cell types in the heart. The authors employed a Pdgfra-CreER-controlled diphtheria toxin A (DTA) system to ablate fibroblasts at various embryonic and postnatal stages, characterizing the resulting cardiac defects, particularly in myocardium and vasculature development. Single-cell RNA sequencing (scRNA-seq) analysis of the ablated hearts identified collagen as a crucial signaling molecule from fibroblasts that influences the development of cardiomyocytes and vascular endothelial cells.
This is an interesting manuscript; however, …
Reviewer #2 (Public review):
This study aims to elucidate the role of fibroblasts in regulating myocardium and vascular development through signaling to cardiomyocytes and endothelial cells. This focus is significant, given that fibroblasts, cardiomyocytes, and vascular endothelial cells are the three primary cell types in the heart. The authors employed a Pdgfra-CreER-controlled diphtheria toxin A (DTA) system to ablate fibroblasts at various embryonic and postnatal stages, characterizing the resulting cardiac defects, particularly in myocardium and vasculature development. Single-cell RNA sequencing (scRNA-seq) analysis of the ablated hearts identified collagen as a crucial signaling molecule from fibroblasts that influences the development of cardiomyocytes and vascular endothelial cells.
This is an interesting manuscript; however, there are several major issues, including an over-reliance on the scRNA-seq data, which shows inconsistencies between replicates.
Some of the major issues are described below.
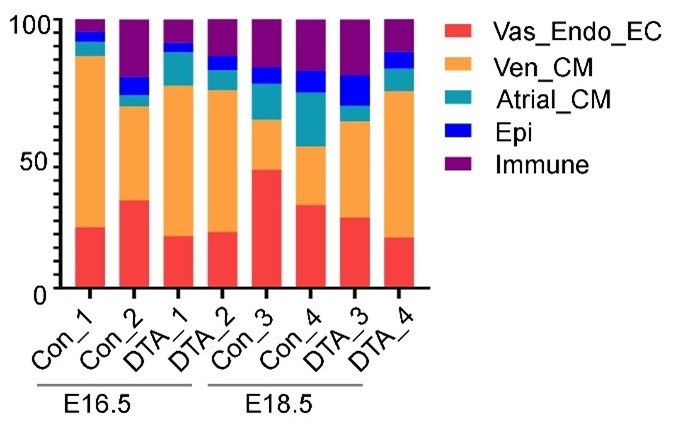
(1) The CD31 immunostaining data (Figure 3B-G) indicate a reduction in endothelial cell numbers following fibroblast deletion using PdgfraCreER+/-; RosaDTA+/- mice. However, the scRNA-seq data show no percentage change in the endothelial cell population (Figure 4D). Furthermore, while the percentage of Vas_ECs decreased in ablated samples at E16.5, the results at E18.5 were inconsistent, showing an increase in one replicate and a decrease in another, raising concerns about the reliability of the RNA-seq findings.
(2) Similarly, while the percentage of Ven_CMs increased at E18.5, it exhibited differing trends at E16.5 (Fig. 4E), further highlighting the inconsistency of the scRNA-seq analysis with the other data.
(3) Furthermore, the authors noted that the ablated samples had slightly higher percentages of cardiomyocytes in the G1 phase compared to controls (Fig. 4H, S11D), which aligns with the enrichment of pathways related to heart development, sarcomere organization, heart tube morphogenesis, and cell proliferation. However, it is unclear how this correlates with heart development, given that the hearts of ablated mice are significantly smaller than those of controls (Figure 3E). Additionally, the heart sections from ablated samples used for CD31/DAPI staining in Figure 3F appear much larger than those of the controls, raising further inconsistencies in the manuscript.
(4) The manuscript relies heavily on the scRNA-seq dataset, which shows inconsistencies between the two replicates. Furthermore, the morphological and histological analyses do not align with the scRNA-seq findings.
(5) There is a lack of mechanistic insight into how collagen, as a key signaling molecule from fibroblasts, affects the development of cardiomyocytes and vascular endothelial cells.
(6) In Figure 1B, Col1a1 expression is observed in the epicardial cells (Figure 1A, E11.5), but this is not represented in the accompanying cartoon.
(7) Do the PdgfraCreER+/-; RosaDTA+/- mice survive after birth when induced at E15.5, and do they exhibit any cardiac defects?
Comments on Revised Version (from BRE):
The manuscript has greatly improved following the revision, and I have no additional comments to offer.
-

Reviewer #3 (Public review):
Summary:
The authors investigated fibroblasts' communication with key cell types in developing and neonatal hearts, with focus on critical roles of fibroblast-cardiomyocyte and fibroblast-endothelial cells network in cardiac morphogenesis. They tried to map the spatial distribution of these cell types and reported the major pathways and signaling molecules driving the communication. They also used Cre-DTA system to ablate Pdgfra labeled cells and observed myocardial and endothelial cell defects at development. They screened the pathways and genes using sequencing data of ablated heart. Lastly they reported a compensatory collagen expression in long term ablated neonate heart. Overall, this study provides us with important insight on fibroblasts' roles in cardiac development and will be a powerful resource …
Reviewer #3 (Public review):
Summary:
The authors investigated fibroblasts' communication with key cell types in developing and neonatal hearts, with focus on critical roles of fibroblast-cardiomyocyte and fibroblast-endothelial cells network in cardiac morphogenesis. They tried to map the spatial distribution of these cell types and reported the major pathways and signaling molecules driving the communication. They also used Cre-DTA system to ablate Pdgfra labeled cells and observed myocardial and endothelial cell defects at development. They screened the pathways and genes using sequencing data of ablated heart. Lastly they reported a compensatory collagen expression in long term ablated neonate heart. Overall, this study provides us with important insight on fibroblasts' roles in cardiac development and will be a powerful resource for collagens and ECM focused research.
Strengths:
The authors utilized good analyzing tools to investigate on multiple database of single cell sequencing and Multi-seq. They identified significant pathways, cellular and molecular interactions of fibroblasts. Additionally, they compared some of their analytic findings with human database, and identified several groups of ECM genes with varying roles in mice.
Weaknesses:
This study is majorly based on sequencing data analysis. At the bench, they used very strident technique to study fibroblast functions by ablating one of the major cell population of heart. Also, experimental validation of their analyzed downstream pathways will be required eventually.
Comments on Revised Version (from BRE):
The authors did a good job addressing the questions asked at first review. However, I have some minor concerns.
(1) The paper notes that collagen signaling is observed in FB-VasEC in humans, but not in FB-VenCM, unlike mice. Did the authors analyze predictive ligand receptor interaction as they did with control and ablated mice heart? This could add valuable new insights that how FB regulate ventricular CM in human heart.
