Monocyte-derived macrophage recruitment mediated by TRPV1 is required for eardrum wound healing
Curation statements for this article:-
Curated by eLife
eLife Assessment
This study exploring the role of TRPV1 signaling in recruiting macrophages and promoting angiogenesis during tympanic membrane wound healing presents useful findings. However, the strength of evidence supporting the central claims is incomplete, as the mechanistic links between TRPV1 activation and immune cell recruitment remain largely correlative and rely heavily on previously published datasets without sufficient functional validation. The work will be of interest to researchers studying wound healing and sensory-immune interactions, though substantial revisions are needed to support its broader significance.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Abstract
The tympanic membrane (TM), or eardrum, is a thin, sensitive tissue critical for hearing by vibrating and transmitting sound waves to the inner ear. TM perforation and development of otitis media and conductive hearing loss are commonly seen in the clinic. In this study, we demonstrate the role of TRPV1 signaling mediated macrophage recruitment and angiogenesis in TM repair. By creating a wounded TM mouse model with a perforation in the anteroinferior region of the pars tensa — a region in humans often damaged in traumatic injury, we observed a massive accumulation of macrophages in the vicinity of the acutely wounded TM. Using 5-Ethynyl-2’-deoxyuridine pause labeling and a chimeric bone marrow transplant model, we found that most of the recruited macrophages did not originate from local tissue-resident macrophages but rather from blood-circulating monocytes. Parallel to macrophage recruitment, angiogenesis was observed near the wound on day 3 after perforation and further progressed by day 7. The angiogenic process was strongly associated with the recruited macrophages, as macrophage depletion resulted in a notable reduction in angiogenesis. At the transcriptional level, we found that macrophages facilitate angiogenesis through several signaling pathways. Additionally, we identified direct intercellular communication between macrophages and endothelial cells mediated by phosphoprotein 1 signaling. Furthermore, Gene Ontology analysis of bulk RNA sequencing data from TMs revealed that the macrophage recruitment is associated with neuroinflammatory responses. Using a fluorescence reporter mouse driven by TRPV1, we discovered that the TM contains rich sensory nerve fibers expressing TRPV1. A genetic mutation in the Trpv1 gene resulted in a marked decrease in the expression of neuroinflammatory genes, such as Tac1. This decrease subsequently resulted in reduced macrophage recruitment, impaired angiogenesis, and delayed wound healing. Together, these findings highlight the crucial role of TRPV1 signaling in monocyte migration and macrophage-related angiogenesis, both of which are crucial for facilitating healing of the TM. These results also open new opportunities for clinical interventions. Targeting TRPV1 signaling could enhance TM immunity, improve blood circulation, promote the repair of damaged TM, and ultimately prevent middle ear infections.
Article activity feed
-

eLife Assessment
This study exploring the role of TRPV1 signaling in recruiting macrophages and promoting angiogenesis during tympanic membrane wound healing presents useful findings. However, the strength of evidence supporting the central claims is incomplete, as the mechanistic links between TRPV1 activation and immune cell recruitment remain largely correlative and rely heavily on previously published datasets without sufficient functional validation. The work will be of interest to researchers studying wound healing and sensory-immune interactions, though substantial revisions are needed to support its broader significance.
-

Reviewer #1 (Public review):
Summary:
This study reveals that TRPV1 signaling plays a key role in tympanic membrane (TM) healing by promoting macrophage recruitment and angiogenesis. Using a mouse TM perforation model, researchers found that blood-derived macrophages accumulated near the wound, driving angiogenesis and repair. TRPV1-expressing nerve fibers triggered neuroinflammatory responses, facilitating macrophage recruitment. Genetic Trpv1 mutation reduced macrophage infiltration, angiogenesis, and delayed healing. These findings suggest that targeting TRPV1 or stimulating sensory nerve fibers could enhance TM repair, improve blood flow, and prevent infections. This offers new therapeutic strategies for TM perforations and otitis media in clinical settings. This is an excellent and high-quality study that provides valuable insights …
Reviewer #1 (Public review):
Summary:
This study reveals that TRPV1 signaling plays a key role in tympanic membrane (TM) healing by promoting macrophage recruitment and angiogenesis. Using a mouse TM perforation model, researchers found that blood-derived macrophages accumulated near the wound, driving angiogenesis and repair. TRPV1-expressing nerve fibers triggered neuroinflammatory responses, facilitating macrophage recruitment. Genetic Trpv1 mutation reduced macrophage infiltration, angiogenesis, and delayed healing. These findings suggest that targeting TRPV1 or stimulating sensory nerve fibers could enhance TM repair, improve blood flow, and prevent infections. This offers new therapeutic strategies for TM perforations and otitis media in clinical settings. This is an excellent and high-quality study that provides valuable insights into the mechanisms underlying TM wound healing.
