C-C chemokine receptor 4 deficiency exacerbates early atherosclerosis in mice
Curation statements for this article:-
Curated by eLife
eLife Assessment
This valuable study provides in-vivo evidence that CCR4 regulates the early inflammatory response during atherosclerotic plaque formation. The authors propose that altered T-cell response plays a role in this process, shedding light on mechanisms that may be of interest to medical biologists, biochemists, cell biologists, and immunologists. The work is currently considered incomplete pending textual changes and the inclusion of proper controls.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Chronic inflammation via dysregulation of T cell immune responses is critically involved in the pathogenesis of atherosclerotic cardiovascular disease. Improving the balance between proinflammatory T cells and anti-inflammatory regulatory T cells (Tregs) may be an attractive approach for treating atherosclerosis. Although C-C chemokine receptor 4 (CCR4) has been shown to mediate the recruitment of T cells to inflamed tissues, its role in atherosclerosis is unclear. Here, we show that genetic deletion of CCR4 in hypercholesterolemic mice accelerates the development of early atherosclerotic lesions characterized by an inflammatory plaque phenotype. This was associated with the augmentation of proinflammatory T helper type 1 (Th1) cell responses in peripheral lymphoid tissues, para-aortic lymph nodes, and atherosclerotic aorta. Mechanistically, CCR4 deficiency in Tregs impaired their suppressive function and tended to inhibit their migration to the atherosclerotic aorta, and subsequently augmented Th1 cell-mediated immune responses through defective regulation of dendritic cell function, which accelerated aortic inflammation and atherosclerotic lesion development. Thus, we revealed a previously unrecognized role for CCR4 in controlling the early stage of atherosclerosis via Treg-dependent regulation of proinflammatory T cell responses. Our data suggest that CCR4 is an important negative regulator of atherosclerosis.
Article activity feed
-

-

-

eLife Assessment
This valuable study provides in-vivo evidence that CCR4 regulates the early inflammatory response during atherosclerotic plaque formation. The authors propose that altered T-cell response plays a role in this process, shedding light on mechanisms that may be of interest to medical biologists, biochemists, cell biologists, and immunologists. The work is currently considered incomplete pending textual changes and the inclusion of proper controls.
-

Reviewer #2 (Public review):
Summary:
Tanaka et al. investigated the role of CCR4 in early atherosclerosis, focusing on the immune modulation elicited by this chemokine receptor under hypercholesterolemia. The study found that Ccr4 deficiency led to qualitative changes in atherosclerotic plaques, characterized by an increased inflammatory phenotype. The authors further analyzed the CD4 T cell immune response in para-aortic lymph nodes and atherosclerotic aorta, showing an increase mainly in Th1 cells and the Th1/Treg ratio in Ccr4-/-Apoe-/- mice compared to Apoe-/- mice. They then focused on Tregs, demonstrating that Ccr4 deficiency impaired their immunosuppressive function in in vitro assays. Authors also states that Ccr4-deficient Tregs had, as expected, impaired migration to the atherosclerotic aorta. Adoptive cell transfer of …
Reviewer #2 (Public review):
Summary:
Tanaka et al. investigated the role of CCR4 in early atherosclerosis, focusing on the immune modulation elicited by this chemokine receptor under hypercholesterolemia. The study found that Ccr4 deficiency led to qualitative changes in atherosclerotic plaques, characterized by an increased inflammatory phenotype. The authors further analyzed the CD4 T cell immune response in para-aortic lymph nodes and atherosclerotic aorta, showing an increase mainly in Th1 cells and the Th1/Treg ratio in Ccr4-/-Apoe-/- mice compared to Apoe-/- mice. They then focused on Tregs, demonstrating that Ccr4 deficiency impaired their immunosuppressive function in in vitro assays. Authors also states that Ccr4-deficient Tregs had, as expected, impaired migration to the atherosclerotic aorta. Adoptive cell transfer of Ccr4-/- Tregs to Apoe-/- mice mimicked early atherosclerosis development in Ccr4-/-Apoe-/- mice. Therefore, this work shows that CCR4 plays an important role in early atherosclerosis but not in advanced stages.
Strengths:
Several in vivo and in vitro approaches were used to address the role of CCR4 in early atherosclerosis. Particularly, through the adoptive cell transfer of CCR4+ or CCR4- Tregs, the authors aimed to demonstrate the role of CCR4 in Tregs' protection against early atherosclerosis.
Weaknesses:
Flow cytometry experiments are not well controlled. Dead cells and doublets were not excluded from analysis.
Clinical relevance is unclear.
Comments on revisions:
I thank the authors for addressing my suggestions.
I understand that excluding dead cells would require repeating the entire experiment. However, the authors can at least exclude doublets from the existing flow cytometry data.
I also agree with the more cautious claim regarding the role of CCR4 in Treg migration. -

Reviewer #3 (Public review):
Summary
Tanaka and colleagues addressed the role of the C-C chemokine receptor 4 (CCR4) in early atherosclerotic plaque development using ApoE-deficient mice on a standard chow diet as a model. Because several CD4+ T cell subsets express CCR4, they examined whether CCR4-deficiency alters the immune response mediated by CD4+ T cells. By histological analysis of aortic lesions, they demonstrated that the absence of CCR4 promoted the development of early atherosclerosis, with heightened inflammation linked to increased macrophages and pro-inflammatory CD4+ T cells, along with reduced collagen content. Flow cytometry and mRNA expression analysis for identifying CD4+ T cell subsets showed that CCR4 deficiency promoted higher proliferation of pro-inflammatory effector CD4+ T cells in peripheral lymphoid tissues …
Reviewer #3 (Public review):
Summary
Tanaka and colleagues addressed the role of the C-C chemokine receptor 4 (CCR4) in early atherosclerotic plaque development using ApoE-deficient mice on a standard chow diet as a model. Because several CD4+ T cell subsets express CCR4, they examined whether CCR4-deficiency alters the immune response mediated by CD4+ T cells. By histological analysis of aortic lesions, they demonstrated that the absence of CCR4 promoted the development of early atherosclerosis, with heightened inflammation linked to increased macrophages and pro-inflammatory CD4+ T cells, along with reduced collagen content. Flow cytometry and mRNA expression analysis for identifying CD4+ T cell subsets showed that CCR4 deficiency promoted higher proliferation of pro-inflammatory effector CD4+ T cells in peripheral lymphoid tissues and accumulation of Th1 cells in the atherosclerotic lesions. Interestingly, the increased pro-inflammatory CD4+ T cell response occurred despite the expansion of T CD4+ Foxp3+ regulatory cells (Tregs), found in higher numbers in lymphoid tissues of CCR4-deficient mice, suggesting that CCR4 deficiency interfered with Treg's regulatory actions. The findings contrast with earlier studies in a murine model of advanced atherosclerosis, where CCR4 deficiency did not alter the development of the aortic lesions. The authors included a thoughtful discussion about hypothetical mechanisms explaining these contrasting results, including putative differences in the role played by the CCL17/CCL22-CCR4 axis along the stages of atherosclerosis development in this murine model.
Major strengths
• Demonstration of CCR4 deficiency's impact on early atherosclerosis. CCR4 deficiency effects on the early atherosclerosis development in the Apoe-/-mice model were demonstrated by a quantitative analysis of the lesion area, inflammatory cell content and the expression profile of several pro- and anti-inflammatory markers.
• Analysis of the T CD4+ response in various lymphoid tissues (peripheral and para-aortic lymph nodes and spleen) and the atherosclerotic aorta during the early phase of atherosclerosis in the Apoe-/-mice model. This analysis, combining flow cytometry and mRNA expression, showed that CCR4 deficiency enhanced T CD4+ cell activation, favouring the amplification of the typical biased Th1-mediated inflammatory response observed in the lymphoid tissues of hypercholesterolemic mice.
• Treg transference experiments. Transference of Treg from Apoe-/- or Ccr4-/- Apoe-/- mice to Apoe-/- mice under a standard chow diet was useful for addressing the relevance of CCR4 expression on Tregs for the atheroprotective effect of this regulatory T cell subset during early atherosclerosis.Major weaknesses
• Methodological Limitations: The controls used in the flow cytometry analysis were suboptimal, as neither cell viability nor doublets were assessed. This may have introduced artifacts, particularly when measuring less-represented cell populations within complex samples, such as in assays evaluating Treg migration to the aorta in atherosclerotic mice.
• Incomplete understanding of CCR4-Mediated Mechanisms: The mechanisms by which CCR4 regulates early inflammation and the development of atherosclerosis were not fully clarified.I have previously addressed the study limitations and their global impact in my earlier reviews.
-