(2) The authors provided data on Defect in CD31 expression in several models. Did they observe any other phenotypes associated with defective endothelial or vascular system? Such as, blood accumulation in pericardium, larger/smaller capillaries? Did they also examine percentage of Cdh5+ cells?
(3) Please mention the sample age of Figure 2A-C.
(4) Please follow the same style to describe X axis in graphs in Figure 3D (and all similar graphs in the manuscript) as followed in 3G.
(5) It is important to provide echocardiographic M mode images with a comparable number of cardiac cycles in control and ablated (Fig. 6H).
(6) In the long-term neonatal ablation experiments, collagen expressions return to normal. The manuscript attributes this to possible "compensatory expression," Do they have any thoughts how this is regulated? Are other cell types stepping in, or are surviving FBs proliferating?
(7) While collagen is shown to be a dominant signaling molecule, its centrality is inferred primarily from scRNA-seq and ligand-receptor predictions. Did authors try any functional rescue experiment (e.g., exogenous collagen supplementation or receptor blockade) to directly validate this pathway's role in vivo?
-

Author response:
The following is the authors’ response to the previous reviews.
Public Reviews:
Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. The work includes extensive gene expression profiling and bioinformatic analysis. The prenatal fibroblast ablation studies show new information on the requirement of these cells on heart maturation before birth.
The strengths of the manuscript are the new single cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice. Extensive data are presented on mouse embryo fibroblast diversity and morphology in response to fibroblast ablation. Histological data support localization of …
Author response:
The following is the authors’ response to the previous reviews.
Public Reviews:
Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. The work includes extensive gene expression profiling and bioinformatic analysis. The prenatal fibroblast ablation studies show new information on the requirement of these cells on heart maturation before birth.
The strengths of the manuscript are the new single cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice. Extensive data are presented on mouse embryo fibroblast diversity and morphology in response to fibroblast ablation. Histological data support localization of major cardiac cell types and effects of fibroblast ablation on cardiac gene expression at different times of development.
A weakness of the study is that the major conclusions regarding collagen signaling and heart maturation are based on gene expression patterns and are not functionally validated.
Reviewer #2 (Public review):
This study aims to elucidate the role of fibroblasts in regulating myocardium and vascular development through signaling to cardiomyocytes and endothelial cells. This focus is significant, given that fibroblasts, cardiomyocytes, and vascular endothelial cells are the three primary cell types in the heart. The authors employed a Pdgfra-CreER-controlled diphtheria toxin A (DTA) system to ablate fibroblasts at various embryonic and postnatal stages, characterizing the resulting cardiac defects, particularly in myocardium and vasculature development. Single-cell RNA sequencing (scRNA-seq) analysis of the ablated hearts identified collagen as a crucial signaling molecule from fibroblasts that influences the development of cardiomyocytes and vascular endothelial cells.
This is an interesting manuscript; however, there are several major issues, including an over-reliance on the scRNA-seq data, which shows inconsistencies between replicates.
We thank the reviewer for carefully reading our revised manuscript. All of the questions listed below were raised in the previous round and have been addressed in the current revision. As noted in the “Recommendations for the Authors” section, the reviewer has no additional comments at this time.
Some of the major issues are described below.
(1) The CD31 immunostaining data (Figure 3B-G) indicate a reduction in endothelial cell numbers following fibroblast deletion using PdgfraCreER+/-; RosaDTA+/- mice. However, the scRNA-seq data show no percentage change in the endothelial cell population (Figure 4D). Furthermore, while the percentage of Vas_ECs decreased in ablated samples at E16.5, the results at E18.5 were inconsistent, showing an increase in one replicate and a decrease in another, raising concerns about the reliability of the RNA-seq findings.
(2) Similarly, while the percentage of Ven_CMs increased at E18.5, it exhibited differing trends at E16.5 (Fig. 4E), further highlighting the inconsistency of the scRNA-seq analysis with the other data.
(3) Furthermore, the authors noted that the ablated samples had slightly higher percentages of cardiomyocytes in the G1 phase compared to controls (Fig. 4H, S11D), which aligns with the enrichment of pathways related to heart development, sarcomere organization, heart tube morphogenesis, and cell proliferation. However, it is unclear how this correlates with heart development, given that the hearts of ablated mice are significantly smaller than those of controls (Figure 3E). Additionally, the heart sections from ablated samples used for CD31/DAPI staining in Figure 3F appear much larger than those of the controls, raising further inconsistencies in the manuscript.
(4) The manuscript relies heavily on the scRNA-seq dataset, which shows inconsistencies between the two replicates. Furthermore, the morphological and histological analyses do not align with the scRNA-seq findings.
(5) There is a lack of mechanistic insight into how collagen, as a key signaling molecule from fibroblasts, affects the development of cardiomyocytes and vascular endothelial cells.
(6) In Figure 1B, Col1a1 expression is observed in the epicardial cells (Figure 1A, E11.5), but this is not represented in the accompanying cartoon.
(7) Do the PdgfraCreER+/-; RosaDTA+/- mice survive after birth when induced at E15.5, and do they exhibit any cardiac defects?
Reviewer #3 (Public review):
Summary:
The authors investigated fibroblasts' communication with key cell types in developing and neonatal hearts, with focus on critical roles of fibroblast-cardiomyocyte and fibroblast-endothelial cells network in cardiac morphogenesis. They tried to map the spatial distribution of these cell types and reported the major pathways and signaling molecules driving the communication. They also used Cre-DTA system to ablate Pdgfra labeled cells and observed myocardial and endothelial cell defects at development. They screened the pathways and genes using sequencing data of ablated heart. Lastly they reported a compensatory collagen expression in long term ablated neonate heart. Overall, this study provides us with important insight on fibroblasts' roles in cardiac development and will be a powerful resource for collagens and ECM focused research.
Strengths:
The authors utilized good analyzing tools to investigate on multiple database of single cell sequencing and Multi-seq. They identified significant pathways, cellular and molecular interactions of fibroblasts. Additionally, they compared some of their analytic findings with human database, and identified several groups of ECM genes with varying roles in mice.
Weaknesses:
This study is majorly based on sequencing data analysis. At the bench, they used very strident technique to study fibroblast functions by ablating one of the major cell population of heart. Also, experimental validation of their analyzed downstream pathways will be required eventually.
Recommendations for the authors:
Reviewer #1 (Recommendations for the authors):
Most of my comments have been adequately addressed. Additional comments on new data in the revised manuscript are below.
(1) In the new figure S11, it is not really possible to draw major conclusions on mitral valve morphology and maturation since the planes of sections to not seem comparable. Observations regarding attachment to the papillary muscle might be dependent on the particular section being evaluated. However, it is useful to see that the valves are not severely affected in the ablated animals.