Strengths:
The work is particularly important for elucidating the cellular and molecular processes involved in TM repair. However, there are several concerns about the current version.
Weaknesses:
Major concerns
(1) The method of administration will be a critical factor when considering potential therapeutic strategies to promote TM healing. It would be beneficial if the authors could discuss possible delivery methods, such as topical application, transtympanic injection, or systemic administration, and their respective advantages and limitations for targeting TRPV1 signaling. For example, Dr. Kanemaru and his colleagues have proposed the use of Trafermin and Spongel to regenerate the eardrum.
(2) The authors appear to have used surface imaging techniques to observe the TM. However, the TM consists of three distinct layers: the epithelial layer, the fibrous middle layer, and the inner mucosal layer. The authors should clarify whether the proposed mechanism involving TRPV1-mediated macrophage recruitment and angiogenesis is limited to the epithelial layer or if it extends to the deeper layers of the TM.
Minor concerns
In Figure 8, the schematic illustration presents a coronal section of the TM. However, based on the data provided in the manuscript, it is unclear whether the authors directly obtained coronal images in their study. To enhance the clarity and impact of the schematic, it would be helpful to include representative images of coronal sections showing macrophage infiltration, angiogenesis, and nerve fiber distribution in the TM.
-

Reviewer #2 (Public review):
Summary:
This study examines the role of TRPV1 signaling in the recruitment of monocyte-derived macrophages and the promotion of angiogenesis during tympanic membrane (TM) wound healing. The authors use a combination of genetic mouse models, macrophage depletion, and transcriptomic approaches to suggest that neuronal TRPV1 activity contributes to macrophage-driven vascular responses necessary for tissue repair.
Strengths:
(1) The topic of neuroimmune interactions in tissue regeneration is of interest and underexplored in the context of the TM, which presents a unique model due to its anatomical features.
(2) The use of reporter mice and bone marrow chimeras allows for some dissection of immune cell origin.
(3) The authors incorporate transcriptomic data to contextualize inflammatory and angiogenic processes …
Reviewer #2 (Public review):
Summary:
This study examines the role of TRPV1 signaling in the recruitment of monocyte-derived macrophages and the promotion of angiogenesis during tympanic membrane (TM) wound healing. The authors use a combination of genetic mouse models, macrophage depletion, and transcriptomic approaches to suggest that neuronal TRPV1 activity contributes to macrophage-driven vascular responses necessary for tissue repair.
Strengths:
(1) The topic of neuroimmune interactions in tissue regeneration is of interest and underexplored in the context of the TM, which presents a unique model due to its anatomical features.
(2) The use of reporter mice and bone marrow chimeras allows for some dissection of immune cell origin.
(3) The authors incorporate transcriptomic data to contextualize inflammatory and angiogenic processes during wound healing.
Weaknesses:
(1) The primary claims of the manuscript are not convincingly supported by the evidence presented. Most of the data are correlative in nature, and no direct mechanistic experiments are included to establish causality between TRPV1 signaling and macrophage recruitment or function.
(2) Functional validation of key molecular players (such as Tac1 or Spp1) is lacking, and their roles are inferred primarily from gene expression data rather than experimentally tested.
(3) The reuse of publicly available scRNA-seq data is not sufficiently integrated or extended to yield new biological insights, and it remains largely descriptive.
(4) The macrophage depletion model (CX3CR1CreER; iDTR) lacks specificity, and possible off-target or systemic effects are not addressed.
