14-3-3 protein augments the protein stability of phosphorylated spastin and promotes the recovery of spinal cord injury through its agonist intervention
Curation statements for this article:-
Curated by eLife
eLife assessment
The finding that Fusicoccin (FC-A) promotes locomotor recovery after spinal cord injury is supported by solid data, and the idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is valuable. The evidence showing that 14-3-3 and spastin interact and that 14-3-3 enhances spastin function and stability in cells is also solid.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Axon regeneration is abortive in the central nervous system following injury. Orchestrating microtubule dynamics has emerged as a promising approach to improve axonal regeneration. The microtubule severing enzyme spastin is essential for axonal development and regeneration through remodeling of microtubule arrangement. To date, however, little is known regarding the mechanisms underlying spastin action in neural regeneration after spinal cord injury. Here, we use glutathione transferase pulldown and immunoprecipitation assays to demonstrate that 14-3-3 interacts with spastin, both in vivo and in vitro, via spastin Ser233 phosphorylation. Moreover, we show that 14-3-3 protects spastin from degradation by inhibiting the ubiquitination pathway and upregulates the spastin-dependent severing ability. Furthermore, the 14-3-3 agonist Fusicoccin (FC-A) promotes neurite outgrowth and regeneration in vitro which needs spastin activation. Western blot and immunofluorescence results revealed that 14-3-3 protein is upregulated in the neuronal compartment after spinal cord injury in vivo. In addition, administration of FC-A not only promotes locomotor recovery, but also nerve regeneration following spinal cord injury in both contusion and lateral hemisection models; however, the application of spastin inhibitor spastazoline successfully reverses these phenomena. Taken together, these results indicate that 14-3-3 is a molecular switch that regulates spastin protein levels, and the small molecule 14-3-3 agonist FC-A effectively mediates the recovery of spinal cord injury in mice which requires spastin participation.
Article activity feed
-

-

-

-

Author Response
The following is the authors’ response to the previous reviews.
eLife assessment
The finding that Fusicoccin (FC-A) promotes locomotor recovery after spinal cord injury is useful, and the idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. However, the main methods, data, and analyses are inadequate to support the primary claim of the manuscript that a 14-3-3-Spastin complex is necessary for the observed FC-A effects.
Response: We appreciate the eLife editorial and review team for consideration and evaluation of our manuscript. In light of the feedback from the editors and reviewers, we recognize that certain aspects of the title and key conclusions require further refinement. We have shown that 14-3-3, through its …
Author Response
The following is the authors’ response to the previous reviews.
eLife assessment
The finding that Fusicoccin (FC-A) promotes locomotor recovery after spinal cord injury is useful, and the idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. However, the main methods, data, and analyses are inadequate to support the primary claim of the manuscript that a 14-3-3-Spastin complex is necessary for the observed FC-A effects.
Response: We appreciate the eLife editorial and review team for consideration and evaluation of our manuscript. In light of the feedback from the editors and reviewers, we recognize that certain aspects of the title and key conclusions require further refinement. We have shown that 14-3-3, through its interaction with phosphorylated spastin, inhibits the degradation of spastin. Also, we have demonstrated that 14-3-3 can enhance spastin's microtubule-severing ability in cell lines. Furthermore, our work has illustrated the significant roles of 14-3-3 and spastin in the repair process of spinal cord injury. However, there is currently insufficient direct evidence to confirm the cooperation between 14-3-3 and spastin during axon regeneration and the recovery of spinal cord injury. Moreover, we have not provided conclusive evidence of their simultaneous action in injured axons, mediating changes in microtubule dynamics. Consequently, we have re-evaluated the manuscript's title and primary conclusions, and have made relevant modifications. For more detailed information, please refer to the reviewer's comments.
Public Reviews:
Reviewer #1 (Public Review):
The present work establishes 14-3-3 proteins as binding partners of spastin and suggests that this binding is positively regulated by phosphorylation of spastin. The authors show evidence that 14-3-3 - spastin binding prevents spastin ubiquitination and final proteasomal degradation, thus increasing the availability of spastin. The authors measured microtubule severing activity in cell lines and axon regeneration and outgrowth as a prompt to spastin activity. By using drugs and peptides that separately inhibit 14-3-3 binding or spastin activity, they show that both proteins are necessary for axon regeneration in cell culture and in vivo models in rats.
The following is an account of the major strengths and weaknesses of the methods and results.
Major strengths
-The authors performed pulldown assays on spinal cord lysates using GST-spastin, then analyzed pulldowns via mass spectrometry and found 3 peptides common to various forms of 14-3-3 proteins. In co-expression experiments in cell lines, recombinant spastin co-precipitated with all 6 forms of 14-3-3 tested. The authors could also co-immunoprecipitate spastin-14-3-3 complexes from spinal cord samples and from primary neuronal cultures.
-By protein truncation experiments they found that the Microtubule Binding Domain of spastin contained the binding capability to 14-3-3. This domain contained a putative phosphorylation site, and substitutions that cannot be phosphorylated cannot bind to 14-3-3.
-Overexpression of GFP-spastin shows a turn-over of about 12 hours when protein synthesis is inhibited by cycloheximide. When 14-3-3 is co-overexpressed, GFP-spastin does not show a decrease by 12 hours. When S233A is expressed, a turn-over of 9 hours is observed, suggesting that phosphorylation increases the stability of the protein. In support of that notion, the phospho-mimetic S233D makes it more stable, lasting as much as the over-expression of 14-3-3.
-By combining FCA with Spastazoline, authors claim that FCA increased regeneration is due to increased spastin activity in various models of neurite outgrowth and regeneration in cell culture and in vivo, the authors show impressive results on the positive effect of FCA in regeneration, and that this is abolished when spastin is inhibited.
Major weaknesses
- The present manuscript suggests that 14-3-3 and spastin work in the same pathway to promote regeneration. Although the manuscript contains valuable evidence in support for a role of 14-3-3 and spasting in regeneration, the conclusive evidence is difficult to generate, and is missing in the present manuscript. For example, there are simpler explanations for the combined effect of FC-A and spastazoline. The FC-A mechanism of action can be very broad, since it will increase the binding of all 14-3-3 proteins with presumably all their substrates, hence the pathways affected can rise to the hundreds. The fact that spastazoline abolishes FC-A effect, may not be because of their direct interaction, but because spastin is a necessary component of the execution of the regeneration machinery further downstream, in line with the fact that spastazoline alone prevented outgrowth and regeneration, and in agreement with previous work showing that normal spastin activity is necessary for regeneration.
With this in mind, I consider the title and most major conclusions of the manuscript related to these two proteins acting together for the observed effects are overstated.
Response: We appreciate and acknowledge the reviewers' considerations. Our results demonstrated that the spastin inhibitor, spastazolin, almost completely inhibited axon regeneration and the spinal cord injury repair process. This, in turn, leads to the disappearance of any promoting effect on spinal cord injury repair when spastin function is compromised. While we have provided evidence that the expression levels of spastin are moderately increased at the injury site in mice after treatment with FC-A following spinal cord injury, the conclusion that FC-A promotes spinal cord injury repair through the direct interaction between 14-3-3 and spastin still lacks direct evidence. Therefore, we have made appropriate modifications to the manuscript's title and main conclusions.