We appreciate the reviewer’s comment and agree with the reviewer’s observation. Accordingly, we have updated the manuscript by removing the original conclusion-related statement and instead highlighting that the valves were not severely affected in the ablated animals (page 6).
(2) In the last supplemental figure S19, it is not possible to determine if results are or are not statistically significant for n=2 as shown for FS and EF for the ablated animals and controls. The text says that there is a trend of improved heart function, but evaluation of additional animals is needed to support this conclusion.
We thank the reviewer for the comment and agree that a sample size of n = 2 is too small to draw meaningful conclusions. As previously suggested by the reviewer, we have removed this result from the manuscript (page 10).
Reviewer #2 (Recommendations for the authors):
The manuscript has greatly improved following the revision, and I have no additional comments to offer.
Thanks!
Reviewer #3 (Recommendations for the authors):
Authors did a good job addressing questions asked at first review. However, I have some minor concerns.
(1) The paper notes that collagen signaling is observed in FB-VasEC in humans, but not in FB-VenCM, unlike mice. Did authors analyze predictive ligand receptor interaction as they did with control and ablated mice heart? This could add valuable new insights that how FB regulate ventricular CM in human heart.
Thank you. We have analyzed the predicted ligand-receptor interactions between Fb and Ven_CM, as well as between Fb and Vas_EC, using human scRNA-seq data. The results are provided as a supplemental figure (Fig. S8C).
(2) The authors provided data on Defect in CD31 expression in several models. Did they observed any other phenotypes associated with defective endothelial or vascular system? Such as, blood accumulation in pericardium, larger/smaller capillaries? Did they also examined percentage of Cdh5+ cells?
We thank the reviewer for the questions. We did not observe clear evidence of blood accumulation in the pericardium of the ablated hearts, as shown in figure 3B, 3E, 6B, and 6F. Additionally, we did not perform Cdh5 staining in either the control or ablated hearts.
(3) Please mention the sample age of Figure 2A-C.
These are single-cell mRNA sequencing data from CD1 mice across 18 developmental stages, ranging from E9.5 to P9. We have added this information to the manuscript (page 4).
(4) Please follow the same style to describe X axis in graphs in Figure 3D (and all similar graphs in manuscript) as followed in 3G.
Thank you. We assume the reviewer was referring to the descriptions in the relevant figure legends. We have updated the legend for Figure 3D to ensure consistency with the description provided for Figure 3G (page 15).
(5) It is important to provide echocardiographic M mode images with a comparable number of cardiac cycles in control and ablated (Fig. 6H).
We thank the reviewer for the comment. As explained in our previous response, the echocardiographic data for both control and mutant mice were collected in conscious animals. The differences in their cardiac cycles reflect variations in heart rate, which represent a disease phenotype and cannot be altered. Therefore, we are unable to provide M-mode images with a similar number of cardiac cycles for control and ablated mice.
(6) In the long-term neonatal ablation experiments, collagen expressions return to normal. The manuscript attributes this to possible "compensatory expression," Do they have any thoughts how this is regulated? Are other cell types stepping in, or are surviving FBs proliferating?
We thank the reviewer for the question. As suggested, the compensatory collagen expression could be driven by surviving fibroblasts or other cell types. Since we currently lack evidence to exclude either possibility, we believe both could be contributing factors.
(7) While collagen is shown to be a dominant signaling molecule, its centrality is inferred primarily from scRNAseq and ligand-receptor predictions. Did authors try any functional rescue experiment (e.g., exogenous collagen supplementation or receptor blockade) to directly validate this pathway's role in vivo?
We thank the reviewer for the comment. As noted in our previous revision in response to similar questions from the other two reviewers, we agree that these rescue experiments are of interest but are beyond the scope of the current study. We plan to pursue these investigations in future work and share our findings when available.
-

-

eLife Assessment
The study presents extensive gene expression profiling and bioinformatic analyses, offering insights into the roles of fibroblasts in cardiac development. The large volume of scRNA-seq data is both compelling and important to the scientific community. All three reviewers agree that the revised manuscript represents a significant improvement and addresses most, if not all, of their previous concerns. The reviewers also acknowledge that detailed mechanistic studies on how fibroblast-derived collagen regulates myocardial and coronary vasculature development are beyond the scope of the current study.
-

Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. The work includes extensive gene expression profiling and bioinformatic analysis. The prenatal fibroblast ablation studies show new information on the requirement of these cells on heart maturation before birth.
The strengths of the manuscript are the new single cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice. Extensive data are presented on mouse embryo fibroblast diversity and morphology in response to fibroblast ablation. Histological data support localization of major cardiac cell types and effects of fibroblast ablation on cardiac gene expression at different times …
Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. The work includes extensive gene expression profiling and bioinformatic analysis. The prenatal fibroblast ablation studies show new information on the requirement of these cells on heart maturation before birth.
The strengths of the manuscript are the new single cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice. Extensive data are presented on mouse embryo fibroblast diversity and morphology in response to fibroblast ablation. Histological data support localization of major cardiac cell types and effects of fibroblast ablation on cardiac gene expression at different times of development.
A weakness of the study is that the major conclusions regarding collagen signaling and heart maturation are based on gene expression patterns and are not functionally validated.
-

Reviewer #2 (Public review):
This study aims to elucidate the role of fibroblasts in regulating myocardium and vascular development through signaling to cardiomyocytes and endothelial cells. This focus is significant, given that fibroblasts, cardiomyocytes, and vascular endothelial cells are the three primary cell types in the heart. The authors employed a Pdgfra-CreER-controlled diphtheria toxin A (DTA) system to ablate fibroblasts at various embryonic and postnatal stages, characterizing the resulting cardiac defects, particularly in myocardium and vasculature development. Single-cell RNA sequencing (scRNA-seq) analysis of the ablated hearts identified collagen as a crucial signaling molecule from fibroblasts that influences the development of cardiomyocytes and vascular endothelial cells.
This is an interesting manuscript; however, …
Reviewer #2 (Public review):
This study aims to elucidate the role of fibroblasts in regulating myocardium and vascular development through signaling to cardiomyocytes and endothelial cells. This focus is significant, given that fibroblasts, cardiomyocytes, and vascular endothelial cells are the three primary cell types in the heart. The authors employed a Pdgfra-CreER-controlled diphtheria toxin A (DTA) system to ablate fibroblasts at various embryonic and postnatal stages, characterizing the resulting cardiac defects, particularly in myocardium and vasculature development. Single-cell RNA sequencing (scRNA-seq) analysis of the ablated hearts identified collagen as a crucial signaling molecule from fibroblasts that influences the development of cardiomyocytes and vascular endothelial cells.