(5) Several interpretations of the data appear overstated, particularly regarding the necessity of TRPV1 for monocyte recruitment and wound healing.
(6) Overall, the study appears to apply known concepts - namely, TRPV1-mediated neurogenic inflammation and macrophage-driven angiogenesis - to a new anatomical site without providing new mechanistic insight or advancing the field substantially.
Overall:
While the study addresses an interesting topic, the current version does not provide sufficiently strong or novel evidence to support its major conclusions. Additional mechanistic experiments and more rigorous validation would be necessary to substantiate the proposed model and clarify the relevance of the findings beyond this specific tissue context.
-

Author response:
Public Reviews:
Reviewer #1 (Public review):
Summary:
This study reveals that TRPV1 signaling plays a key role in tympanic membrane (TM) healing by promoting macrophage recruitment and angiogenesis. Using a mouse TM perforation model, researchers found that blood-derived macrophages accumulated near the wound, driving angiogenesis and repair. TRPV1-expressing nerve fibers triggered neuroinflammatory responses, facilitating macrophage recruitment. Genetic Trpv1 mutation reduced macrophage infiltration, angiogenesis, and delayed healing. These findings suggest that targeting TRPV1 or stimulating sensory nerve fibers could enhance TM repair, improve blood flow, and prevent infections. This offers new therapeutic strategies for TM perforations and otitis media in clinical settings. This is an excellent and high-quality …
Author response:
Public Reviews:
Reviewer #1 (Public review):
Summary:
This study reveals that TRPV1 signaling plays a key role in tympanic membrane (TM) healing by promoting macrophage recruitment and angiogenesis. Using a mouse TM perforation model, researchers found that blood-derived macrophages accumulated near the wound, driving angiogenesis and repair. TRPV1-expressing nerve fibers triggered neuroinflammatory responses, facilitating macrophage recruitment. Genetic Trpv1 mutation reduced macrophage infiltration, angiogenesis, and delayed healing. These findings suggest that targeting TRPV1 or stimulating sensory nerve fibers could enhance TM repair, improve blood flow, and prevent infections. This offers new therapeutic strategies for TM perforations and otitis media in clinical settings. This is an excellent and high-quality study that provides valuable insights into the mechanisms underlying TM wound healing.
Strengths:
The work is particularly important for elucidating the cellular and molecular processes involved in TM repair. However, there are several concerns about the current version.
We sincerely thank Reviewer #1 for their time and effort in evaluating and improving our study. Below, we are pleased to address the Reviewer's concerns point by point.
Weaknesses:
Major concerns
(1) The method of administration will be a critical factor when considering potential therapeutic strategies to promote TM healing. It would be beneficial if the authors could discuss possible delivery methods, such as topical application, transtympanic injection, or systemic administration, and their respective advantages and limitations for targeting TRPV1 signaling. For example, Dr. Kanemaru and his colleagues have proposed the use of Trafermin and Spongel to regenerate the eardrum.
We are grateful to the reviewer for raising this important point. While the present study primarily focuses on the mechanistic role of TRPV1 in TM repair, we agree that the mode of therapeutic delivery will be pivotal in translating these findings into clinical practice. In response, we will expand the discussion to explore possible delivery methods—such as topical application, transtympanic injection, and systemic routes—along with their respective benefits and challenges. We will also cite the work by Dr. Kanemaru and colleagues as an example of how local delivery systems may facilitate TM regeneration.
(2) The authors appear to have used surface imaging techniques to observe the TM. However, the TM consists of three distinct layers: the epithelial layer, the fibrous middle layer, and the inner mucosal layer. The authors should clarify whether the proposed mechanism involving TRPV1-mediated macrophage recruitment and angiogenesis is limited to the epithelial layer or if it extends to the deeper layers of the TM.