- Authors show that S233D increases MT severing activity, and explain that it is related to increased binding to 14-3-3. An alternative explanation is that phosphorylation at S233 by itself could increase MT severing activity. The authors could test if purified spastin S233D alone could have more potent enzymatic activity.
Response: We appreciate the considerations of the reviewer. We believe that supplementing in vitro experiments to assess whether S233D affects spastin's microtubule severing function can more intuitively demonstrate whether phosphorylation of spastin at S233 affects its microtubule severing function; however, spastin forms hexamers through its AAA domain to exert ATPase activity and cut microtubules. Current research has reported that mutation sites leading to changes in microtubule severing function are mainly located within spastin's AAA domain (affecting spastin's ATPase activity, amino acids 342-599), such as E356A, G370R, N386K, K388R, E442Q, K427R and R562Q. Furthermore, studies have shown that mutating 11 phosphorylation sites in spastin's MIT and MTBD regions to alanine does not affect spastin's microtubule severing function, including human S268 (Rat Ser233) (Phosphorylation mutation impairs the promoting effect of spastin on neurite outgrowth without affecting its microtubule severing ability. Eur J Histochem. doi: 10.4081/ejh.2023.3594). Additionally, we also provided supplementary experiments in cell lines which showed that both spastin S233A and S233D could effectively sever microtubules (Fig.S2).
- The interpretation of the authors cannot explain how Spastin can engage in MT severing while bound to 14-3-3 using its Microtubule Binding Domain.
Response: We appreciate the considerations of the expert reviewer. The IP experiments with truncated fragments suggest that the binding region of 14-3-3 with spastin is located within the region (215-336 amino acids) in spastin. Furthermore, experiments involving site-directed mutagenesis confirm that the actual binding site of 14-3-3 with spastin is the S233 site, rather than its MTBD region (270-328). Therefore, we have made corrections in the manuscript. We also indicate that 14-3-3 enhances spastin's protein levels by binding to the S233 site, which may be due to 14-3-3 masking the ubiquitination sites near spastin S233 (K206 or K254). Our further experiments also demonstrate that 14-3-3 inhibits the ubiquitination degradation pathway of phosphorylated spastin.
- Also, the term "microtubule dynamics", which is present in the title and in other major conclusions, is overstated. Although authors show, in cell lines, changes in microtubule content, it is far from evidence for changes in "MT dynamics" in the settings of interest (i.e. injured axons).
Response: We appreciate and acknowledge the rigorous feedback. While our manuscript demonstrated the regulatory role of 14-3-3 and spastin in microtubule dynamics in cell lines, we lack direct evidence of these changes in microtubule dynamics within injured axons. Therefore, we have made appropriate modifications to the title, main conclusions, and related statements in our manuscript.
- In the same lines, the manuscript lacks evidence for the changes of MT content and/dynamics as a function of the proposed 14-3-3 - Spastin pathway.
Response: We appreciate and concur with the opinions of the expert reviewer. The observed changes in microtubule dynamics in spinal cord injury were related to the overall alterations in microtubule dynamics within the spinal cord injury site. We still lack direct evidence that 14-3-3, in conjunction with spastin, alters the microtubule dynamics within axons during the process of regeneration. Therefore, we have made modifications to the manuscript.
Reviewer #2 (Public Review):
Summary:
The idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. In this manuscript Liu et al. explore a 14-3-3-Spastin complex and its role in axon regeneration.
Strengths:
Some of the effects of FC-A on locomotor recovery after spinal cord contusion look interesting
Weaknesses:
The manuscript falls short of establishing that a 14-3-3-Spastin complex is important for any FC-A-dependent effects and there are several issues with data quality that make it difficult to interpret the results. Importantly, the effects of the spastin inhibitor has a major impact on neurite outgrowth suggesting that cells simply cannot grow in the presence of the inhibitor and raising serious questions about any selectivity for FC-A - dependent growth. Aspects of the histology following spinal cord injury were not convincing.
Response: We appreciate the rigorous review by the expert reviewers. In response to the feedback from reviewer 1, we lack direct evidence to demonstrate that the reparative effect of FC-A on spinal cord injury is mediated by the combined action of 14-3-3 and spastin. We have accordingly made the necessary changes to our manuscript. Additionally, due to upload limitations, the resolution of our tissue slices related to spinal cord injury in the manuscript is relatively low. To address this, we have supplemented relevant images which was enlarged in the supplementary materials (Fig. S7-9), Also, the original confocal files and images were uploaded.
Furthermore, our manuscript does not suggest that the reparative effect of FC-A in spinal cord injury selectively impacts the interaction between 14-3-3 and spastin. Therefore, we have modified our claims (title and conclusions) to ensure a more precise statement. Despite the fact that our axonal markers do not fully align, our evidence still strongly supports the role of FC-A in promoting nerve regeneration after spinal cord injury. Additionally, we will further optimize our immunohistochemistry methods.
Reviewer #3 (Public Review):
Summary:
The current manuscript shows that 14-3-3 are binding partners of spastin, preventing its degradation. It is additionally shown, using complementary methods, that both 14-3-3 and spastin are necessary for axon regeneration in vitro and in vivo. While interesting in vitro and vivo data is provided, some of the claims of the authors are not convincingly supported.
Major strengths:
Very interesting effect of FC-A in functional recovery after spinal cord injury.
Major Weaknesses:
Some of the in vitro data, including colocalizations, and analysis of microtubule severing fall short to support the claims of the authors.
The in vivo selectivity of FC-A towards spastin is not adequately supported by the data presented. There are aspects of the spinal cord injury site histology that are unclear.
Response: Reviewer 3's comments align with those of Reviewers 1 and 2.
Reviewer #1 (Recommendations For The Authors):
-The new blots presented in Fig. 3N lacks corresponding labels as for antibodies used for IP and IB and molecular weight markers.
Response: We appreciate the reviewer's feedback. We have made the corresponding modifications in the figure.
Reviewer #2 (Recommendations For The Authors):
The authors have addressed many of the specific concerns shared with the authors in the first round of review but several issues remain with the manuscript.
- Fig. 1D - the interpretation that spastin co-localizes with 14-3-3 proteins in hippocampal neurons is still tenuous since 14-3-3 uniformly labels the cell.
Response: We appreciate the reviewer's consideration. Upon re-examining the source files, we found that the predominant reason for 14-3-3 showing a ubiquitous cellular distribution was excessive brightness and insufficient contrast. After appropriate adjustments, we discovered that 14-3-3 exhibits characteristic distribution in axons, including aggregation at growth cone and specific locations in the axon shaft. We have made the relevant changes in the revised version.