This is an interesting manuscript; however, there are several major issues, including an over-reliance on the scRNA-seq data, which shows inconsistencies between replicates.
Some of the major issues are described below.
(1) The CD31 immunostaining data (Figure 3B-G) indicate a reduction in endothelial cell numbers following fibroblast deletion using PdgfraCreER+/-; RosaDTA+/- mice. However, the scRNA-seq data show no percentage change in the endothelial cell population (Figure 4D). Furthermore, while the percentage of Vas_ECs decreased in ablated samples at E16.5, the results at E18.5 were inconsistent, showing an increase in one replicate and a decrease in another, raising concerns about the reliability of the RNA-seq findings.
(2) Similarly, while the percentage of Ven_CMs increased at E18.5, it exhibited differing trends at E16.5 (Fig. 4E), further highlighting the inconsistency of the scRNA-seq analysis with the other data.
(3) Furthermore, the authors noted that the ablated samples had slightly higher percentages of cardiomyocytes in the G1 phase compared to controls (Fig. 4H, S11D), which aligns with the enrichment of pathways related to heart development, sarcomere organization, heart tube morphogenesis, and cell proliferation. However, it is unclear how this correlates with heart development, given that the hearts of ablated mice are significantly smaller than those of controls (Figure 3E). Additionally, the heart sections from ablated samples used for CD31/DAPI staining in Figure 3F appear much larger than those of the controls, raising further inconsistencies in the manuscript.
(4) The manuscript relies heavily on the scRNA-seq dataset, which shows inconsistencies between the two replicates. Furthermore, the morphological and histological analyses do not align with the scRNA-seq findings.
(5) There is a lack of mechanistic insight into how collagen, as a key signaling molecule from fibroblasts, affects the development of cardiomyocytes and vascular endothelial cells.
(6) In Figure 1B, Col1a1 expression is observed in the epicardial cells (Figure 1A, E11.5), but this is not represented in the accompanying cartoon.
(7) Do the PdgfraCreER+/-; RosaDTA+/- mice survive after birth when induced at E15.5, and do they exhibit any cardiac defects?
-

Reviewer #3 (Public review):
Summary:
The authors investigated fibroblasts' communication with key cell types in developing and neonatal hearts, with focus on critical roles of fibroblast-cardiomyocyte and fibroblast-endothelial cells network in cardiac morphogenesis. They tried to map the spatial distribution of these cell types and reported the major pathways and signaling molecules driving the communication. They also used Cre-DTA system to ablate Pdgfra labeled cells and observed myocardial and endothelial cell defects at development. They screened the pathways and genes using sequencing data of ablated heart. Lastly they reported a compensatory collagen expression in long term ablated neonate heart. Overall, this study provides us with important insight on fibroblasts' roles in cardiac development and will be a powerful resource …
Reviewer #3 (Public review):
Summary:
The authors investigated fibroblasts' communication with key cell types in developing and neonatal hearts, with focus on critical roles of fibroblast-cardiomyocyte and fibroblast-endothelial cells network in cardiac morphogenesis. They tried to map the spatial distribution of these cell types and reported the major pathways and signaling molecules driving the communication. They also used Cre-DTA system to ablate Pdgfra labeled cells and observed myocardial and endothelial cell defects at development. They screened the pathways and genes using sequencing data of ablated heart. Lastly they reported a compensatory collagen expression in long term ablated neonate heart. Overall, this study provides us with important insight on fibroblasts' roles in cardiac development and will be a powerful resource for collagens and ECM focused research.
Strengths:
The authors utilized good analyzing tools to investigate on multiple database of single cell sequencing and Multi-seq. They identified significant pathways, cellular and molecular interactions of fibroblasts. Additionally, they compared some of their analytic findings with human database, and identified several groups of ECM genes with varying roles in mice.
Weaknesses:
This study is majorly based on sequencing data analysis. At the bench, they used very strident technique to study fibroblast functions by ablating one of the major cell population of heart. Also, experimental validation of their analyzed downstream pathways will be required eventually.
-

Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single-cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. Much of this work is overlapping with previous studies, but the single-cell gene expression data may be useful to investigators in the field. The significance and scope of new findings are limited and major conclusions are largely based on correlative data.
Strengths:
The strengths of the manuscript are the new single-cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice.
Weaknesses:
There are several major weaknesses in the analysis and interpretation of the results.
(1) The major …
Author response:
The following is the authors’ response to the original reviews
Public Reviews:
Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single-cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. Much of this work is overlapping with previous studies, but the single-cell gene expression data may be useful to investigators in the field. The significance and scope of new findings are limited and major conclusions are largely based on correlative data.
Strengths:
The strengths of the manuscript are the new single-cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice.
Weaknesses:
There are several major weaknesses in the analysis and interpretation of the results.
(1) The major conclusions regarding collagen signaling and heart maturation are based on gene expression patterns and are not functionally validated. The potential downstream signaling pathways were not examined and known structural contributions of fibrillar collagen to heart maturation are not discussed.
We thank the reviewer for the comment. In this study, we mainly focused on the functional analysis of fibroblasts in heart development at embryonic and neonatal stages by using cell ablation system and single cell mRNA sequencing analysis. The further functional analysis of collagen pathway is interesting but out of the scope of this study. We will continue this line of research and share the results in the future. Moreover, through the analysis of single cell mRNA-sequencing data, we have predicted the downstream genes that are regulated by the collagen pathway in Fig 5C. We have also added sentences to highlight the structural role of collagen in affecting the related heart developmental processes.
(2) The heterogeneity of fibroblast populations and contributions to multiple structures in the developing heart are not well-considered in the analysis. The developmental targeting of fibroblasts will likely affect multiple structures in the embryonic heart and other organs. Lethality is described in some of these studies, but additional analysis is needed to determine the effects on heart morphogenesis or other organs beyond the focus on cardiomyocyte maturation being reported. In particular, the endocardial cushions and developing valves are likely to be affected in the prenatal ablations, but these structures are not included in the analyses.
We thank the reviewer for the comment. We have included a new figure presenting the fibroblast heterogeneity in developing hearts (Fig S3). We have also compared the valve structural differences at E18.5 (Fig S11).
(3) ECM complexity and extensive previous work on specific ECM proteins in heart development and maturation are not incorporated into the current study. Different types of collagen (basement membrane Col4, filamentous Col6, and fibrillar Col1) are known to be expressed in fibroblast populations in the developing heart and have been studied extensively. Much also has been reported for other ECM components mentioned in the current work.
We thank the reviewer for the comment. We agree that the ECM is complex, and the functions of many of its components have been previously reported, as mentioned in the introduction. In this study, our focus is to analyze the spatial and temporal expression patterns of various ECM genes in fibroblasts throughout developmental progression (Fig. S5–7). To further acknowledge previous work, we have added additional sentences and cited relevant literature on the role of collagen genes in developing hearts (page 4).