Author response:
The following is the authors’ response to the previous reviews
Response to the reviewer #2 (Public review):
We greatly appreciate the reviewer’s high evaluation of our paper and helpful comments and suggestions.
Regarding in vivo Treg homing assay, we did not exclude doublets and dead cells from the analysis of Kaede-expressing Tregs migrated to the aorta, which may affect the results. We described this issue as the limitation of this study in the revised manuscript. Nonetheless, we believe the reliability of our findings because we repeated this experiment three times and obtained similar results.
There is no evidence to support the clinical relevance of our findings. Future clinical research on this topic is highly desired.
Response to the reviewer #3 (Public review):
We greatly appreciate the reviewer’s high …
Author response:
The following is the authors’ response to the previous reviews
Response to the reviewer #2 (Public review):
We greatly appreciate the reviewer’s high evaluation of our paper and helpful comments and suggestions.
Regarding in vivo Treg homing assay, we did not exclude doublets and dead cells from the analysis of Kaede-expressing Tregs migrated to the aorta, which may affect the results. We described this issue as the limitation of this study in the revised manuscript. Nonetheless, we believe the reliability of our findings because we repeated this experiment three times and obtained similar results.
There is no evidence to support the clinical relevance of our findings. Future clinical research on this topic is highly desired.
Response to the reviewer #3 (Public review):
We greatly appreciate the reviewer’s high evaluation of our paper and helpful comments and suggestions.
Despite the controversial role of Th17 cells in atherosclerosis, we understand the possible involvement of Th17 cells and the Th1 cell/Th17 cell balance in lymphoid tissues and aortic lesions in accelerated inflammation and atherosclerosis in Ccr4-/-Apoe-/- mice. Although we could not completely evaluate the changes in these immune responses in detail, future study may elucidate interesting mechanisms mediated by Th17 cell responses.
As the reviewer suggested, we understand that it is necessary to provide in vivo evidence for the Treg suppressive effects on DC activation. Based on the results of in vitro experiments, we described the discussion on the in vivo evidence in the revised manuscript.
We understand methodological limitations for flow cytometric analysis of immune cells in the aorta and in vivo Treg homing assay. We described this issue as the limitation of this study in the revised manuscript. Regarding in vivo Treg homing assay, we statistically re-analyzed the combined data from multiple experiments and observed a tendency toward reduction in the proportion of CCR4-deficient Kaede-expressing Tregs in the aorta of recipient Apoe-/- mice, though there was no statistically significant difference in the migratory capacity of CCR4-intact or CCR4-deficient Kaede-expressing Tregs. Accordingly, we toned down our claim that CCR4 expression on Tregs plays a critical role in mediating Treg migration to the atherosclerotic aorta under hypercholesterolemia.
The reviewer requested us to evaluate aortic inflammation in Ccr4-/-Apoe-/- mice injected with CCR4-intact or CCR4-deficient Tregs. However, we think that this experiment will provide marginal information because Treg transfer experiments in Apoe-/- mice have already shown the protective role of CCR4 in Tregs against aortic inflammation and early atherosclerosis.
Recommendations for the authors:
Reviewer #2 (Recommendations for the authors):
(1) #1 and #2: CD103 and CD86 expression should be discussed on the text and not only in the response to reviewer.
In accordance with the reviewer’s suggestion, we added a discussion on the downregulated CD103 expression in peripheral LN Tregs and upregulated CD86 expression on DCs in Ccr4-/-Apoe-/- mice in the discussion section in the revised manuscript.
(2) #5: Authors response is not satisfactory. No gate percentage is shown. As it currently is, the difference in the number of cells shown in the figure could be due to differences in events recorded. Furthermore, the gate strategy is not thorough. Considering the very low frequency of Kaede + cells detected, it is crucial to properly exclude doublets and dead cells.
Authors reported a dramatic difference in Kaede + Tregs cells in the aorta across experiments. This could be addressed by normalization followed by appropriate statistical analysis (One sample t-test).
The data shown is not strong enough to conclude that there is a reduced migration to the aorta.
We understand the importance of reviewer’s suggestion. We described the percentage of Kaede+ Tregs in the aorta of Apoe-/- mice receiving transfer of Kaede-expressing CCR4-intact or CCR4-deficient Tregs in Figure 5I.
As the reviewer pointed out, we understand that it would be important to properly exclude doublets and dead cells in in vivo Treg homing assay. However, it is difficult for us to resolve this issue because we need to perform the same experiments again which will require a great number of additional mice and substantial amount of time. We deeply regret that these important experimental procedures were not performed. We described this issue as the limitation of this study.
In accordance with the reviewer’s suggestion, we re-analyzed the combined data from multiple experiments using one-sample t-test. We observed a tendency toward reduction in the proportion of CCR4-deficient Kaede-expressing Tregs in the aorta of recipient Apoe-/- mice, though there was no statistically significant difference in the migratory capacity of CCR4-intact or CCR4-deficient Kaede-expressing Tregs. By modifying the corresponding descriptions in the manuscript, we toned down our claim that CCR4 expression on Tregs plays a critical role in mediating Treg migration to the atherosclerotic aorta under hypercholesterolemia.
(3) #8: There are still several not shown data
In accordance with the reviewer’s suggestion, we showed the data on the responses of Tregs and effector memory T cells in 8-week-old wild-type or Ccr4-/- mice and Ccr4 mRNA expression in Tregs and non-Tregs from Apoe-/- or Ccr4-/-Apoe-/- mice in Supplementary Figures 4 and 7.
Reviewer #3 (Recommendations for the authors):
(1) Issue 1. For future studies, I recommend not omitting viability controls during cell staining. Removal of dead cells and doublets should always be included during the gating strategy to avoid undesirable artefacts, especially when analysing less-represented cell populations. According to your previous report (ref #40), I agree that isotype controls were unnecessary using the same staining protocol. FMO controls should always be included in flow cytometry analysis (not mentioned in the methodology description and ref#40).
As the reviewer suggested, we understand that it would be important to properly exclude dead cells and doublets and to prepare FMO controls in flow cytometric analysis. We deeply regret that these important experimental procedures were not performed. We described this issue as the limitation of this study.
(2) Issue 3. Although Th17's role in atherosclerosis remains controversial, the data obtained in this work could provide valuable insights if discussed appropriately. As noted in my public review, I found it noteworthy that ROR γ t+ cells represented around 13% of effector TCD45+CD3+CD4+ lymphocytes in the aorta of Apoe-/- mice while Th1 less than 5% (Fig 4H and F, respectively). I recognise that differences in cell staining sensibility and robustness for different transcription factors may influence these percentages. However, analysing how CCR4 deficiency influences the Th1/TI h17 balance would yield interesting data, similar to what was done for the Th1/Treg ratio.
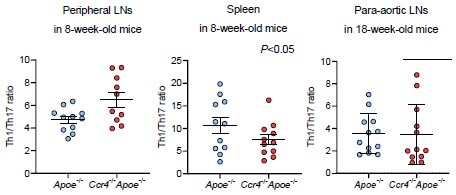
Considering the higher proportion of Th17 cells than Th1 or Th2 cells in atherosclerotic aorta, we understand the importance of reviewer’s suggestion. However, we could not evaluate the effect of CCR4 deficiency on the Th1/Th17 balance in aorta because we did not perform flow cytometric analysis of aortic Th1 and Th17 cells in the same mice. Meanwhile, we could examine the Th1/Th17 balance in peripheral lymphoid tissues by flow cytometry. We found a significant increase in the Th1/Th17 ratio in the peripheral LNs of Ccr4-/-Apoe-/- mice, while there were no changes in its ratio in the spleen or para-aortic LNs of these mice, which limits the contribution of the Th1/Th17 balance to exacerbated atherosclerosis. We showed these data below.
Author response image 1.
(3) Issue 4. I appreciate the authors for sharing data on the flow cytometry analysis of Tregs in para-aortic LNs of Apoe-/- and Ccr4-/- Apoe-/- mice, which would have been included as a Supplementary figure. These results reinforce the notion that Treg dysfunction in CCR4-deficient mice may not be due to the downregulation of regulatory cell surface receptors.
We showed the data on the expression of CTLA-4, CD103, and PD1 in Tregs in the para-aortic LNs of Apoe-/- and Ccr4-/-Apoe-/- mice in Supplementary Figure 8.
(4) Issue 5. I agree that CD4+ T cell responses are substantially regulated by DCs. While CD80 and CD86 on DC primarily serve as costimulatory signals for T-cell activation, cytokines secreted by DCs are primordial signals for determining the differentiation phenotype of effector Th cells. Since the analysis of DC phenotype in lymphoid tissues of Apoe-/- and Ccr4-/- Apoe-/- mice could not be addressed in this study, it is not possible to differentiate which processes may be mainly affected by CCR4-deficiency during CD4+ T cell activation. In this scenario, and considering in vitro studies, the results suggest a possible role of CCR4 in controlling the extent of activation of CD4+T cells rather than shifting the CD4+T cell differentiation profile in peripheral lymphoid tissues, where a predominant Th1 profile was already established in Apoe-/- mice. Therefore, I advise caution when concluding about shifts in CD4+ T cell responses.
We thank the reviewer for providing us thoughtful comments. As the reviewer pointed out, we understand that we should carefully interpret the mechanisms for the shift of CD4+ T cell responses by CCR4 deficiency.
(5) Regarding migration studies in the revised manuscript. I fully understand that Treg transference assays are challenging. The results do not suggest that CCR4 was critical for Treg migration to lymphoid tissues in the conditions assayed. Concerning migration to the aorta, I found the results inconclusive since the authors mention that: i) there was a dramatic difference in the absolute numbers of Kaede-expressing Tregs that migrated to the aorta impairing statistical analysis; ii) the number of Kaede-expressing Tregs that migrated to the aorta was extremely low; iii) dead cells and doublets were not removed in the flow cytometry analysis. In this context, I do not agree with the following statements and recommend revising them:
- "CCR4 deficiency in Tregs impaired their migration to the atherosclerotic aorta" (lines 36-7),
- "…we found a significant reduction in the proportion of CCR4 deficient Kaede-expressing Tregs in the aorta of recipient Apoe-/- mice" (lines 356-7),
- "CCR4 expression on Tregs regulates the development of early atherosclerosis by....... mediating Treg migration to the atherosclerotic aorta" (lines 409-411),
- "…we found that CCR4 expression on Tregs is critical for regulating atherosclerosis by mediating their migration to the atherosclerotic aorta" (lines 437-438),
- "CCR4 protects against early atherosclerosis by mediating Treg migration to the aorta.... (lines 464-465),
- "We showed that CCR4 expression on Tregs is critical for ...... mediating Treg migration to the atherosclerotic aorta" (503-505).
We understand the importance of the reviewer’s suggestion. We described this issue as the limitation of this study. In accordance with the reviewer’s suggestion, we modified the above descriptions and toned down our claim that CCR4 expression on Tregs plays a critical role in mediating Treg migration to the atherosclerotic aorta under hypercholesterolemia.
(6) Line 206: Mention the increased expression of CD86 by DCs
We mentioned this result in the revised manuscript. We also added a discussion on the upregulated CD86 expression on DCs in Ccr4-/-Apoe-/- mice in the discussion section in the revised manuscript.
(7) Lines 304-305. According to Fig 4F-H, a selective accumulation of Th1 cells seems to have occurred only in the aorta, coinciding with a higher Th1/Treg ratio. No selective accumulation of Th1 cells was observed in para-aortic lymph nodes. These results could be clarified.
We modified the above description in the revised manuscript.
-