- Line 336. The meaning of the following statement is unclear "To further identify which isoform of 14-3-3 interacts with spastin, we generated six 14-3-3 isoforms in rats (β、γ、ε、ζ、η、θ ), then purified GST fusion 14-3-3 proteins (Figure 1G).
Response: Sorry for any confusing statement. We obtained gene fragments of six 14-3-3 isoforms from rat brain cDNA and inserted these fragments into the pEGX-5X-3 vector. Subsequently, GST 14-3-3 fusion proteins were expressed and purified in vitro. We have made the corresponding revisions in the revised version.
- Line 341. The authors still fall short of showing that spastin and 14-3-3 interact directly thus it may be more accurate to say that they form a complex.
Response: Thank you for the reviewer's advice. We have made the corresponding corrections in the manuscript.
- Line 388. Please clarify 2th and the meaning of "moderately" - "S233D) was moderately expressed in primary hippocampal neurons at 2th DIV." While it is specified that the transfection dosage and duration were meticulously controlled - it is unclear what the criteria was for establishing the appropriate moderate dosage.
Response: Sorry about the mistake, it should be "2nd" instead of "2th". In order to establish a model for overexpressing spastin to promote neuronal neurite growth, we transfected 0.2 µg of plasmid into 1 well (1×104 cells/cm2, 24-well plate), with a transfection duration controlled at 24 hours.
- Line 395 - It is unclear if S233D is toxic as there seem to be no measurements of cell survival.
Response: We have supplemented relevant experiments (See comment 6) based on comment 6 and found that Spastin S233D can promote neuronal neurite growth. The corresponding descriptions have been revised.
- The pro-growth effects of S233A still does not seem to fit the narrative and the results would have been more convincing if dosage was better controlled to establish any differences between WT and S233A Spastin.
Response: We appreciate the constructive comments from the reviewer. In order to better illustrate the role of spastin S233 in neuronal growth, we have made appropriate adjustments to our experimental conditions based on previous experiments. Cells were transfected with plasmids expressing non-fused GFP and spastin and the relevant S233 mutants at a transfection dose of 0.2 µg into 1 well (1×104 cells/cm2, 24-well plate), duration was controlled at 12 hours. Due to the low expression state of the overexpressed protein, GFP (ab290 antibody for IF) was then stained to trace neuronal morphology. The experimental results demonstrate that spastin promotes neuronal neurite growth, and the dephosphorylation mutant of spastin (spastin S233A) significantly attenuates its neurite-promoting effect compared to wild-type spastin. Conversely, the phosphorylation mutant spastin S233D further enhances the promotion of neuronal neurite growth. We have also made corrections to the relevant statements in the manuscript.
- The reason for examining protection in response to glutamate is not well rationalized based on known spastin functions. The interpretation of this experiment is unclear with respect to effects on protection vs repair.
Response: Thank you for the reviewer's consideration. We suppose that spastin may be involved in both protective and repair processes. Existing studies suggest that spastin can control store-operated calcium entry (SOCE) by altering endoplasmic reticulum morphology (doi: 10.1093/brain/awac122, doi: 10.3389/fphys.2019.01544), which may indicate its role in regulating calcium overload. Additionally, due to the critical role of spastin in axon growth, it is also essential for neuronal repair after injury. Therefore, we have not strictly distinguished between these two concepts here.
- It is unclear if Spastazoline simply blocks any type of growth and it is thus difficult to conclude that FC-A functions through a 14-3-3-spastin effect based on the current data.
Response: We have re-evaluated and modified the title and main conclusions of the manuscript based on the reviewer's comments and the existing evidence, as responded to in reviewer 1's comments.
- The access of FC-A to the CNS with the current protocol has not been clearly established and the effects of FC_A on spastin expression seem to mirror the profile of the control condition.
Response: We agree with the reviewer's comments. The expression trend of spastin after FC-A treatment is consistent with that of the control group, with a slight increase in its expression level compared to the control group.
- The NF and 5-HT staining is not convincing labelling fibres.
Response: We appreciate the reviewer's comments. We believe that the reason for the incomplete axon staining is closely related to the thickness of the tissue sections. In our future research, we will further optimize our axon labeling methods.
Reviewer #3 (Recommendations For The Authors):
Figure 1D: Both spastin and 14-3-3 label the entire neuron which is rather unusual. Conditions of immunfluorescence should be improved. As it is, this image should not be used to claim colocalization.
Response: We appreciate the reviewer's consideration. In response to comment 1 from the expert reviewer 2, we have re-examined the source files and identified that the primary reason for the overall cell-wide distribution of 14-3-3 and spastin is due to excessive brightness and a lack of sufficient contrast. After making appropriate adjustments, we found that 14-3-3 and spastin exhibit characteristic localization within the axon (concentrated in a particular region of the axon shaft and the growth cone). We have made corresponding revisions in the revised version of the manuscript.
Figure S2: The experimental setup and data provided is not adequate to infer microtubule severing.
Response: We appreciate the reviewer's guidance. We have improved the relevant experiments and used a 100X objective lens to observe the microtubule structures more clearly.
Figure 2 I-K: The functional effect of spastin S233A and S233D on neurite outgrowth does not correlate with a function of 14-3-3 and thus does not support the central hypothesis of the manuscript. Minor: The images selected as representative show differences in neurite length and branching that are not portrayed in the graphs.
Response: Thank you for the reviewer’s comment. Similar to the response to the reviewer 2's comment 6, in order to better illustrate the role of spastin S233 in neurite outgrowth, we made corresponding adjustments to our experimental conditions. Cells were transfected with plasmids expressing non-fused GFP and spastin and the relevant S233 mutants at a transfection dose of 0.2 µg into 1 well (1×104 cells/cm2, 24-well plate), duration was controlled at 12 hours. Due to the low expression state of the overexpressed protein, GFP (ab290 antibody for IF) was then stained to trace neuronal morphology. The experimental results demonstrate that spastin promotes neuronal neurite growth, and the dephosphorylation mutant of spastin (spastin S233A) significantly attenuates its neurite-promoting effect compared to wild-type spastin. Conversely, the phosphorylation mutant spastin S233D further enhances the promotion of neuronal neurite growth. We have also made corrections to the relevant statements in the manuscript.
Figure 5 J and L: The quality, resolution and size of the images is insufficient to support the claims of the authors. As it is, one cannot interpret the data. It is very hard to envisage, even considering the explanation provided by the authors, that spinal cords where spastazoline was used correspond to contusion as a complete discontinuity between the rostral and caudal spinal cord tissue is present.
Response: Due to limitations in file uploads, we encountered issues with the resolution of the tissue slices related to spinal cord injury. To address this, we have adjusted the size and resolution of the corresponding images in the supplementary materials (Fig.S7-S9 ) and included the original confocal files and images.
Additionally, it's important to note that the tissue slices we presented do not represent all layers of the spinal cord, and not all layers exhibit discontinuity. Our slices are taken longitudinally at the dorsal site of the lesion area. The dorsal slices represent areas closer to the injury site, while deeper slices correspond to areas distant from the injury site. Therefore, we selected areas closer to the injury site to reflect the repair process following injury.