Reviewer #2 (Public review):
This study aims to elucidate the role of fibroblasts in regulating myocardium and vascular development through signaling to cardiomyocytes and endothelial cells. This focus is significant, given that fibroblasts, cardiomyocytes, and vascular endothelial cells are the three primary cell types in the heart. The authors employed a Pdgfra-CreER-controlled diphtheria toxin A (DTA) system to ablate fibroblasts at various embryonic and postnatal stages, characterizing the resulting cardiac defects, particularly in myocardium and vasculature development. scRNA-seq analysis of the ablated hearts identified collagen as a crucial signaling molecule from fibroblasts that influences the development of cardiomyocytes and vascular endothelial cells. This is an interesting manuscript; however, there are several major issues, including an over-reliance on the scRNA-seq data, which shows inconsistencies between replicates. Some of the major issues are described below.
The comments are the same as the comments for “Recommendations for the authors”. Please see the responses below.
Reviewer #3 (Public review):
The authors investigated fibroblasts' communication with key cell types in developing and neonatal hearts, with a focus on the critical roles of fibroblast-cardiomyocyte and fibroblast-endothelial cell networks in cardiac morphogenesis. They tried to map the spatial distribution of these cell types and reported the major pathways and signaling molecules driving the communication. They also used Cre-DTA system to ablate Pdgfra labeled cells and observed myocardial and endothelial cell defects at development. They screened the pathways and genes using sequencing data of ablated hearts. Lastly, they reported compensatory collagen expression in long-term ablated neonate hearts. Overall, this study provides us with important insight into fibroblasts' roles in cardiac development and will be a powerful resource for collagens and ECM-focused research.
Strengths:
The authors utilized good analyzing tools to investigate multiple databases of single-cell sequencing and Multiseq. They identified significant pathways and cellular and molecular interactions of fibroblasts. Additionally, they compared some of their analytic findings with a human database, and identified several groups of ECM genes with varying roles in mice.
Weaknesses:
This study is majorly based on sequencing data analysis. At the bench, they used a very strident technique to study fibroblast functions by ablating one of the major cell populations of the heart. Considering the importance of the fibroblast population, intriguing in vivo findings were expected. Also, they analyzed the downstream genes in ablated hearts, but did not execute any experimental validation for any of the targets.
Recommendations for the authors:
Reviewing Editor Comments:
All three reviewers found the large amount of scRNA-Seq data compelling and valuable, and they noted that the study's conclusions based on the scRNA Seq and fibroblast ablating align closely with previously published studies. Therefore, a more thorough discussion and integration of the current findings with prior studies are recommended. Each reviewer provided specific feedback to improve the manuscript, correct errors, and strengthen the overall presentation, and please edit the manuscript accordingly. Additionally, further validation of the scRNA-Seq data through more data analysis, reference comparisons, or additional experiments is encouraged.
Reviewer #1 (Recommendations for the authors):
(1) The heterogeneity of fibroblasts and ECM components in the developing heart needs to be considered in the analysis and description of results. There are extensive reports in both of these areas that would inform the gene expression and ablation studies being reported.
We thank the reviewer for the comment. We have added a supplemental figure (Fig. S3) analyzing the heterogeneity of fibroblasts during development and described the results on page 3 and 4. Through the analysis of single-cell mRNA sequencing data, we identified four distinct populations of fibroblasts and further performed RNA scope to examine their spatial locations. Additionally, we agree with the reviewer that there are many types of ECM components, which we have addressed in the introduction (page 2). Furthermore, we have conducted a detailed analysis of the spatial and temporal expression patterns of ECM genes throughout developmental progression (Figs. S5–7).
(2) One of the novel aspects of the work is the prenatal ablation of cardiac fibroblasts. Embryonic lethality was observed in some cases, but the specific cardiac structural anomalies or potential vascular effects were not described. The contributing role of cardiac fibroblasts to valvuloseptal development, which was likely affected in these studies, was not described.
We thank the reviewer for the comment. Since the heart sections were not initially prepared to compare valve differences between control and ablation conditions, most sections do not include valve structures. However, in the small subset of sections that do contain valves, we have compared valve structures in control and ablated hearts at E18.5 following three doses of tamoxifen treatment from E15.5 to E17.5. In mutants, the valves appear shorter compared to controls. Specifically, we observed that in control hearts, the mitral valve was already connected to the papillary muscle, whereas in ablated hearts, the valve leaflet at similar position was not. We have included these images as a new supplemental figure (Fig. S11). Regarding vascular defects, we have described them in Fig. 3C and 3F.
(3) The major conclusions regarding collagen signaling and heart development are based on correlations in gene expression and are not validated by functional data. What are the downstream signaling pathways affected and are they affected during development or with ablation? The main conclusions of the study do not take into account well-known structural functions of collagen in the developing heart.
We thank the reviewer for the comment. Through regulatory prediction analysis, we identified the collagen ligands Col1a1, Col5a1, and Col4a1 from the collagen family (Fig. 5C), which regulate multiple genes in cardiomyocytes, including Masp1. Masp1 is a member of the lectin complement pathway and potentially regulates cardiomyocyte migration during development. These collagen ligands also regulate multiple mitochondria-related genes, such as Etfa, Ndufb10, Ndufs6, and Slc25a4, which are potentially important for cardiomyocyte development and maturation. Moreover, we agree with the reviewer that collagen is an important structural ECM protein, and its deletion or reduction could cause heart developmental defects due to its structural role. We have added a discussion on this possibility (page 8).
(4) The postnatal ablation studies are very similar to studies with the same mouse lines reported by Kurabara et al 2022 in JMCC (PMID 35569524) which came to similar conclusions and was not cited in the current work.
We thank the reviewer for the comment and apologize for overlooking this study. We have now included the citation on page 8.
(5) The discussion of a regenerative response with DTA ablation of fibroblasts is confusing. Proliferation was examined in cardiomyocytes which lose their regenerative capacity after birth in mice. However, cardiac fibroblasts can proliferate in response to injury throughout life which is not really a regenerative process.
We appreciate the reviewer’s comment. To avoid confusion, we have replaced the term "regeneration" with "response to cell loss" and "compensation."
(6) Some of the descriptions of single-cell expression data are overstated (Page 7). Regulatory interactions, signaling pathway activation, or function cannot be determined from gene expression data alone.
We thank the reviewer for the comment. We agree that these conclusions rely on results from multiple assays. We have weakened the description of the analysis by emphasizing that the findings are predictive results from scRNA-seq analysis.