-

eLife Assessment
This valuable study provides in-vivo evidence that CCR4 regulates the early inflammatory response during atherosclerotic plaque formation. The authors propose that altered T-cell response plays a role in this process, shedding light on mechanisms that may be of interest to medical biologists, biochemists, cell biologists, and immunologists. The work is currently considered incomplete pending textual changes and the inclusion of proper controls.
-

Reviewer #2 (Public review):
Summary:
Tanaka et al. investigated the role of CCR4 in early atherosclerosis, focusing on the immune modulation elicited by this chemokine receptor under hypercholesterolemia. The study found that Ccr4 deficiency led to qualitative changes in atherosclerotic plaques, characterized by an increased inflammatory phenotype. The authors further analyzed the CD4 T cell immune response in para-aortic lymph nodes and atherosclerotic aorta, showing an increase mainly in Th1 cells and the Th1/Treg ratio in Ccr4-/-Apoe-/- mice compared to Apoe-/- mice. They then focused on Tregs, demonstrating that Ccr4 deficiency impaired their immunosuppressive function in in vitro assays. Authors also states that Ccr4-deficient Tregs had, as expected, impaired migration to the atherosclerotic aorta. Adoptive cell transfer of …
Reviewer #2 (Public review):
Summary:
Tanaka et al. investigated the role of CCR4 in early atherosclerosis, focusing on the immune modulation elicited by this chemokine receptor under hypercholesterolemia. The study found that Ccr4 deficiency led to qualitative changes in atherosclerotic plaques, characterized by an increased inflammatory phenotype. The authors further analyzed the CD4 T cell immune response in para-aortic lymph nodes and atherosclerotic aorta, showing an increase mainly in Th1 cells and the Th1/Treg ratio in Ccr4-/-Apoe-/- mice compared to Apoe-/- mice. They then focused on Tregs, demonstrating that Ccr4 deficiency impaired their immunosuppressive function in in vitro assays. Authors also states that Ccr4-deficient Tregs had, as expected, impaired migration to the atherosclerotic aorta. Adoptive cell transfer of Ccr4-/- Tregs to Apoe-/- mice mimicked early atherosclerosis development in Ccr4-/-Apoe-/- mice. Therefore, this work shows that CCR4 plays an important role in early atherosclerosis but not in advanced stages.
Strengths:
Several in vivo and in vitro approaches were used to address the role of CCR4 in early atherosclerosis. Particularly, through the adoptive cell transfer of CCR4+ or CCR4- Tregs, the authors aimed to directly demonstrate the role of CCR4 in Tregs' protection against early atherosclerosis.
Weaknesses:
Flow cytometry experiments are not well controlled. Dead cells and doublets were not excluded from analysis.
Clinical relevance is unclear.
-