Figure 7B: Similar comment to spianl cord images provided in Figure 5. NF and MBP are not supposed to colocalize as they label different cell types...
Response: We appreciate the comments from the expert reviewer, and we agree with their suggestions. We will further optimize our axon labeling methods. The excessive brightness and lack of contrast primarily led to the non-specific labeling of other cell types with the MBP antibody. In fact, our primary goal was to highlight the injured areas by enhancing the fluorescence intensity of the images, which inadvertently resulted in neglecting the exclusion of non-specific staining. Therefore, we have made appropriate adjustments to the images to better visualize the distribution of myelin sheaths.
-

eLife assessment
The finding that Fusicoccin (FC-A) promotes locomotor recovery after spinal cord injury is supported by solid data, and the idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is valuable. The evidence showing that 14-3-3 and spastin interact and that 14-3-3 enhances spastin function and stability in cells is also solid.
-

Joint Public Review:
The present work establishes 14-3-3 proteins as binding partners of spastin and suggests that this binding is positively regulated by phosphorylation of spastin. The authors show evidence that 14-3-3 - spastin binding prevents spastin ubiquitination and final proteasomal degradation. By using drugs and peptides that separately inhibit 14-3-3 binding or spastin activity, they show that both proteins are necessary for axon regeneration in cell culture and in vivo models in rats.
Major strengths
-The data establishing 14-3-3 and spastin as binding partners is convincing, as is its regulation by phosphorylation and its impact on protein levels related to the activity of the ubiquitin-proteasome system.-The effects of FC-A on locomotor recovery after spinal cord contusion is very interesting.
Major weaknesses
-Given …Joint Public Review:
The present work establishes 14-3-3 proteins as binding partners of spastin and suggests that this binding is positively regulated by phosphorylation of spastin. The authors show evidence that 14-3-3 - spastin binding prevents spastin ubiquitination and final proteasomal degradation. By using drugs and peptides that separately inhibit 14-3-3 binding or spastin activity, they show that both proteins are necessary for axon regeneration in cell culture and in vivo models in rats.
Major strengths
-The data establishing 14-3-3 and spastin as binding partners is convincing, as is its regulation by phosphorylation and its impact on protein levels related to the activity of the ubiquitin-proteasome system.-The effects of FC-A on locomotor recovery after spinal cord contusion is very interesting.
Major weaknesses
-Given that spastazoline has a major impact on neurite outgrowth suggests that cells simply cannot grow in the presence of the inhibitor and raises serious questions about any selectivity for the concomitant effect FC-A - dependent growth.-The histological data and analyses following spinal cord injury are not convincing. For example, the colabeling of NF and 5-HT is not convincingly labeling fibers. Also, the quality, resolution and size of the images is insufficient to support the quantitative data and it is hence difficult to interpret the data. Reviewers recognize that during the review process, efforts were made to improve the quality of the images.
-Reviewers also observed that the data to infer Spastin actions on Microtubules across different experimental models is weak and that claims about "MT severing" and "microtubules dynamics" were wrongly used given the provided evidence.
-The manuscript lacks direct evidence that a 14-3-3 and spastin function as a complex in the same pathway to promote regeneration. It is recognized, however, that the authors had made changes in the manuscript title and claims not to imply that the current evidence is sufficient in that matter.
-

-

Author Response
The following is the authors’ response to the original reviews.
Reviewer #1 (Public Review):
The present work establishes 14-3-3 proteins as binding partners of spastin and suggests that this binding is positively regulated by phosphorylation of spastin. The authors show evidence that 14-3-3 >- spastin binding prevents spastin ubiquitination and final proteasomal degradation, thus increasing the availability of spastin. The authors measured microtubule severing activity in cell lines and axon regeneration and outgrowth as a prompt to spastin activity. By using drugs and peptides that separately inhibit 14-3-3 binding or spastin activity, they show that both proteins are necessary for axon regeneration in cell culture and in vivo models in rats.
The following is an account of the major strengths and weaknesses of the …
Author Response
The following is the authors’ response to the original reviews.
Reviewer #1 (Public Review):
The present work establishes 14-3-3 proteins as binding partners of spastin and suggests that this binding is positively regulated by phosphorylation of spastin. The authors show evidence that 14-3-3 >- spastin binding prevents spastin ubiquitination and final proteasomal degradation, thus increasing the availability of spastin. The authors measured microtubule severing activity in cell lines and axon regeneration and outgrowth as a prompt to spastin activity. By using drugs and peptides that separately inhibit 14-3-3 binding or spastin activity, they show that both proteins are necessary for axon regeneration in cell culture and in vivo models in rats.
The following is an account of the major strengths and weaknesses of the methods and results.
Major strengths
-The authors performed pulldown assays on spinal cord lysates using GST-spastin, then analyzed pulldowns via mass spectrometry and found 3 peptides common to various forms of 14-3-3 proteins. In co-expression experiments in cell lines, recombinant spastin co-precipitated with all 6 forms of 14-3-3 tested.
-By protein truncation experiments they found that the Microtubule Binding Domain of spastin contained the binding capability to 14-3-3. This domain contained a putative phosphorylation site, and substitutions that cannot be phosphorylated cannot bind to spastin.
-spastin overexpression increased neurite growth and branching, and so did the phospho null spastin. On the other hand, the phospho mimetic prevents all kinds of neurite development.
-Overexpression of GFP-spastin shows a turn-over of about 12 hours when protein synthesis is inhibited by cycloheximide. When 14-3-3 is co-overexpressed, GFP-spastin does not show a decrease by 12 hours. When S233A is expressed, a turn-over of 9 hours is observed, indicating that the ability to be phosphorylated increases the stability of the protein.
-In support of that notion, the phospho-mimetic S233D makes it more stable, lasting as much as the over-expression of 14-3-3.
-Authors show that spastin can be ubiquitinated, and that in the presence of ubiquitin, spastin-MT severing activity is inhibited.
-By combining FCA with Spastazoline, the authors claim that FCA increased regeneration is due to increased spastin Activity in various models of neurite outgrowth and regeneration in cell culture and in vivo, the authors show impressive results on the positive effect of FCA in regeneration, and that this is abolished when spastin is inhibited.
Major weaknesses
-However convincing the pull-downs of the expressed proteins, the evidence would be stronger if a co-immunoprecipitation of the endogenous proteins were included.
We thank the reviewer for their succinct summary of the main results and strengths of our study. We acknowledge the reviewers' valuable suggestions and agree that performing endogenous co-immunoprecipitation (co-IP) experiments in neurons is crucial for supporting our conclusions. To address this question, cortical neurons were cultured in vitro for endogenous IP experiment. The cortical neurons were cultured using a neurobasal medium supplemented with 2% B27, and using cytarabine to inhibit the proliferation of glial cells. The proteins were then extracted and subjected to the immunoprecipitation experiments using antibodies against spastin. The results, as shown in Fig.1C in the revised manuscript, clearly demonstrate that 14-3-3 protein indeed interacts with spastin within neurons.