(7) In the last paragraph of the discussion "data not shown" should be shown or this information should be deleted. As written, the discussion does not present a clear description of what major new findings are being reported or why they are significant. The new insights into heart development are not specified.
We thank the reviewer for the comment. We have added the data as a supplemental figure (Fig. S19). Since this paragraph is part of the discussion, we believe the results are not conclusive at this stage and require further research to explore the potential protective role of fibroblast ablation in neonatal hearts.
Minor comments.
(1) Figure legends are missing information needed to understand what is being shown. For example, in Figure 2, collagen is visualized using CHP staining.
Thanks. We have gone through all figure legends to ensure that all necessary information has been provided.
(2) The hearts in Figure S15 are upside down.
Thanks. We have updated the figure.
(3) In Figure S16A, "brian" should be "brain".
Thanks. We have updated it.
Reviewer #2 (Recommendations for the authors):
This is an interesting manuscript; however, there are several major issues, including an overreliance on the scRNA-seq data, which shows inconsistencies between replicates. Some of the major issues are described below.
(1) The CD31 immunostaining data (Figures 3B-G) indicate a reduction in endothelial cell numbers following fibroblast deletion using PdgfraCreER+/-; RosaDTA+/- mice. However, the scRNA-seq data show no percentage change in the endothelial cell population (Figure 4D). Furthermore, while the percentage of Vas_ECs decreased in ablated samples at E16.5, the results at E18.5 were inconsistent, showing an increase in one replicate and a decrease in another, raising concerns about the reliability of the RNA-seq findings.
We thank the reviewer for the comment. We believe that measuring cell proportions in scRNA-seq results is sensitive and relies on a high number of total and target cells, similar to other cell counting assays such as FACS. As the reviewer pointed out, the proportions of Vas_EC in E18.5 replicates are inconsistent. Specifically, Col_4 at E18.5 showed a relatively low proportion of Vas_EC. Upon examining the cell numbers in each sample, we found that Col_4 had the lowest number of recovered cells, with approximately 760 in total, whereas the other samples had more than 920 cells each. Additionally, since immunofluorescence staining for CD31 marks both Vas_EC and Endo_EC, we combined these two cell types to increase the number of targeted cells. This analysis consistently showed that the ablated samples had lower proportions. However, given that the quantifications have also produced inconsistent results for other cell types, such as Ven_CM, as mentioned in the reviewer’s next question, we have decided to delete this plot to avoid confusion.
Author response image 1.
(2) Similarly, while the percentage of Ven_CMs increased at E18.5, it exhibited differing trends at E16.5 (Figure 4E), further highlighting the inconsistency of the scRNA-seq analysis with the other data.
We thank the reviewer for the comment. Please see the response above.
(3) Furthermore, the authors noted that the ablated samples had slightly higher percentages of cardiomyocytes in the G1 phase compared to controls (Figures 4H, S11D), which aligns with the enrichment of pathways related to heart development, sarcomere organization, heart tube morphogenesis, and cell proliferation. However, it is unclear how this correlates with heart development, given that the hearts of ablated mice are significantly smaller than those of controls (Figure 3E). Additionally, the heart sections from ablated samples used for CD31/DAPI staining in Figure 3F appear much larger than those of the controls, raising further inconsistencies in the manuscript.
We thank the reviewer for the comment. We observed changes in G1-phase cardiomyocytes at both E16.5 and E18.5, with pathway enrichment primarily identified in E16.5 cardiomyocytes. At E16.5, the ablated hearts exhibited myocardial defects, including an increased trabecular-to-compact myocardium ratio and reduced vascular density. By E18.5, the ablated embryos had smaller hearts with reduced vascular density, although the trabecular-to-compact myocardium ratio showed no obvious changes. Regarding the larger section size in the ablated hearts compared to the control hearts, there are two reasons contributing to this discrepancy. First, the control and ablated heart sections have different scale bars. The ablated hearts were enlarged compared to control section. Secondly, the heart sections vary in size depending on their position. Sections taken from the middle of the heart are larger than those from the edges. In our initial comparison, we used an edge-positioned section from the control hearts and a middle-positioned section from the ablated hearts. To avoid confusion, we have now updated the control section to match the position of the ablated embryos more closely and used the same size of scale bars in the two images (Fig 3F).
(4) The manuscript relies heavily on the scRNA-seq dataset, which shows inconsistencies between the two replicates. Furthermore, the morphological and histological analyses do not align with the scRNA-seq findings.
We respectfully disagree with this comment from the reviewer. As shown in Figure 4B, the scRNAseq data from the two replicates are highly consistent. For inconsistencies in cell proportions and tissue section sizes, please refer to our responses above.
(5) There is a lack of mechanistic insight into how collagen, as a key signaling molecule from fibroblasts, affects the development of cardiomyocytes and vascular endothelial cells.
We thank the reviewer for the comment. In this study, we primarily focused on analyzing fibroblast function in heart development using cell ablation and single-cell mRNA sequencing. While further mechanistic analysis of the collagen pathway is intriguing, it falls outside the scope of this study. Additionally, our scRNAseq analysis identified multiple collagen ligands derived from fibroblasts that may regulate gene expression in Ven_CM and influence their development, as shown in Figure 5C. Although validating these predictions would be valuable, it is beyond the scope of this study. We will continue this line of research and share our findings in the future.
(6) In Figure 1B, Col1a1 expression is observed in the epicardial cells (Figure 1A, E11.5), but this is not represented in the accompanying cartoon.
We thank the reviewer for the comment. As stated in the main text (page 3), based on scRNA-seq and IF staining results, we observed that Col1a1 is also expressed in epicardial cells. In the cartoon, we depicted the pattern of fibroblasts rather than Col1a1-positive cells, which is why we did not include epicardial cells.
(7) What is the genotype of the control animals used in the study?
We thank the reviewer for the comment. We have added the genotype information for the control embryos in the legends of the relevant figures.
(8) Do the PdgfraCreER+/-; RosaDTA+/- mice survive after birth when induced at E15.5, and do they exhibit any cardiac defects?
We thank the reviewer for the comment. This is an interesting question; however, we did not perform the experiment because administering tamoxifen to pregnant mice from E15.5 to E18.5 causes delivery complications, as reported in the literature (PMID: 23139287). Unfortunately, this prevents us from exploring this question further.
Reviewer #3 (Recommendations for the authors):
Overall, this is a comprehensive study substantiated by the evidence the authors provided in their findings. However, I have a few concerns to be addressed.
(1) The claim by the authors that "at E17.5 and P3, each FB was in contact with approximately one Vas_EC and four CMs at both stages" is not fully convincing. RNA scope images for Actn2 are not clear enough to lead the quantification (RNA scope images for Cdh5 look better). I suggest performing imaging at higher magnification and the Z stack technique to provide a better understanding of their localization. Also, no changes in FBs adjacent cell numbers (CM&EC) with ages (P3) compared to E17.5? Any thoughts on the explanation?