Reviewer #3 (Public review):
Summary:
Tanaka and colleagues addressed the role of the C-C chemokine receptor 4 (CCR4) in early atherosclerotic plaque development using ApoE-deficient mice on a standard chow diet as a model. Because several CD4+ T cell subsets express CCR4, they examined whether CCR4-deficiency alters the immune response mediated by CD4+ T cells. By histological analysis of aortic lesions, they demonstrated that the absence of CCR4 promoted the development of early atherosclerosis, with heightened inflammation linked to increased macrophages and pro-inflammatory CD4+ T cells, along with reduced collagen content. Flow cytometry and mRNA expression analysis for identifying CD4+ T cell subsets showed that CCR4 deficiency promoted higher proliferation of pro-inflammatory effector CD4+ T cells in peripheral lymphoid tissues …
Reviewer #3 (Public review):
Summary:
Tanaka and colleagues addressed the role of the C-C chemokine receptor 4 (CCR4) in early atherosclerotic plaque development using ApoE-deficient mice on a standard chow diet as a model. Because several CD4+ T cell subsets express CCR4, they examined whether CCR4-deficiency alters the immune response mediated by CD4+ T cells. By histological analysis of aortic lesions, they demonstrated that the absence of CCR4 promoted the development of early atherosclerosis, with heightened inflammation linked to increased macrophages and pro-inflammatory CD4+ T cells, along with reduced collagen content. Flow cytometry and mRNA expression analysis for identifying CD4+ T cell subsets showed that CCR4 deficiency promoted higher proliferation of pro-inflammatory effector CD4+ T cells in peripheral lymphoid tissues and accumulation of Th1 cells in the atherosclerotic lesions. Interestingly, the increased pro-inflammatory CD4+ T cell response occurred despite the expansion of T CD4+ Foxp3+ regulatory cells (Tregs), found in higher numbers in lymphoid tissues of CCR4-deficient mice, suggesting that CCR4 deficiency interfered with Treg's regulatory actions. In addition, CCR4 deficiency induced an augmented Th1/Treg ratio in the aortic lesions. The CCR4-mediated mechanisms underlying the control of early inflammation and atherosclerosis development were not completely elucidated. In vitro studies suggest that CCR4 expression in Tregs plays a role in controlling DC activation and, in turn, the extent of CD4+T cell activation and proliferation. Dependence on CCR4 expression for Treg migration to the atherosclerotic aorta was not proved. The findings contrast with earlier studies in a murine model of advanced atherosclerosis, where CCR4 deficiency did not alter the development of the aortic lesions. The authors included a thoughtful discussion about hypothetical mechanisms explaining these contrasting results, including putative differences in the role played by the CCL17/CCL22-CCR4 axis along the stages of atherosclerosis development in this murine model.
Major strengths:
• Demonstration of CCR4 deficiency's impact on early atherosclerosis. CCR4 deficiency effects on the early atherosclerosis development in the Apoe-/-mice model were demonstrated by a quantitative analysis of the lesion area, inflammatory cell content and the expression profile of several pro- and anti-inflammatory markers.
• Analysis of the T CD4+ response in various lymphoid tissues (peripheral and para-aortic lymph nodes and spleen) and the atherosclerotic aorta during the early phase of atherosclerosis in the Apoe-/-mice model. This analysis, combining flow cytometry and mRNA expression, showed that CCR4 deficiency enhanced T CD4+ cell activation, favouring the amplification of the typical biased Th1-mediated inflammatory response observed in the lymphoid tissues of hypercholesterolemic mice.
• Treg transference experiments. Transference of Treg from Apoe-/- or Ccr4-/- Apoe-/- mice to Apoe-/- mice under a standard chow diet was useful for addressing the relevance of CCR4 expression on Tregs for the atheroprotective effect of this regulatory T cell subset during early atherosclerosis.Major weaknesses:
• The effect of CCR4 deficiency on the Th1/Th17 balance was not evaluated. Although the role of Th17 cells in atherosclerosis remains controversial, RORγt+ cells constituted, on average, more than 10% of the effector TCD45+CD3+CD4+ lymphocytes in the aorta of Apoe-/- mice (Fig 4H). Changes in the Th1/Th17 balance in lymphoid tissues and aortic lesions may influence the type and functional properties of inflammatory cells recruited to the atherosclerotic aorta.
• Lack of in vivo evidence for Treg suppressive effects on DC activation. The proposed CCR4 requirement for the Treg suppressive activity on DC activation is supported by in vitro co-culture assays, in which CCR4-deficiency partially reverted Treg regulatory actions. Higher expression of CD86, a DC activation marker, was found in spleen DCs from Ccr4-/- Apoe-/- mice compared to Apoe-/- mice (Supplementary Fig 5), which would be worth commenting on and discussing.
• Methodological limitations. Controls in flow cytometry analysis were suboptimal (no viability and doublets were checked) which may have introduced artefacts, especially when measuring less-represented cell populations within complex samples. In addition, assessing Treg migration to the aorta in atherosclerotic mice faced methodological limitations that hindered statistical comparisons between Tregs from Apoe-/- and Ccr4-/- Apoe-/- mice, leading to inconclusive results. The dependence on CCR4 expression for Treg migration to the atherosclerotic aorta was not established.
• Treg transference experiments did not allow the detection of a reduction in the aortic lesion area by transferred CCR4 expressing Tregs (comparison between saline and Apoe-/- Tregs groups). Using Apoe-/- mice as recipients, the CCR4-dependent protective effect of Tregs was mostly evidenced by analysis of aortic inflammation, which was valuable. When using Ccr4-/- Apoe-/- mice as recipients, analysis of aortic inflammation was not mentioned.
Study limitations:
This investigation has some limitations. Current tools for single-cell characterization have revealed the phenotypic heterogeneity and dynamics of aortic leukocytes, including T cells, which are among the principal aortic leukocytes found in mouse and human atherosclerotic lesions (doi:10.1161/CIRCRESAHA.117.312513). The flow cytometry analysis applied in this study cannot distinguish the generation of particular phenotypes within T CD4+ subsets, including putative phenotypes of no-suppressive T cells expressing low levels of Foxp3, as seems could occur in other chronic inflammatory disorders (doi: 10.1038/nm.3432; doi: 10.1172/JCI79014). Limitations due to the use of a complete CCR4 knockout mouse and putative differences in CCR4-mediated mechanisms along atherosclerosis stages and in human atherosclerosis were commented on by the authors in the discussion.
Global Impact:
This work opens the way for a deeper analysis of the contribution of CCR4 and its ligands to the activation and differentiation of T CD4+ lymphocytes during atherosclerosis development, with these lymphocytes being fundamental players in the generation of pro-atherogenic and anti-atherogenic immune responses. Differences in the mechanisms mediated by the CCL17/CCL22-CCR4 axis among early and advanced atherosclerosis highlight the complex landscape to examine and validate in human samples and the need to achieve a deep knowledge for identifying genuine and safe targets capable of promoting protective anti-atherogenic immune responses.
-