-To better establish the impact of spastin phosphorylation in the interaction, there is no indication that the phosphomimetic (S233D) can better bind spastin, and this result is contradicting to the conclusion of the authors that spastin-14-3-3 interaction is necessary for (or increases) spastin function.
Thank you for your valuable and constructive comments. We agree with your consideration. To reinforce the importance of phosphorylated spastin in this binding model, we conducted additional experiments by transfecting S233D into 293T cells and performed immunoprecipitation experiments (Fig.2H). The results clearly demonstrate that spastin (S233D) exhibits enhanced binding to spastin, indicating that phosphorylation at the S233 site is critical for this interaction. Additionally, we observed that spastin (S233D) maintains its binding to 14-3-3 even in the presence of staurosporine. This data further supports and strengthens our conclusions.
-To fully support the authors' suggestion that 14-3-3 and spastin work in the same pathway to promote regeneration, I believe that some key observations are missing.
1-There is no evidence showing that 14-3-3 overexpression increases the total levels of spastin, not only its turnover.
Thank you for your consideration and valuable input. We have previously demonstrated that overexpression of 14-3-3 leads to an increase in the protein levels of spastin in the absence of CHX (Fig.3E&F). Furthermore, we also observed an upregulated protein levels of spastin S233D compared to the wild-type (Fig.3G). We have now included these results in the revised manuscript.
2- There is no indication that increasing the ubiquitination of spastin decreases its levels. To suggest that proteasomal activity is affecting the levels of a protein, one would expect that proteasomal inhibition (with bortezomib or epoxomycin), would increase its levels.
Thanks for your concern. We believe that this evidence is critical. Indeed, another study by our team is working to elucidate the ubiquitination degradation pathway of spastin. In addition, a previous study has shown that phosphorylation of the S233 site of spastin can affect its protein stability (Spastin recovery in hereditary spastic paraplegia by preventing neddylation-dependent degradation, doi:10.26508/lsa.202000799.). To better support our conclusions, we have supplemented the results in Fig.3L&M. The results showed that the proteasome inhibitor MG132 could significantly increase the protein level of spastin, whereas CHX could significantly decrease the protein level of spastin, and the degradation of spastin is significantly hindered in the presence of both CHX and MG132. This experiment also further showed that ubiquitination of spastin reduced its protein level.
3- Authors show that S233D increases MT severing activity, and explain that it is related to increased binding to 14-3-3. An alternative explanation is that phosphorylation at S233 by itself could increase MT severing activity. The authors could test if purified spastin S233D alone could have more potent enzymatic activity.)
We appreciate the reviewer’s consideration. After investigating the interaction between 14-3-3 and spastin, we first aimed to determine whether the S233 phosphorylation mutation of spastin influenced its microtubule-severing activity. We found that overexpression of both S233A and S233D mutants resulted in significant microtubule severing (as indicated by a significant decrease in microtubule fluorescence intensity) (Fig.S2). Furthermore, it is noteworthy that S233 is located outside the microtubule-binding domain (MTBD, 270-328 amino acids) and the AAA region (microtubule-severing region, 342-599 amino acids) of spastin. Based on our initial observations, we believe that the phosphorylation of the S233 residue in spastin does not impact its microtubule-severing function. Additionally, under the same experimental conditions, we observed that the green fluorescence intensity of GFP-spastin S233D was significantly higher than that of GFP-spastin S233A. Based on these phenomena, we speculated that phosphorylation of the S233 residue of spastin might affect its protein stability, leading us to conduct further experiments. Furthermore, we fully acknowledge the reviewer's concern; however, due to technical limitations, we were unable to perform an in vitro assay to test the microtubule-severing activity of spastin. We have provided an explanation for this consideration in the revised version.
-Finally, I consider that there are simpler explanations for the combined effect of FC-A and spastazoline. FC-A mechanism of action can be very broad, since it will increase the binding of all 14-3-3 proteins with presumably all their substrates, hence the pathways affected can rise to the hundreds. The fact that spastazoline abolishes FC-A effect, may not be because of their direct interaction, but because spastin is a necessary component of the execution of the regeneration machinery further downstream, in line with the fact that spastizoline alone prevented outgrowth and regeneration, and in agreement with previous work showing that normal spastin activity is necessary for regeneration.
We appreciate the considerations raised by the reviewer. It is evident that spastin is not the exclusive substrate protein for 14-3-3, and it is challenging to demonstrate that 14-3-3 promotes nerve regeneration and recovery of spinal cord injury directly through spastin in vivo. However, we have identified the importance of 14-3-3 and spastin in the process of nerve regeneration. Importantly, we have conducted supplementary experiments to support the stabalization of spastin by FC-A treatment within neurons (Fig.4M), as well as the repair process of spinal cord injury in vivo (Fig.5D). The results showed that FC-A treatment in cortical neurons could enhance the stability of spastin protein levels, and we also demonstrated a consistent trend of upregulated protein levels of spastin and 14-3-3 following spinal cord injury. Moreover, the protein levels were significantly elevated in the the FC-A group of mice. These results also support that 14-3-3 enhances spastin protein stability to promote spinal cord injury repair. The manuscript was revised accordingly.
Reviewer #2 (Public Review):
Summary:
The idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. In this manuscript, Liu et al. explore a 14-3-3-spastin complex and its role in axon regeneration.
Strengths:
Some of the effects of FC-A on locomotor recovery after spinal cord contusion look interesting.
Weaknesses:
The manuscript falls short of establishing that a 14-3-3-spastin complex is important for any FC-A-dependent effects and there are several issues with data quality that make it difficult to interpret the results. Importantly, the effects of the spastin inhibitor have a major impact on neurite outgrowth suggesting that cells simply cannot grow in the presence of the inhibitor and raising serious questions about any selectivity for FC-A - dependent growth. Aspects of the histology following spinal cord injury were not convincing.
We sincerely appreciate the reviewer for evaluating our manuscript. Given the multitude of substrates that interact with 14-3-3, and considering spastin's indispensable role in neuroregeneration, it is indeed challenging to experimentally establish that FC-A's neuroregenerative effect is directly mediated through spastin in vivo. Therefore, we have provided additional crucial evidence regarding the changes in spastin protein levels following spinal cord injury, as well as the application of FC-A after spinal cord injury. Furthermore, we have made relevant adjustments to the uploaded images to enhance the resolution of the presented figures, as detailed in the subsequent response.
Reviewer #3 (Public Review):
Summary: The current manuscript c laims that 14-3-3 interacts with spastin and that the 14-3-3/spastin interaction is important to regulate axon regeneration after spinal cord injury.
Strengths:
In its present form, this reviewer identified no clear strengths for this manuscript.