We thank the reviewer for the comment. We imaged the staining results using a confocal microscope at 20X resolution. We also considered imaging them at 40X; however, due to the large areas that need to be imaged in these sections, it was challenging to do so. Additionally, we identified each CM based on Actn2 and DAPI staining information and are confident in the accuracy of our quantification results. Moreover, since each FB interacts with multiple CMs and Vas_ECs in 3D projections, but our calculations are based only on 2D imaging sections, there may be discrepancies compared to a true 3D environment. We have added a sentence to address this limitation (page 9). Regarding the similar number of interactions observed at E17.5 and P3, we think there are two possibilities. First, the three cell types may proliferate in a synchronized manner, maintaining a consistent number of interactions. Second, these cell types may exhibit minimal proliferation during late embryonic and early neonatal stages. Instead, heart growth primarily occurs through CM hypertrophy, which does not significantly alter the number of interactions.
(2) Fix the Capitalized font of RNA markers in Figure S2.
Thanks. We have updated them.
(3) I appreciate the visualization of ligand-receptor interactions in collagen network comparison between FB to CM and FB to EC, and predictive analysis on the FB ligands that regulate differentially expressed genes in ablated heart CM and ECs.
We appreciate the reviewer for the comment.
(4) The authors depleted Pdgfra-Cre cells at E10.5, and reported 100% DTA+ lethality after 3 days. Induction at E13.5 to ablate Pdgfra-Cre cells resulted in survival at least up to E16.5 age. What could be the possible reasons authors think that lead to embryo lethality when induced at E10.5? Did the authors analyze the expression of Pdgfra at E10.5 to E13.5 using Pdgfra antibody or Pdgfra-Cre labeling, or using the ScRNA seq data?
We thank the reviewer for the comment. The expression pattern of Pdgfra at E10.5 has been previously reported (PMID: 18297729) and shown to be highly expressed in the atrioventricular region, consistent with the Col1a1 expression pattern we profiled in this study. Therefore, we believe the embryonic lethality observed in the ablated embryos at E10.5 was likely due to the disruption of the atrioventricular structure. However, since Pdgfra is also expressed in other tissues at this stage, we cannot rule out the possibility that the ablation of non-cardiac tissues also contributed to the lethality.
(5) In terms of the findings on the trabeculation and compaction defects, please provide the images of the ventricles with markers to indicate the compact and trabecular zones and their defects.
Thanks! We have included images that illustrate the quantification of compact and trabecular myocardium thickness in control and ablated hearts (FigS10C).
(6) Did the author check the expression of any other marker for the vascular system in addition to CD31 to see the effects of ablated FB on coronary vasculature development?
We thank the reviewer for the comment. We analyzed only Cd31 to assess the effects of fibroblast ablation on the overall endothelial cell population. We did not separately examine the subpopulations, but this would be an interesting direction for future studies.
(7) Can the authors interpret how findings from PHH3 proliferation explain thinner compact and thicker trabeculae in ablated hearts?
We thank the reviewer for the comment and apologize for the misinterpretation of the results. We observed that the ablated hearts have a thinner compact myocardium, while the thickness of the trabecular myocardium remains unchanged, leading to an increased trabecular-to-compact myocardium ratio (Fig 3D). We have corrected the description in the manuscript accordingly. Moreover, since the compact myocardium has a higher proliferation rate than the trabecular myocardium, a reduction in overall cell proliferation is expected to have a more pronounced impact on the compact myocardium. Inhibition of compact myocardium proliferation has been reported to lead thinner compact myocardium and non-compaction defects (PMID: 31342111).
(8) The authors did not execute experiments to find the downstream target that causes compaction defects and endothelial cell density defects upon ablation of FBs. Can you project from your sequencing analysis what could be the potential downstream if you could execute bench-side experiments on this?
We appreciate the reviewer for the comment. We believe that the regulatory predictive results in Figures 5C and D from the scRNA-seq data analysis have provided a set of downstream candidates for validation. We could select some of the ligands, such as the collagen ligands Col1a1, Col4a1, and Col5a1, to treat the ablated embryos in vivo to assess whether they could partially rescue the myocardium defects. Additionally, we could conduct ex vivo experiments by co-culturing CM and FB, comparing them with CM alone and CM treated with the identified ligands. This would allow us to evaluate CM proliferation and the expression of downstream genes identified in the prediction results. However, as the reviewer suggested, these experiments are planned for future studies.
(9) Please provide the echocardiographic M mode images with a comparable number of cardiac cycles in control and ablated (Fig. 6H). Also, the heart rate of the ablated heart is too low to compare other parameters with the control. If you could stabilize the heart rate at comparable values to control the heart, it is possible that EF and FS values will be largely changed.
We thank the reviewer for the comment. As the echocardiographic analysis was performed on conscious mice, the lower heart rates in the ablated mice are a phenotype associated with the ablation. Unfortunately, we are unable to adjust them to the same as the control mice.
(10) Can you provide a numerical dataset for any one of the cell chat figures? Like in figure 2A, supporting the claim "However, in terms of interaction strength, FB exhibited the highest values compared to those of other cell types (Fig. 2A)".
Yes, we have added a supplemental table (Table S2) containing the numerical interaction weights. As shown in the table, the interactions between FB and other cell types have the highest values.
-

-

eLife Assessment
This study provides abundant valuable scRNA-Seq data that profiles fibroblasts involved in myocardium and coronary vasculature development. However, the evidence supporting the authors' claims is currently incomplete. The inclusion of additional citations, more in-depth discussions, and further analyses or experiments to validate the scRNA-Seq data would have significantly strengthened the study. Nonetheless, the scRNA-Seq expression data will be a resource that is of value to researchers in the field.
-

Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single-cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. Much of this work is overlapping with previous studies, but the single-cell gene expression data may be useful to investigators in the field. The significance and scope of new findings are limited and major conclusions are largely based on correlative data.
Strengths:
The strengths of the manuscript are the new single-cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice.
Weaknesses:
There are several major weaknesses in the analysis and interpretation of the results.
(1) The major conclusions regarding collagen signaling and heart maturation are based on gene expression …
Reviewer #1 (Public review):
Summary:
The study by Deng et al reports single-cell expression analysis of developing mouse hearts and examines the requirements for cardiac fibroblasts in heart maturation. Much of this work is overlapping with previous studies, but the single-cell gene expression data may be useful to investigators in the field. The significance and scope of new findings are limited and major conclusions are largely based on correlative data.
Strengths:
The strengths of the manuscript are the new single-cell datasets and comprehensive approach to ablating cardiac fibroblasts in pre and postnatal development in mice.