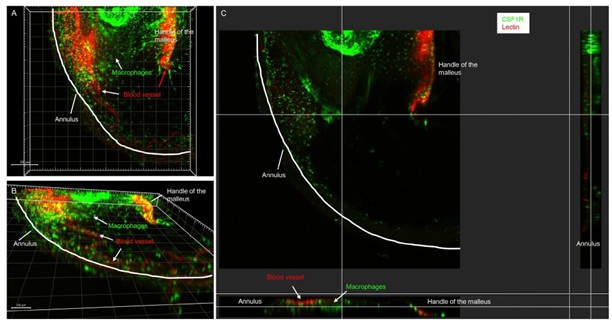
We apologize for any confusion caused by our previous description. In our study, we utilized Z-stack confocal imaging to capture the full thickness of the TM, as illustrated in Author response image 1 (reconstructed from the acquired Z-sections). This imaging technique allowed us to encompass all three layers of the TM entirely. Each sample was imaged using a 10X objective on an Olympus fluorescence microscope. Given the conical shape and size of the TM, we imaged it in four quadrants, acquiring approximately 30 optical sections (with a 3 µm step) per region. Each acquired images were projected and exported using FV10ASW 4.2 Viewer, then stitched together using Photoshop. The resulting Z-stack projections enabled us to visualize the distribution of macrophages, angiogenesis, and the localization of nerve fibers throughout the TM. We will include this detailed methodology in our revision to clarify any potential confusion.
Author response image 1.
Representative confocal images showing one quadrant of the TM collected from collected from CSR1FEGFP bone marrow transplanted mouse at day 7 post-perforation. (A-B) 3D-rendered views from different angles reveal the close spatial relationship between CSF1REGFP cells (green) and blood vessels (red) within the TM. (C) Cross-sectional view highlights the depth-wise distribution of CSF1REGFP cells (green) and blood vessels (red) across the layered TM architecture. All images were processed using Imaris Viewer x64 (version 10.2.0).
Minor concerns
In Figure 8, the schematic illustration presents a coronal section of the TM. However, based on the data provided in the manuscript, it is unclear whether the authors directly obtained coronal images in their study. To enhance the clarity and impact of the schematic, it would be helpful to include representative images of coronal sections showing macrophage infiltration, angiogenesis, and nerve fiber distribution in the TM.
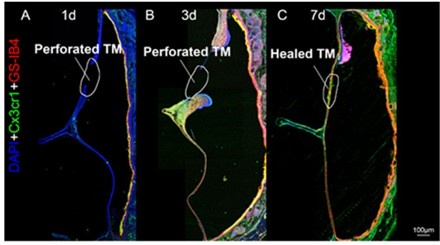
As noted above, we utilized Z-stack confocal imaging to capture the full thickness of the TM, enabling us to visualize structures across all three layers. This approach ensured that all layers were included in our analysis. Due to the thin and curved nature of the TM, traditional cross-sectional imaging often struggles to clearly depict the spatial relationships between macrophages, blood vessels, and nerve fibers, especially at low magnification as shown in Author response image 2. In response to the reviewer's suggestion, we will include representative coronal images in the revised manuscript to better illustrate the distribution of these structures at higher magnification.
Author response image 2.
Confocal images of eardrum cross-sections collected at day 1 (A), 3 (B), and 7 (C) post perforation to demonstrate the wound healing processes.
Reviewer #2 (Public review):
Summary:
This study examines the role of TRPV1 signaling in the recruitment of monocyte-derived macrophages and the promotion of angiogenesis during tympanic membrane (TM) wound healing. The authors use a combination of genetic mouse models, macrophage depletion, and transcriptomic approaches to suggest that neuronal TRPV1 activity contributes to macrophage-driven vascular responses necessary for tissue repair.
Strengths:
(1) The topic of neuroimmune interactions in tissue regeneration is of interest and underexplored in the context of the TM, which presents a unique model due to its anatomical features.
(2) The use of reporter mice and bone marrow chimeras allows for some dissection of immune cell origin.
(3) The authors incorporate transcriptomic data to contextualize inflammatory and angiogenic processes during wound healing.
We sincerely thank Reviewer #2 for their time and effort in improving our study and recognizing its strengths. Below, we are pleased to address the reviewer's concerns point by point.
Weaknesses:
(1) The primary claims of the manuscript are not convincingly supported by the evidence presented. Most of the data are correlative in nature, and no direct mechanistic experiments are included to establish causality between TRPV1 signaling and macrophage recruitment or function.