Weaknesses:
In general, most of the figures lack sufficient quality to allow analyses and support the author's claims (detailed below). The legends also fail to provide enough information on the figures which makes it hard to interpret some of them. Most of the quantifications were done based on pseudo-replication. The number of independent experiments (that should be defined as n) is not shown. The overall quality of the written text is also low and typos are too many to list. The original nature of the spinal cord injury-related experiments is unclear as the role of 14-3-3 (and spastin) in axon regeneration has been extensively explored in the past.
We sincerely appreciate the careful consideration and rigorous evaluation provided by the reviewer. In the revised version, we have made effort to present high-resolution figures and provide more detailed figure legends. Furthermore, we have made relevant adjustments to the statistical methods in accordance with the reviewer's suggestions. The manuscript has also undergone a thorough review and correction process to eliminate any writing-related errors. Please refer to the following response.
To the best of our knowledge, there has been no clear reports on the efficacy of 14-3-3 in the repair of spinal cord injury. Kaplan A et al. (doi: 10.1016/j.neuron.2017.02.018) reported a reduction in die-back of the corticospinal tract following spinal cord injury using FC-A as a filler in situ in the lesion site. However, the specific effects of FC-A on spinal cord injury, such as motor function and neural reactivity, as well as the expression characteristic of 14-3-3 after spinal cord injury, have not been extensively elucidated. Additionally, prior research on spastin's role in axon regeneration primarily focused on the effects in Drosophila, and its regenerative effects in the central nervous system of adult mammals after injury have not been reported. Therefore, our study provides crucial insights into the importance of 14-3-3 and spastin in the process of spinal cord injury repair in mammals.
Reviewer #1 (Recommendations For The Authors):
There are many spelling and grammar errors, please revise. Examples:
-approach revealed14-3-3
-We have detected different many 14-3-3 peptides
-Line 1057 (D) 14-3-3 agnoist FC-A
-There is a discrepancy between panel names and figure legend in Figure 4.
-There is another discrepancy between the color coding of treatments in Figure 7. All panels show "injury" in red and FC-A in orange, but in panel E, these are swapped. This is confusing to readers.
Thank you for the thorough and rigorous review. We have re-colored the relevant chart. The manuscript has also undergone a thorough review to eliminate any writing-related errors.
Most images from confocal microscopy are blurred or low resolution. They should be sharper for the type of microscopy used.
We have adjusted and re-uploaded the images with higher resolution. Additionally, we have enlarged the relevant images.
The list of all peptides retrieved in the Mass-Spec analyses of the GST-spastin pulldown must be publicly available, according to eLife rules.
Thank you for your suggestion. We have now uploaded the mass spectrometry data.
To determine where the 14-3-3/spastin protein142 complex functions in neurons, we double stained hippocampal neurons with spastin143 and 14-3-3 antibody, and found that 14-3-3 was colocalized with spastin in the entire144 cell compartment (Figure 1C).
Colocalization by confocal fluorescence microscopy is not evidence for protein complexes.
While co-localization experiments may not directly demonstrate protein-protein interactions, they can still provide valuable insights into the cellular localization of the proteins and suggest potential interactions between them. Therefore, we adjusted the statement.
Fig1F- Co-immunoprecipitation assay results confirmed that all 14-3-3 isoforms could form direct complexes with spastin.
CoIP in cells overexpressing the proteins is not evidence that it is direct. That they can interact directly with each other can be extracted from the evidence in vitro with purified proteins.
We agree with this and we have changed our statement accordingly.
For a broad audience to have a better understanding, the authors have to explain their a.a. subtitucions of Serine233, one being mimicking phosphorylation (S233D) and the other rendering the protein not being able to be phosphorylated in that position (S233A).
We appreciate the suggestion. We have provided a more detailed explanation in revised manuscript.
The panel of neuronas in Fig2G is mislabeled, because it is twice spastin S233A, instead of S233D.
We apologize for this mistake and we have corrected it in the panel.
FCA may increase the interaction of 14-3-3 with any of its substrates, including spastin. One would appreciate evidence that FCA increases the MT-severing activity of spastin, as assumed by authors
We appreciate the reviewer’s suggestion. In this study, we overexpressed spastin to investigate its microtubule severing activity. It is important to note that overexpressing spastin significantly exceeds the normal physiological concentration of the protein. Using excessive amounts of FC-A to enhance the interaction between 14-3-3 and spastin in cells can lead to cell toxicity. Therefore, we chose to overexpress 14-3-3 instead of employing excessive FC-A.
In Fig2F, the interaction of 14-3-3 with Spas-S233D would have been very informative.
Thank you for the constructive suggestions from the reviewer. We have supplemented the corresponding co-immunoprecipitation experiments (Fig.).
The functional effect of S233A and S233D does not correlate with a function of 14-3-3 in neurite outgrowth. This is because S233A does not interact with 14-3-3, however, it is as good as WT spastin... meaning that binding of 14-3-3 with spastin is not necessary...
We appreciate the reviewer's consideration. The observed phenomenon of spastin WT and S233A promoting axon growth do not align with the physiological state within neurons. This may mask the true effects of S233A or S233D on neuronal axon growth. It is documented that the proper dosage of spastin is essential for neuronal growth and regeneration, as excessive or insufficient amounts can hinder axon growth. Excessive spastin levels can disrupt the overall cellular MTs. Therefore, spastin were moderately expressed by adjusting the transfection dosage and duration. Nevertheless, we were unable to precisely control the expression levels of spastin for both WT and S233A, also resulting in an overexpression state compared to the physiological state. As a result, the crucial role of spastin S233 in neural growth under physiological conditions may be masked. We have addressed this issue in the revised version of our manuscript.
In panels 3C and D it is not clear if it does contain 14-3-3.... it seems it does not... but clarify.
We apologize for any confusion. Since there is endogenous 14-3-3 present in the cells, we utilized spastin S233A and S233D to mimic the binding pattern with 14-3-3 according to the established interaction model. This information has been clarified in the original manuscript.
Line 217 should indicate Figure 3, not Figure 5
We have made the corresponding corrections.
In F3G, it is intriguing that the input blot shows a decrease in Ubiquitin proteins when there is expression of flag ubiquitin...
We apologize for the error in our presentation. In the control group, we actually overexpressed Flag-ubiquitin and GFP instead of Flag and GFP-spastin. Additionally, to further elucidate the impact of different phosphorylation states on spastin ubiquitination and degradation, we have conducted additional ubiquitination experiments (Fig.3N), which are now included in the revised version of our manuscript.
S233 mutations seem to affect the effective turnover of spastin, but does not seem to change the levels of the spastin protein...hence, the conclusion that 14-3-3 protects from degradation is overstated.
We thank the reviewers for the careful review and we have revised the statement accordingly.
The mode of action of R18 FCA should be introduced earlier in the text.
Thank you for the reviewer's correction. We have provided a corresponding description of the effects of FC-A and R18 on the interaction between 14-3-3 and spastin in the ubiquitination experiments section of the manuscript.