Author response:
The following is the authors’ response to the original reviews.
Response to the Reviewer #1 (Public review):
We greatly appreciate the reviewer’s high evaluation of our paper and helpful comments. As expected, we revealed that the CCL17/CCL22–CCR4 axes play an important role in guiding Tregs to the atherosclerotic aorta. Interestingly, we also demonstrated that these axes are critical for Treg-dependent regulation of proinflammatory T cell responses in lymphoid tissues and atherosclerotic aortas, which is a previously unrecognized role for CCR4 in regulating inflammatory immune responses. However, the role of the CCL17/CCL22–CCR4 axes in regulating inflammatory immune responses and atherosclerosis has not been fully elucidated and further investigation is needed.
Response to the reviewer #2 (Public review):
We greatly …
Author response:
The following is the authors’ response to the original reviews.
Response to the Reviewer #1 (Public review):
We greatly appreciate the reviewer’s high evaluation of our paper and helpful comments. As expected, we revealed that the CCL17/CCL22–CCR4 axes play an important role in guiding Tregs to the atherosclerotic aorta. Interestingly, we also demonstrated that these axes are critical for Treg-dependent regulation of proinflammatory T cell responses in lymphoid tissues and atherosclerotic aortas, which is a previously unrecognized role for CCR4 in regulating inflammatory immune responses. However, the role of the CCL17/CCL22–CCR4 axes in regulating inflammatory immune responses and atherosclerosis has not been fully elucidated and further investigation is needed.
Response to the reviewer #2 (Public review):
We greatly appreciate the reviewer’s high evaluation of our paper and helpful comments and suggestions. We isolated CD4+CD25+ T cells and used them as Tregs in several experiments. As the reviewer pointed out, we realize that CD4+CD25+ T cell population contains some activated effector T cells. However, in consideration of the high expression levels of the most reliable Treg marker Foxp3 in isolated CD4+CD25+ T cells determined by flow cytometry, we believe that our method for separating Tregs would be acceptable.
Regarding the role of Th17 cells in atherosclerosis, conflicting results have been reported. Therefore, it is unclear whether augmented Th17 cell immune responses contribute to accelerated atherosclerosis in Ccr4-/-Apoe-/- mice.
As the reviewer pointed out, it is important to consider the clinical relevance of our findings. We analyzed public database to determine if Ccr4 single nucleotide polymorphisms correlate with a higher incidence of atherosclerotic cardiovascular disease. However, no evidence supporting the clinical relevance of our findings was found.
Response to the Reviewer #3 (Public review):
We greatly appreciate the reviewer’s high evaluation of our paper and helpful comments and suggestions. In accordance with the reviewer’s suggestion, we described the detailed methods and carefully performed data analysis regarding flow cytometry, which would strengthen the conclusion of this study.
We understood the importance of reviewer’s claim that CCR4 deficiency does not shift the Th1 cell/Treg balance toward Th1 cell responses in all lymphoid tissues. CCR4 deficiency promoted the accumulation of Th1 cells but did not affect the accumulation of Tregs in the atherosclerotic aorta, which led to the shift of the Th1 cell/Treg balance toward Th1 cell responses. The frequencies of both Tregs and Th1 cells in peripheral lymphoid tissues were increased by CCR4 deficiency, while these CCR4-deficient Tregs exhibited impaired suppressive function. Given this, we speculate that CCR4 deficiency may shift the Th1 cell/Treg balance toward Th1 cell responses in peripheral lymphoid tissues. However, it is difficult to clearly show this. We revised the manuscript accordingly.
Although the reviewer pointed out the possibility that modulation of the Th1 cell/Th17 cell balance might be responsible for the changes in aortic inflammatory cells in Ccr4-/-Apoe-/- mice, the role of Th17 cells in atherosclerosis remain controversial. However, we cannot completely exclude the possibility of the involvement of the Th17 response modulation in accelerated atherosclerosis in Ccr4-/-Apoe-/- mice.
As the limitation of this study, the phenotypic heterogeneity and dynamics of aortic leukocytes could not be revealed by flow cytometric analysis. Single-cell proteomic and transcriptomic approaches would provide additional important information on various aortic cells including immune cells and vascular cells.
Reviewer #1 (Recommendations for the authors):
Issue (1) Ideally, CCR4 could be deleted on Foxp3+ cells and some staining on double positive Rorg+Foxp3+ done. On the other side, a whole gene expression of infiltrated Foxp3 and effector could be also helpful. More challenging, it would be important to see whether those CCR4-specific Trges could or not regulate effector infiltrating cells.
As the reviewer suggested, single-cell proteomic and transcriptomic approaches would be helpful to reveal the phenotypic heterogeneity and dynamics of aortic leukocytes including Tregs. Also, the use of conditional knockout mice would reveal the precise role of CCR4-expressing Tregs in regulating aortic immune cell infiltration and atherosclerosis.
Reviewer #2 (Recommendations for the authors):
Minor Suggestions:
Issue (1) In supplementary Figure 1, CCR4 expression would be better represented by dot plots rather than histograms.
We revised Supplementary Figure 1A through 1C.
Issue (2) The reduction in CD103 expression shown in Figure 2E at 8 weeks should be discussed.
In Figure 2E, we found that the expression of CD103 in peripheral LN Tregs was slightly lower in 8-week-old Ccr4-/-Apoe-/- mice than in age-matched Apoe-/- mice, while there was no difference in its expression levels between 18-week-old Apoe-/- and Ccr4-/-Apoe-/- mice. In addition, there was no significant difference in the mRNA expression of this molecule in splenic Tregs between 8-week-old Apoe-/- and Ccr4-/-Apoe-/- mice. Based on the minor effect of CCR4 deficiency on CD103 expression in Tregs, reduced CD103 expression in Ccr4-/-Apoe-/- mice does not seem to be an important change.
Issue (3) The increased expression of CD86 by DCs should be discussed.
The upregulated CD86 expression on DCs in Ccr4-/-Apoe-/- mice might be explained by the data on a Treg-DC coculture experiment showing the impaired cell–cell contacts between CCR4-deficient Tregs and DCs. On the other hand, the expression of another important costimulatory molecule CD80 on DCs was not altered in these mice, which is not consistent with the data on the above coculture experiment. The reason why only CD86 expression on DCs was upregulated in Ccr4-/-Apoe-/- mice remains unclear.
Issue (4) In Figures 5F-H, using larger dots would enhance visibility.
We revised the graphs in Figure 5F-H.
Issue (5) In Figure 5I, since the data is normalized, a one-sample t-test is more appropriate.
In accordance with the reviewer’s suggestion, we reconsidered the data analysis. Because there was a dramatic difference in the absolute number of Kaede-expressing Tregs accumulated in the aorta among experiments, we were worried that the statistical analysis of the combined data from multiple experiments might draw a wrong conclusion. We have decided to show the representative data from 3 independent experiments in Figure 5I.
Issue (6) On page 11, line 256, the text mentions IL4 and IL10 being detected by cytokine array; however, the figures do not show these cytokines.
We are afraid that the reviewer might have misunderstood the data. The cytokine levels of IL-4 and IL-10 could not be detected by cytokine array analysis. Accordingly, we carefully revised the text in the manuscript.
Issue (7). On page 14, lines 326-330, the text should be revised for clarity.