We appreciate Reviewer #2's perspective on the lack of molecular mechanisms linking TRPV1 signaling and macrophages. However, our data demonstrates that TRPV1 mutations significantly affect macrophage recruitment and angiogenesis. This initial study primarily focuses on the intriguing phenomenon of how sensory nerve fibers are involved in eardrum immunity and wound healing, an area that has not been clearly reported in the literature before. We believe that further research is necessary to explore this topic in greater depth.
(2) Functional validation of key molecular players (such as Tac1 or Spp1) is lacking, and their roles are inferred primarily from gene expression data rather than experimentally tested.
Although we have identified the TAC1 and SPP1 signals as potentially important for TM wound healing for the first time, we agree with the Reviewer's view regarding the lack of molecular mechanisms explored in this study. We have not yet tested the downstream signaling pathways, but we plan to investigate them in a series of future studies. As this is an early report, we will continue to explore these signals and their potential clinical applications based on our initial findings moving forward.
(3) The reuse of publicly available scRNA-seq data is not sufficiently integrated or extended to yield new biological insights, and it remains largely descriptive.
We appreciate Reviewer #2 for highlighting this point. Leveraging publicly available scRNA-seq databases and established analysis pipelines not only saves time and resources—my lab recently collected macrophages from the eardrums of postnatal P15 mice, with each trial requiring 20 eardrums from 10 animals to obtain a sufficient number of cells—but also allows researchers to build on previous work and focus on new biological questions without the need to repeat experiments. A prior study conducted by Dr. Tward and his team utilized scRNA-seq data to make initial discoveries related to eardrum wound healing, primarily focusing on epithelial cells rather than macrophages. We are building on their raw data to uncover new biological insights regarding macrophages, even though we have not yet tested the unidentified signals, which we believe will be valuable to our peers.
(4) The macrophage depletion model (CX3CR1CreER; iDTR) lacks specificity, and possible off-target or systemic effects are not addressed.
We agree with reviewer #2, although macrophage depletion model used in our study is a standard and well-used animal model (Shi, Hua et al. 2018), which has been used by many other laboratories, it is important to note that any macrophage depletion model may have potential issues. We will discuss this in our revision.
(5) Several interpretations of the data appear overstated, particularly regarding the necessity of TRPV1 for monocyte recruitment and wound healing.
We thank the reviewer for pointing this out. We will revise our manuscript where it is overstated accordingly.
(6) Overall, the study appears to apply known concepts - namely, TRPV1-mediated neurogenic inflammation and macrophage-driven angiogenesis - to a new anatomical site without providing new mechanistic insight or advancing the field substantially.
Although our study may not seem highly innovative at first glance, it reveals a previously unknown role of the TRPV1 pain signaling pathway in promoting eardrum healing for the first time. This healing process includes the recruitment of monocyte-derived macrophages and the formation of new blood vessels (angiogenesis). While this process has been documented in other organs, most research on macrophage-driven angiogenesis has been conducted using in vitro models, with very few studies demonstrating this process in vivo. Our findings could lead to new translational opportunities, especially considering that tympanic membrane perforation, along with damage-induced otitis media and conductive hearing loss, are common clinical issues affecting millions of people worldwide. Targeting TRPV1 signaling could enhance tympanic membrane immunity, improve blood circulation, promote the repair of damaged tympanic membranes, and ultimately prevent middle ear infections—an idea that has not been previously proposed.
Overall:
While the study addresses an interesting topic, the current version does not provide sufficiently strong or novel evidence to support its major conclusions. Additional mechanistic experiments and more rigorous validation would be necessary to substantiate the proposed model and clarify the relevance of the findings beyond this specific tissue context.
We greatly thank the two reviewers for their helpful critiques to improve our study. We especially thank the Section Editors for their insightful and constructive comments on this initial study.
References:
Shi, J., L. Hua, D. Harmer, P. Li and G. Ren (2018). "Cre Driver Mice Targeting Macrophages." Methods Mol Biol 1784: 263-275.
-

-

-