Line 296 reads: Our results revealed that levels of 14-3-3 protein remained high even at 30 DPI, indicating that 14-3-3 plays an important role in the recovery of spinal cord injury.
This is overstated since it can well be that an upregulated protein is inhibitory. We thank the reviewers for their consideration and we have made adjustments accordingly.
It is not clear if 14-3-3 prevents ubiquitination of spastin, then its levels should be higher... it is noteworthy that they did not measure its levels in nerve tissue after injury. For example, in experiments shown in Figure 5A, it would have been very useful the observation of the levels of spastin.
We appreciate the reviewer's consideration. We have now included the assessment of spastin protein levels following spinal cord injury. Additionally, we have collected the injured spinal cord lysates in mice treated with FC-A for western blot analysis. The results revealed that the expression trend of 14-3-3 protein is largely consistent with spastin after spinal cord injury. Furthermore, the treatment with FC-A was found to enhance the expression of spastin after spinal cord injury (Fig. 5C&D)."
Panel 5G reads "nerve regeneration across the lesion site", but it actually measured NF levels, according to the legend.
Thanks to the reviewers for the critical review. We have revised the chart accordingly.
361 "BMS" should be explained in the results section for a better understanding of the results by non-experts.
Thank you to the reviewers for their suggestions. We have explained this in the results section accordingly.
Reviewer #2 (Recommendations For The Authors):
- The results of the mass spec and co-IP in Figure 1 are unclear.
a) Are all of the peptides in Fig. 1A from 14-3-3 and were there only 3 14-3-3 peptides that were identified?
The mass spectrum results did identify only three 14-3-3 peptides, and these three peptides were highly conserved across all isoforms.
b) The blot in panel B needs to show the input band for spastin and 14-3-3 from the same gel and not spliced so that the level of enrichment can be evaluated in the co-IP.
Thanks to the reviewer's comments, we have presented the whole gel (Fig.1B)
c) Further, does an IP for 14-3-3 co-precipitate spastin?
Thank you for your concern. We appreciate your feedback. Our 14-3-3 antibody is capable of Western blot experiments and recognizes all subtypes (Pan 14-3-3, Cell Signaling Technology, Cat #8312). Unfortunately, it is not suitable for immunoprecipitation (IP) experiments. Therefore, we have employed additional approaches, namely immunoprecipitation and pull-down assays, to further investigate the interaction between 14-3-3 and spastin.
- It is difficult to say anything about 14-3-3 - spastin co-localization in hippocampal neurons (1c) since 14-3-3 labels the entire hippocampal neuron so any protein will co-localize.
We appreciate the comments. The co-localization experiments have provided evidence of the relative expression of both 14-3-3 and spastin in neurons, suggesting their potential interaction within neuronal cells. We have made the necessary revisions to accurately describe the results of the co-localization experiments in the manuscript.
To further investigate the interaction between 14-3-3 and spastin within neurons, we have conducted additional co-immunoprecipitation (Co-IP) experiments using cortical neuron lysates (Fig.1C).
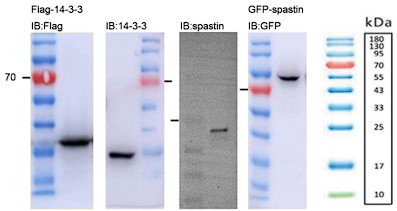
- The molecular weight of 14-3-3 is 25-28 kDa but the band in panel 1B and in subsequent figures it is below 15 kDa. Fig. 1F - the spastin band also seems to be low compared to predicted molecular weight and other W. Blot reports in the literature so some indication of how the antibody was validated would be important.
Apologies for the mistakes. We have carefully re-evaluated the western blot images (See Author response image 1). We have confirmed that the molecular weight of the 14-3-3 protein is approximately 33 kDa. In the case of spastin, its molecular weight is around 55-70 kDa. Additionally, the GFP-spastin fusion protein has an estimated molecular weight of approximately 90 kDa. We have conducted a thorough verification and made appropriate adjustments to the molecular weight labels in all western blot images.
Author response image 1.
- Fig 1G is a co-immunoprecipitation and it is not clear what the authors mean by "direct complexes" as claimed in line 150 of the results since this does not show direct binding between 14-3-3 and spastin. None of the assays in Fig. 1 assess "direct" binding between the two proteins and the authors should be clear in their interpretation.
We agree with the reviewer's comments and have removed the word "direct" from the text.
- Fig. 1D - there is no validation that staurosporine (protein kinase inhibitor, not protein kinase as per typo in Line 167) affects the phosphorylation levels of spastin.
Thank you for your valuable comments. In our group, we have conducted another study that has confirmed the involvement of CAMKII in mediating spastin phosphorylation. Furthermore, we have found that the addition of staurosporine significantly reduces the phosphorylation levels of spastin (unpublished results). In response to the reviewer's comment, we are pleased to provide western blot experiments demonstrating the effect of staurosporine on reducing spastin phosphorylation. The phosphorylation levels of spastin were assessed using a Pan Phospho antibody (Fig.2D).
- Fig. 2F - it would be important to test if spastin S233D interacts more robustly with 14-3-3 and if this is insensitive to staurosporine.
Thank you for your comments. The suggestion provided by the reviewer is highly significant for supporting our conclusion that "phosphorylation of spastin is a prerequisite for its interaction with 14-3-3." Therefore, we have conducted additional immunoprecipitation experiments to further supplement our findings (Fig.2H). The experimental results demonstrate that the binding affinity between spastin S233D and 14-3-3 is stronger compared to spastin WT.
- Line 179 "Next, we transfected Ser233 mutation of spastin (spastin S233A or spastin S233D) with flag tagged 14-3-3 and generated Pearson's correlation coefficients. Results revealed that spastin 181 S233D was markedly colocalized with 14-3-3, with minimal colocalization with spastin S233A (Figure 2A-B)." Assuming the authors are referring to supplemental Figure 2, the 14-3-3 covers the entire cell thus I think measures of co-localization are uninterpretable.
We agree with the reviewer's comment. We realize that 14-3-3θ exhibits a ubiquitous cellular distribution, which renders the measurement of its co-localization coefficients inconclusive. Therefore, we have decided to remove Supplementary Figure 2 from the manuscript.
- Line 189 "Consistent with earlier results, spastin promoted neurite outgrowth, as evidenced by both the length and total branches of neurite." - It is unclear what earlier results the authors are referring to. The authors should clarify how they determined the "moderate" expression level.
We thank the review’s suggestions. The "earlier results" mentioned here refers to previously published articles, we now have added relevant references. Existing literature indicates that an appropriate dosage of spastin is necessary for neuronal growth and regeneration. However, both excessive and insufficient amounts of spastin are detrimental to axonal growth. Excessive spastin disrupts the overall microtubule network within cells. We controlled plasmid transfection dosage and transfection durations to achieve moderate expression. We have provided an explanation of these details in the revised version.