We revised the text in the manuscript.
Issue (8) Several data are marked as "not shown"; some of this information is relevant and should be included in the supplementary figures.
We showed the data on CCL17 and CCL22 expression in peripheral LNs in Supplementary Figure 2.
Major Suggestions:
Issue (1) FoxP3 expression should be evaluated post-isolation of CD4+CD25+ T cells, and FoxP3- CD4+CD25+ T cells should be characterized. Tregs could be more effectively isolated using FoxP3eGFP mice.
After isolation of CD4+CD25+ T cells (the purity was >95%), we examined Foxp3 expression by flow cytometry and found that most of these cells express Foxp3 (Supplementary Figure 10). Therefore, CD4+CD25+ T cells without Foxp3 expression, which are considered contaminated effector T cells, are minor cells and would not substantially affect the results. Nonetheless, the use of Foxp3-eGFP mice would enable us to isolate Tregs more accurately.
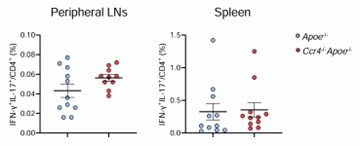
Issue (2) In Figure 3, it would be interesting to evaluate whether there are RORgt+Tbet+ (IL17+IFNg+) cells. These cells would be pathogenic, whereas RORgt+CD73+ cells would be non-pathogenic.
We analyzed CD4+ T cells producing both IL-17 and IFN-γ in the peripheral lymphoid tissues of Apoe-/- and Ccr4-/-Apoe-/- mice. We found that this cell population was quite rare and that there was no significant difference its proportion between the 2 groups, suggesting the possible minor contribution of this cell population to the atherosclerosis phenotype.
Author response image 1.
Issue (3) Different time points after adoptive cell transfer should be evaluated to confirm reduced migration to the atherosclerotic aorta.
It would be interesting to evaluate Treg migration to the atherosclerotic aorta at different time points after Treg transfer. However, it seems difficult to accurately evaluate the migration of Tregs at later time points because they would proliferate in the aorta.
Issue (4) The authors could evaluate whether Ccr4 SNPs correlate with an increased risk of atherosclerosis.
As the reviewer pointed out, it is important to consider the clinical relevance of our findings. However, there is no evidence supporting that Ccr4 single nucleotide polymorphisms correlate with a higher incidence of atherosclerotic cardiovascular disease.
Issue (5) The authors could evaluate if the transfer of Apoe-/- Tregs rescues early atherosclerosis development in Ccr4-/-Apoe-/- mice.
To confirm whether transfer of CCR4-intact Tregs rescues the development of early atherosclerotic lesions in Ccr4-/-Apoe-/- mice, we injected Ccr4-/-Apoe-/- mice with saline or Tregs from Apoe-/- or Ccr4-/-Apoe-/- mice and analyzed the aortic root atherosclerotic lesions of recipient Ccr4-/-Apoe-/- mice. However, we found no significant difference in the aortic sinus plaque area among the 3 groups. We described this result in the results section and included the data in Supplementary Figure 8.
Reviewer #3 (Recommendations for the authors):
Analysis of TCD4+ cell populations in different tissues:
Issue (1) The description of flow cytometry analysis is incomplete and requires clarification. Please detail the use of controls to ensure correct analysis, including the following: i) cell viability; ii) staining controls to define positive and negative cells; iii) the gating strategy used to identify cell populations in each lymphoid tissue and aorta (please provide them as supplementary figures).
As we thought that most of the prepared cells would be viable, we did not check their viability. Based on our previous work where various immune cells including Tregs, effector memory T cells, and helper T cell subsets were clearly detected, in this study we performed flow cytometric analysis of these immune cells without preparing negative controls stained with isotype control antibodies. The gating strategy of flow cytometric analysis of various immune cells in peripheral lymphoid tissues was reported in our previous report (J Am Heart Assoc 2024; 13: e031639). We provided the gating strategy of flow cytometric analysis of helper T cells and Tregs in the aorta in Supplementary Figure 9.
Issue (2) The phenotype/differentiation markers used for analysing T CD4+ cell subsets differ between lymphoid tissues and aortic lesions; might this influence results? If so, please comment on that.
As the number of aortic T cells was quite few compared with that in peripheral lymphoid tissues, it seemed difficult to precisely detect aortic T cells including various helper T cell subsets and Tregs by intracellular cytokine staining. Therefore, we decided to analyze these cells by evaluating transcription factors specific for helper T cell subsets. The difference in the markers used for analyzing T cell subsets would not considerably influence the results.
Issue (3) Considering my observations about the effect of CCR4 deficiency on the T CD4+ differentiation profile in different tissues, I suggest comparing Th1/Treg and Th17/Treg ratios in all examined tissues. The modulation of the Th17/Th1 balance could shape inflammation.
The Th1 cell/Treg balance is shifted toward Th1 cell responses in the atherosclerotic aorta of Ccr4-/-Apoe-/- mice, while this balance would not be altered in the peripheral lymphoid tissues. It remains unclear whether CCR4 deficiency affects the Th17 cell/Treg ratio. We do not think that it is important to investigate the effect of CCR4 deficiency on the balance of Th17 cell/Treg or Th17 cell/Th1 cell because the role of Th17 cell responses in atherosclerosis remains controversial.
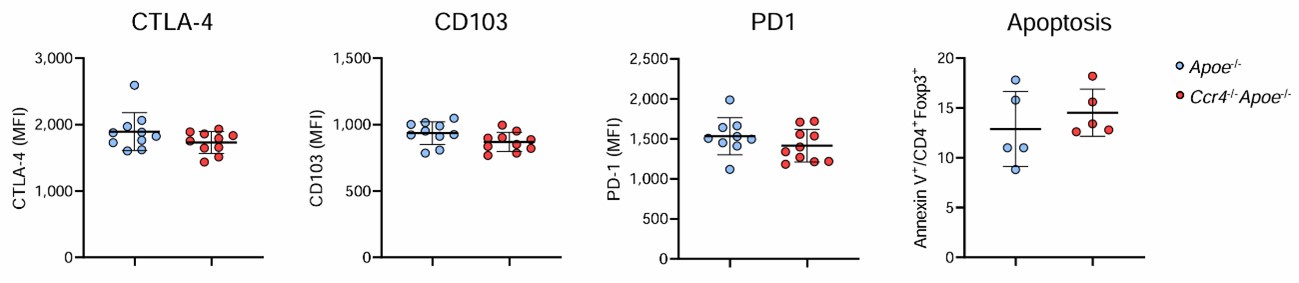
Issue (4) Cell numbers of recovered Treg from para-aortic lymphoid nodes and aortic tissues might not allow Treg functional assays. Analysis by flow cytometry of biomarkers of Treg activation state would be more informative than by quantifying mRNA expression levels. In particular, TGFβ analysis at the mRNA level does not provide much more information about the suppressive activity of Treg, and even at the protein level, the recognition of the active form of this cytokine is required. Analysis of PD1 (for exhausted cell phenotype) and Treg apoptosis along the stages of atherosclerosis could also yield useful information.
We performed flow cytometric analysis of activation markers CTLA-4 and CD103, cell exhaustion marker PD1, and apoptosis in Tregs in the para-aortic LNs of Apoe-/- or Ccr4-/-Apoe-/- mice, and found no major differences in the expression levels of these molecules or the proportion of apoptotic cells between the 2 groups. We showed these data below.
Author response image 2.
Unfortunately, we failed to evaluate the activity of TGF-β in Tregs because an appropriate experimental method for precisely detecting its active form was unavailable.
Issue (5) Regarding the result´s interpretation, I recommend being precise when concluding to avoid misunderstanding. A shift in the T CD4+ response in lymphoid tissues might be interpreted as a modulation of the T cell differentiation process, which strongly depends on signals derived from DCs, which were not the focus of this study.
There are two possible mechanisms for the altered CD4+ T cell responses in peripheral lymphoid tissues, which include the modulation of their differentiation and proliferation processes. These processes are substantially regulated by DCs whose function could be favorably modulated by CCR4-expressing Tregs as described in the manuscript. Therefore, we think that the interactions between Tregs and DCs are crucial for shifting the CD4+ T cell responses in peripheral lymphoid tissues, though it remains unclear which process plays a major role in regulating CD4+ T cell polarization.
Suppression studies:
Issue (1) In vitro assays. According to the methodology suppression studies were performed using Treg collected from peripheral lymphoid nodes and spleen, but it is unclear whether these cells were analysed separately or as a pool (this was not clarified in the legend of Figure 5 either). Besides, be precise about which cells were used as antigen-presenting cells in the Treg suppression assay.
In in vitro Treg suppression assay, we used Tregs purified from peripheral lymph nodes and spleen as a pool. We used splenocytes as antigen-presenting cells in Treg suppression assay. We revised the manuscript accordingly.
Issue (2) Obtaining CD4+CD25+ and CD4+CD25-. The control of the purity and viability of cell preparations from CCR4 deficient and CCR4 sufficient Apoe-/- mice should be included as a supplementary material; these purified cells were used in in vitro suppressive assays and in vivo cell transfer experiments, being relevant information to guarantee results. Since this control was performed by flow cytometry, I wonder whether Foxp3 levels were also checked.
We included the data on the purity and viability of CD4+CD25+ Tregs and CD4+CD25- T cells from Apoe-/- or Ccr4-/-Apoe-/- mice in Supplementary Figure 10. After the isolation of CD4+CD25+ T cells, we examined Foxp3 expression by flow cytometry and found that most of these cells express Foxp3.
Issue (3) For in vitro assays, IL-2, IL-10, and TGFβ measurement in culture supernatants could confirm and provide more information about Treg function.
As both CD4+CD25+ Tregs and CD4+CD25- T cells would produce various cytokines in in vitro Treg suppression assay, it is difficult to determine which cells mainly produce the above cytokines. Therefore, measurement of these cytokines would not provide more information about Treg function.
Issue (4) It would be interesting to assess whether CCR4-mediated DC-Treg interaction is equally important to regulate Th1 than Th17 and Th2 activation; this likely requires using different settings to favour each activation profile.
Based on our findings, we speculate that CCR4 may play an important role in regulating not only Th1 cell responses but also Th2 and Th17 cell responses by maintaining the interactions between Tregs and DCs. However, it may not be meaningful to investigate the effect of CCR4 deficiency on these T cell responses because the roles of Th2 and Th17 cell responses in atherosclerosis remain controversial.
Issue (5) The authors showed that the presence of Treg decreased CD80 and CD86 surface levels in DCs in vitro, remarking a lower capacity of Treg derived from CCR4-deficient mice (Figure 5B). However, the fact that CD86 on splenic CD11c+MHC-II+ DCs in 8-week-old Ccr4-/-Apoe-/- mice was significantly higher than in Apoe-/- was underestimated (Supplementary Figure 4). This data needs reconsideration as it might indicate an in vivo more permissive activation state of DCs in Ccr4-/-Apoe-/- mice than in Apoe-/- mice, explaining the augmented effector T cell response observed in these mice (Figure 2).
Our finding of the upregulated CD86 expression on DCs in Ccr4-/-Apoe-/- mice could be explained by the data on a Treg-DC coculture experiment showing the impaired ability of CCR4-deficient Tregs to downregulate CD80 and CD86 expression on DCs. As the reviewer pointed out, our data may indicate more permissive activation state of DCs and subsequent augmentation of effector T cell responses in Ccr4-/-Apoe-/- mice, which may be derived from impaired Treg suppressive function.
Assays for chemokine levels and influence on T cell activation and traffic:
Issue (1) Considering the findings described by Döring et al. (reference 24 in the paper), monitoring CCL22, CCL17, and CCL3 levels in the aorta and lymph nodes along atherosclerosis development would help in understanding when and how CCL17/CCL20-CCR4 might influence T cell activation and traffic. I wonder whether these chemokines were assayed by qPCR in lymphoid nodes and aorta from CCR4-deficient and sufficient Apoe-/- mice. The authors report that CCR8 (capable also of binding CCL17) was unaltered by CCR4 deficiency in splenic and para-aortic lymph nodes Treg from 8 and 18 weeks-old mice, respectively (Supplementary Figure 5 and 6), although a trend towards a high-level was observed for splenic Treg. It would be informative to evaluate CCR8 Treg levels along with atherosclerosis progress.
As it is considered that the mRNA expression levels of chemokines do not necessarily reflect their protein expression levels, we did not analyze the mRNA expression of Ccl17 or Ccl22 by quantitative reverse transcription PCR. Instead of this, we evaluated the protein expression of CCL17 and CCL22 not only in the aorta but also in the peripheral lymph nodes of 18-week-old wild-type, Apoe-/-, and Ccr4-/-Apoe-/- mice by immunohistochemistry. We found no marked differences in their expression levels in peripheral lymph nodes among these mice and included the data in Supplementary Figure 2.
As we focused on the role of the CCL17/CCL22–CCR4 axes in atherosclerosis, we did not examine the expression of CCL3 that is not directly related to these axes. The evaluation of CCR8+ Treg proportion is beyond the scope of this study, though we are interested in the change of this population by CCR4 deficiency associated with atherosclerotic lesion development.
Issue (2) According to IFNγ and IL-17 expressing TCD4+ subclasses, Th1 and Th17 cell subset levels increase in the spleen (Figure 3B-D) and para-aortic lymphoid nodes (Figure 4E) in CCR4 absence. A comparison of the CCR4 dependence for the migration of Th17 and Th1 cell subsets to the aorta was not performed in this atherosclerosis model; this study could help to understand the mechanisms associated with the aortic inflammation development.
To evaluate the migration of Th1 or Th17 cells in the aorta, we need to specifically isolate them from the peripheral lymphoid tissues of Apoe-/- or Ccr4-/-Apoe-/- mice and adoptively transfer them into recipient Apoe-/- mice. However, it is impossible to isolate alive Th1 or Th17 cells because specific cell surface markers that enable us to separate these cells are unavailable.
Issue (3) The numbers of Kaede Treg cells detected in the aorta were extremely low in both Apoe-/- and Ccr4-/-Apoe-/- mice (Figure 5I), opening results to question. Besides, the flow cytometry assay used for determining Kaede Treg cells in tissues was not well described. How were cell viability and formation of doublets examined to avoid artefacts? The gating strategy used to ensure a confident analysis of Kaede Tregs, particularly in the aorta, should be included as supplementary material.
The extremely low number of Kaede-expressing Tregs migrated in the aorta of Apoe-/- and Ccr4-/-Apoe-/- mice may be derived from the small number of the transferred Tregs. As another explanation for this finding, Tregs may rarely migrate in the aorta under hypercholesterolemic conditions. We did not check the viability or doublets of Kaede-expressing Tregs because we thought that such experimental procedures would not considerably affect the results. We provided the gating strategy of flow cytometric analysis of Kaede-expressing Tregs in peripheral lymphoid tissues and aortas in Supplementary Figure 11.
Other comments:
Issue (1) As an alternative for statistical data analysis from independent experiments, two-way ANOVA with Tukey's post hoc (for data normally distributed) or the Mack Skillings exact test with Conover´s post hoc multiple comparison test (for a two-way layout in non-parametric conditions) could improve analysis.
We performed statistical analysis in Figure 5A according to the reviewer’s suggestion.
Issue (2) For future work, employing recombinant pseudo-receptor proteins capable of neutralizing chemokines (doi: 10.1016/j.jhep.2021.08.029) might help as an alternative to complete knockout mice.
We thank the reviewer for giving us the information on an interesting approach as an alternative to CCR4-deficient mice.
-