Weaknesses:
There are several major weaknesses in the analysis and interpretation of the results.
(1) The major conclusions regarding collagen signaling and heart maturation are based on gene expression patterns and are not functionally validated. The potential downstream signaling pathways were not examined and known structural contributions of fibrillar collagen to heart maturation are not discussed.
(2) The heterogeneity of fibroblast populations and contributions to multiple structures in the developing heart are not well-considered in the analysis. The developmental targeting of fibroblasts will likely affect multiple structures in the embryonic heart and other organs. Lethality is described in some of these studies, but additional analysis is needed to determine the effects on heart morphogenesis or other organs beyond the focus on cardiomyocyte maturation being reported. In particular, the endocardial cushions and developing valves are likely to be affected in the prenatal ablations, but these structures are not included in the analyses.
(3) ECM complexity and extensive previous work on specific ECM proteins in heart development and maturation are not incorporated into the current study. Different types of collagen (basement membrane Col4, filamentous Col6, and fibrillar Col1) are known to be expressed in fibroblast populations in the developing heart and have been studied extensively. Much also has been reported for other ECM components mentioned in the current work.
-

Reviewer #2 (Public review):
This study aims to elucidate the role of fibroblasts in regulating myocardium and vascular development through signaling to cardiomyocytes and endothelial cells. This focus is significant, given that fibroblasts, cardiomyocytes, and vascular endothelial cells are the three primary cell types in the heart. The authors employed a Pdgfra-CreER-controlled diphtheria toxin A (DTA) system to ablate fibroblasts at various embryonic and postnatal stages, characterizing the resulting cardiac defects, particularly in myocardium and vasculature development. scRNA-seq analysis of the ablated hearts identified collagen as a crucial signaling molecule from fibroblasts that influences the development of cardiomyocytes and vascular endothelial cells.
This is an interesting manuscript; however, there are several major …
Reviewer #2 (Public review):
This study aims to elucidate the role of fibroblasts in regulating myocardium and vascular development through signaling to cardiomyocytes and endothelial cells. This focus is significant, given that fibroblasts, cardiomyocytes, and vascular endothelial cells are the three primary cell types in the heart. The authors employed a Pdgfra-CreER-controlled diphtheria toxin A (DTA) system to ablate fibroblasts at various embryonic and postnatal stages, characterizing the resulting cardiac defects, particularly in myocardium and vasculature development. scRNA-seq analysis of the ablated hearts identified collagen as a crucial signaling molecule from fibroblasts that influences the development of cardiomyocytes and vascular endothelial cells.
This is an interesting manuscript; however, there are several major issues, including an over-reliance on the scRNA-seq data, which shows inconsistencies between replicates.
Some of the major issues are described below.(1) The CD31 immunostaining data (Figures 3B-G) indicate a reduction in endothelial cell numbers following fibroblast deletion using PdgfraCreER+/-; RosaDTA+/- mice. However, the scRNA-seq data show no percentage change in the endothelial cell population (Figure 4D). Furthermore, while the percentage of Vas_ECs decreased in ablated samples at E16.5, the results at E18.5 were inconsistent, showing an increase in one replicate and a decrease in another, raising concerns about the reliability of the RNA-seq findings.
(2) Similarly, while the percentage of Ven_CMs increased at E18.5, it exhibited differing trends at E16.5 (Figure 4E), further highlighting the inconsistency of the scRNA-seq analysis with the other data.
(3) Furthermore, the authors noted that the ablated samples had slightly higher percentages of cardiomyocytes in the G1 phase compared to controls (Figures 4H, S11D), which aligns with the enrichment of pathways related to heart development, sarcomere organization, heart tube morphogenesis, and cell proliferation. However, it is unclear how this correlates with heart development, given that the hearts of ablated mice are significantly smaller than those of controls (Figure 3E). Additionally, the heart sections from ablated samples used for CD31/DAPI staining in Figure 3F appear much larger than those of the controls, raising further inconsistencies in the manuscript.
(4) The manuscript relies heavily on the scRNA-seq dataset, which shows inconsistencies between the two replicates. Furthermore, the morphological and histological analyses do not align with the scRNA-seq findings.
(5) There is a lack of mechanistic insight into how collagen, as a key signaling molecule from fibroblasts, affects the development of cardiomyocytes and vascular endothelial cells.
(6) In Figure 1B, Col1a1 expression is observed in the epicardial cells (Figure 1A, E11.5), but this is not represented in the accompanying cartoon.
(7) What is the genotype of the control animals used in the study?
(8) Do the PdgfraCreER+/-; RosaDTA+/- mice survive after birth when induced at E15.5, and do they exhibit any cardiac defects?
-

Reviewer #3 (Public review):
The authors investigated fibroblasts' communication with key cell types in developing and neonatal hearts, with a focus on the critical roles of fibroblast-cardiomyocyte and fibroblast-endothelial cell networks in cardiac morphogenesis. They tried to map the spatial distribution of these cell types and reported the major pathways and signaling molecules driving the communication. They also used Cre-DTA system to ablate Pdgfra labeled cells and observed myocardial and endothelial cell defects at development. They screened the pathways and genes using sequencing data of ablated hearts. Lastly, they reported compensatory collagen expression in long-term ablated neonate hearts. Overall, this study provides us with important insight into fibroblasts' roles in cardiac development and will be a powerful resource …
Reviewer #3 (Public review):
The authors investigated fibroblasts' communication with key cell types in developing and neonatal hearts, with a focus on the critical roles of fibroblast-cardiomyocyte and fibroblast-endothelial cell networks in cardiac morphogenesis. They tried to map the spatial distribution of these cell types and reported the major pathways and signaling molecules driving the communication. They also used Cre-DTA system to ablate Pdgfra labeled cells and observed myocardial and endothelial cell defects at development. They screened the pathways and genes using sequencing data of ablated hearts. Lastly, they reported compensatory collagen expression in long-term ablated neonate hearts. Overall, this study provides us with important insight into fibroblasts' roles in cardiac development and will be a powerful resource for collagens and ECM-focused research.
Strengths:
The authors utilized good analyzing tools to investigate multiple databases of single-cell sequencing and Multi-seq. They identified significant pathways and cellular and molecular interactions of fibroblasts. Additionally, they compared some of their analytic findings with a human database, and identified several groups of ECM genes with varying roles in mice.
Weaknesses:
This study is majorly based on sequencing data analysis. At the bench, they used a very strident technique to study fibroblast functions by ablating one of the major cell populations of the heart. Considering the importance of the fibroblast population, intriguing in vivo findings were expected. Also, they analyzed the downstream genes in ablated hearts, but did not execute any experimental validation for any of the targets.
-