-

eLife Assessment
This important study provides solid in-vivo evidence that CCR4 regulates the early inflammatory response during atherosclerotic plaque formation. The authors propose that altered T-cell response plays a role in this process, shedding light on mechanisms that may be of interest to medical biologists, biochemists, cell biologists, and immunologists. Further in vivo validation, mechanistic studies, and discussion of results in vitro suggested would be helpful to cement the significance and implications of these findings.
-

Reviewer #1 (Public review):
Summary:
The article provides valuable information on the role of CCR4 in an inflammatory condition, namely, the arteriosclerosis plaque. The data demonstrated that in the absence of CCR4, the Th1 cells infiltrated the plaque and Tregs lost its functions. The data are clear and well-presented. Mostly importantly, the data on CCR4-specific deficiency in Regulatory T cells is more impressive.
Strengths:
The data are clear, well performed, and interesting in focusing on the plaque and compared to peripheral organs. The disease is relevant and the data could be used to understand the risk of patients under immunomodulator use.
Weaknesses:
Still, we don't know the mechanism, besides migration.
-

Reviewer #2 (Public review):
Summary:
Tanaka et al. investigated the role of CCR4 in early atherosclerosis, focusing on the immune modulation elicited by this chemokine receptor under hypercholesterolemia. The study found that Ccr4 deficiency led to qualitative changes in atherosclerotic plaques, characterized by an increased inflammatory phenotype. The authors further analyzed the CD4 T cell immune response in para-aortic lymph nodes and atherosclerotic aorta, showing an increase mainly in Th1 cells and the Th1/Treg ratio in Ccr4-/-Apoe-/- mice compared to Apoe-/- mice. They then focused on Tregs, demonstrating that Ccr4 deficiency impaired their immunosuppressive function in in-vitro assays and elegantly showed that Ccr4-deficient Tregs had, as expected, impaired migration to the atherosclerotic aorta. Adoptive cell transfer of …
Reviewer #2 (Public review):
Summary:
Tanaka et al. investigated the role of CCR4 in early atherosclerosis, focusing on the immune modulation elicited by this chemokine receptor under hypercholesterolemia. The study found that Ccr4 deficiency led to qualitative changes in atherosclerotic plaques, characterized by an increased inflammatory phenotype. The authors further analyzed the CD4 T cell immune response in para-aortic lymph nodes and atherosclerotic aorta, showing an increase mainly in Th1 cells and the Th1/Treg ratio in Ccr4-/-Apoe-/- mice compared to Apoe-/- mice. They then focused on Tregs, demonstrating that Ccr4 deficiency impaired their immunosuppressive function in in-vitro assays and elegantly showed that Ccr4-deficient Tregs had, as expected, impaired migration to the atherosclerotic aorta. Adoptive cell transfer of Ccr4-/- Tregs to Apoe-/- mice mimicked early atherosclerosis development in Ccr4-/-Apoe-/- mice. Therefore, this work shows that CCR4 plays an important role in early atherosclerosis but not in advanced stages.
Strengths:
Several in vivo and in vitro approaches were used to address the role of CCR4 in early atherosclerosis. Particularly, through the adoptive cell transfer of CCR4+ or CCR4- Tregs, the authors aimed to directly demonstrate the role of CCR4 in Tregs' protection against early atherosclerosis.
Weaknesses:
The isolation of Tregs was inadequately controlled; they were isolated based solely on CD4 and CD25 expression. CD25 is also expressed by activated effector T cells, meaning the analyzed cells could be a pool of mainly Tregs but also include effector T cells.
The study primarily focused on Th1 and Tregs without thoroughly investigating other CD4 T cell subsets. Th17 cells are known to play an important role in atherosclerosis; non-pathogenic Th17 cells express CCR4, while pathogenic Th17 cells do not. Considering that Figure 3 shows an increased frequency of IL17-expressing CD4 T cells compared to Apoe-/- mice, and given the imprecise Treg isolation, differences in non-pathogenic Th17 cells could be contributing to the observed effects.
Furthermore, the clinical relevance of these findings is not discussed. As an initial approach, the authors could analyze public datasets to determine if certain Ccr4 single nucleotide polymorphisms correlate with a higher incidence of atherosclerosis.
-

Reviewer #3 (Public review):
Summary:
In this paper, Tanaka and colleagues address the role played by the C-C chemokine receptor 4 (CCR4) in developing early atherosclerotic plaques using ApoE-deficient mice fed with a standard chow diet as a model. Since CCR4 is expressed in several T CD4+ lymphocyte subsets, the authors examined the consequences of CCR4 deficiency on the differentiation profile and traffic of T CD4+ lymphocytes. By histological analysis of aortic lesions, they demonstrated that the absence of CCR4 promoted the development of early atherosclerosis, characterized by an inflammatory reaction with increased levels of macrophages and T CD4+ inflammatory lymphocytes while decreased collagen content. Using flow cytometry together with mRNA expression analysis for identifying T CD4+ cell subsets, the authors found that the …
Reviewer #3 (Public review):
Summary:
In this paper, Tanaka and colleagues address the role played by the C-C chemokine receptor 4 (CCR4) in developing early atherosclerotic plaques using ApoE-deficient mice fed with a standard chow diet as a model. Since CCR4 is expressed in several T CD4+ lymphocyte subsets, the authors examined the consequences of CCR4 deficiency on the differentiation profile and traffic of T CD4+ lymphocytes. By histological analysis of aortic lesions, they demonstrated that the absence of CCR4 promoted the development of early atherosclerosis, characterized by an inflammatory reaction with increased levels of macrophages and T CD4+ inflammatory lymphocytes while decreased collagen content. Using flow cytometry together with mRNA expression analysis for identifying T CD4+ cell subsets, the authors found that the accelerated aortic inflammation induced by CCR4 deficiency correlated with higher proliferation of T CD4+ cells in lymphoid tissues, favouring the expansion of the pro-inflammatory effector Th1 cell subset, typically found in atherosclerotic lesions. Interestingly, the increased T CD4+ cell response occurred despite the expansion of T CD4+ Foxp3+ regulatory cells (Treg), which were in higher numbers in the lymphoid tissues of CCR4-deficient mice, suggesting the absence of CCR4 interfered with the regulatory actions of Treg cells. Using in vitro and or in vivo approaches, the authors found evidence of CCR4 requirement for Treg suppressive activity and migratory capacity to inflamed aortic areas, contributing to why CCR4 deficiency induced an augmented Th1/Treg ratio in the aortic lesions. These findings might not be surprising considering the demonstrated involvement of CCR4 in driving Treg migration to inflamed tissues in immune-related pathological models and Treg-dendritic cell contact for imprinting suppressive signals. However, in previous studies using a murine model of advanced atherosclerosis, neither hematopoietic nor systemic CCR4 deficiency altered the development of the aortic lesions. The authors included a thoughtful discussion about hypothetical mechanisms explaining these contrasting results, highlighting putative differences in the role played by the CCL17/CCL22-CCR4 axis along the stages of atherosclerosis development in this murine model.
Major strengths and weaknesses:
The main effects of CCR4 deficiency on early atherosclerosis development and Treg functional loss are valuable and supported by collected data. In vivo studies for comparing Treg-tissue accumulation or atherosclerotic lesions in Apoe-/- mice that received Treg derived from Apoe-/- or Apoe-/-Ccr4-/- mice, strengthening results. However, an incomplete description of methods (particularly flow cytometry) and data analysis weakens some conclusions of this study. Readers should note some inconsistencies in the T CD4+ response analysis in different tissues. In aortic lesions, but not in lymphoid tissues (peripheral, para-aortic, and spleen), the ratio Th1/Treg was used for evaluating the effect of CCR4 deficiency on the profile of Th cell subsets. In lymphoid tissues, increments in the frequency of both effector Th1 and Treg were observed in CCR4-deficient Apoe-/- mice compared to CCR4-sufficient Apoe-/- mice. Therefore, it is not convincing that CCR4-deficiency shifts Th1 cell/Treg balance toward Th1 cell responses in all lymphoid tissues; this claim needs to be revised by the authors. The Treg dysfunction, caused by CCR4 deficiency, enhanced T CD4+ activation and might have amplified rather than shifted, the typical biased Th1-mediated inflammatory response observed in the lymphoid tissues of hypercholesterolemic mice. A different scenario emerged in aortic lesions, where recruitment of effector Th1 cells, but not of additional effector T CD4+ cell subsets expanded in lymphoid tissues, leading to a higher Th1/Treg balance. Also, effector Th17 cells seem to predominate among effector TCD45+CD3+CD4+ cells in the aorta of Apoe-/- mice, and the Th1/Th17 balance appears to have increased as a consequence of CCR4 deficiency as well. Modulation of Th1/Th17 balance might be responsible for changes in the type and functional properties of recruited inflammatory cells in the aorta.
Study limitations:
This investigation has some limitations. Current tools for single-cell characterization have revealed the phenotypic heterogeneity and dynamics of aortic leukocytes, including T cells, which are among the principal aortic leukocytes found in mouse and human atherosclerotic lesions (doi:10.1161/CIRCRESAHA.117.312513). The flow cytometry analysis applied in this study cannot distinguish the generation of particular phenotypes within T CD4+ subsets, including putative phenotypes of no-suppressive T cells expressing low levels of Foxp3, as seems could occur in other chronic inflammatory disorders (doi: 10.1038/nm.3432; doi: 10.1172/JCI79014). Limitations due to the use of a complete CCR4 knockout mouse and putative differences in CCR4-mediated mechanisms along atherosclerosis stages and in human atherosclerosis were commented on by the authors in the discussion.
Global Impact
This work opens the way for a deeper analysis of the contribution of CCR4 and its ligands to the activation and differentiation of T CD4+ lymphocytes during atherosclerosis development, with these lymphocytes being fundamental players in the generation of pro-atherogenic and anti-atherogenic immune responses. Differences in the mechanisms mediated by the CCL17/CCL22-CCR4 axis among early and advanced atherosclerosis highlight the complex landscape to examine and validate in human samples and the need to achieve a deep knowledge for identifying genuine and safe targets capable of promoting protective anti-atherogenic immune responses.
-

-