- The effects of WT spastin and spastin S233A were similar in spite of the fact that S233A does not bind to 14-3-3, which is inconsistent with the author's model that spastin-14-3-3 binding promotes growth. Line 191 - the authors mention that spastin S233D was toxic but I do not see any cell death measurements. I assume the bottom right panel in Fig. 2G labelled as spastin S233A is mislabeled and should be S233D.
In response to comment 8, the transfection of both wild-type (WT) spastin and S233A mutant failed to precisely control the expression levels around the physiological concentration. Consequently, we observed an overexpression of spastin in both cases, which obscured the critical role of S233 phosphorylation in neurite outgrowth. We have addressed this issue in the revised version of the manuscript.
- Fig. 3. Does spastin(S233D) bind constitutively to 14-3-3? Why is spastin S233A not less stable than WT spastin based on the author's model?
We propose that 14-3-3 is more likely to interact with spastin S233D in a non-constitutive manner. The instability of the S233A protein is attributed to the disruption of its ubiquitination degradation process due to the absence of 14-3-3 binding.
- The ubiquitin blot in Fig. 3G is not convincing and not quantified.
We acknowledge the mislabeling in our figures. In the control group, Flag-Ubiquitin was also overexpressed, and we transfected GFP as a control instead of GFP-spastin. To further enhance the reliability, we conducted additional ubiquitination experiments (Fig.3N), which revealed a significant increase in spastin (S233A) ubiquitination levels compared to the WT group, consistent with previous research findings (Spastin recovery in hereditary spastic paraplegia by preventing neddylation-dependent degradation, doi:10.26508/lsa.202000799). Additionally, we observed that the addition of R18 could partially enhance spastin ubiquitination levels, as quantitatively illustrated in the figure (Fig.3O). This result further underscores the inhibitory role of 14-3-3 in the ubiquitination degradation pathway of spastin.
- I do not understand how the glutamate injury fits with the narrative (Fig. 4C).
Excessive glutamate exposure can induce severe intracellular oxidative stress reactions, leading to the disruption of physiological processes such as mitochondrial energy production. This, in turn, results in the swelling and lysis of neuronal processes, a phenomenon known as neuronal necrosis. During this state, neurite maintenance is obstructed, and neurites exhibit swelling and breakage (Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995 Oct;15(4):961-73). We have provided a more comprehensive explanation of this phenomenon in the revised version of our manuscript.
- Some commentary about the selectivity of spastazoline to inhibit spastin should be included - it would be helpful if the authors could explain that this is a spastin inhibitor in the manuscript. FC-A still seems to promote growth in the presence of spastazoline suggesting that the FC-A effects are not dependent on spastin (Fig. 4E). The statistical analysis section of the materials and methods indicates that multiple groups were analyzed by one-way ANOVA. This seems unusual since the controls for cellular transfection are different than for small molecules (FC-A) and for peptides such as R18. As such, there is no vehicle control for the FC-A condition and it is difficult to assess the FC-A vs Spastazoline vs FA-A + Spastoazoline. The authors should clarify (Fig. 4E-J)
Thank you for the reviewer’s suggestions. In the revised version, we have provided a more detailed explanation of the specific inhibition of spastin's severing function by spastazoline.
We observed that FC-A, in combination with spastazoline, still exhibited a certain degree of promotion in neurite growth compared to the injury group under the glutamate circumstances. Evidently, spastin is not the exclusive substrate for 14-3-3, and FC-A might delay cellular oxidative stress reactions by facilitating the interaction of 14-3-3 with other substrates, such as the FOXO transcription factors as mentioned in the introduction. Nevertheless, our results still demonstrate that the addition of spastazoline significantly diminishes the promoting effect of FC-A on neurite growth, indicating that FC-A affects neuronal growth by impacting spastin.
Furthermore, in the drug-treated groups, we overexpressed GFP to trace the morphology of neurons. Culture media were exchanged following transfection, and during media exchange, drugs were added. And an equivalent amount of DMSO or ethanol were added as controls to rule out the influence of solvents on neurons.
- There is a good possibility that spastin is required for all axon regeneration and that there is no selectivity for the FC-A pathway and this is a major issue with the interpretation of the manuscript (Fig 4K-L).
We acknowledge this point. Clearly, spastin is not the exclusive substrate for 14-3-3, and our experimental evidence does not establish that 14-3-3 solely promotes neuronal regeneration through spastin. Nevertheless, we have identified the significance of 14-3-3 and spastin in the process of neural regeneration. Furthermore, we conducted complementary experiments to support the stability of spastin by FC-A treatment both in vitro and in vivo. We found an enhanced protein expression in cortical neurons after FC-A treatment (Fig.4M). Also, the results indicate a consistent elevation trend in the protein levels of spastin and 14-3-3 following spinal cord injury (Fig.5C&H). Moreover, in the FC-A group of mice, there was a significant increase in spastin protein levels (Fig.5D&I). These results also support that 14-3-3 promotes spinal cord injury repair by enhancing spastin protein stability.
- Fig. 5C- it is unclear where the photomicrographs were taken relative to the lesion.
We obtained tissue sections from the lesion core and the above segments for histological analysis. Given the scarcity of neural compartment at the injury center, we select tissue slices as close as possible to lesion core to illustrate the relationship between 14-3-3 and the injured neurons. We have provided an explanation of this in the revised version of the manuscript.
- The authors need to provide some evidence that the FC-A and spastazoline compounds are accessing the CNS following IP injection.
We thank the review’s suggestion. Although direct visualization evidence of FC-A and spastazoline entering the CNS is challenging to obtain, several indicators suggest drug penetration into spinal cord tissue. Firstly, behavioral and electrophysiological experiments in vivo demonstrate that drug injections indeed affect the neural activity of mice. Secondly, following spinal cord injury, the blood-spinal cord barrier was disrupted at the injury site, combined with the fact that both FC-A (molecular weight: 680.82 Da) and spastazoline (molecular weight: 382.51 Da) are small molecule drugs, these increases the likelihood of these small molecules entering the injured spinal cord tissue. Furthermore, our microtubule staining results indicated that FC-A and spastazoline did influence the acetylation ratio of microtubules. These findings support the drug penetration into spinal cord tissue.
- Some quantification of Fig. 5D would be important to support the contention that the lesion site is impacted by FC-A treatment.
Thank you for the suggestion. We have included quantitative analysis for Figure 5D (Figure) as recommended.
- The NF and 5-HT staining in Fig. 5D and in Fig. 7A and B does not clearly define fibers and is not convincing.
We appreciate the concerns. While we did not present whole nerve fibers, we therefore employed NF and 5-HT immunoreactive fluorescence intensity as an indicator to assess the regeneration of nerve fibers as previously described, but not axons per square millimeter (Baltan S, et, al. J Neurosci. 2011 Mar 16;31(11):3990-9; Iwai M, et, al. Stroke. 2010 May;41(5):1032-7; Wang Y, et, al. Elife. 2018 Sep 12;7:e39016; Altmann C, et, al. Mol Neurodegeneration. 2016 Oct 22;11(1):69).
Our results showed that in the spinal cord injury group, there was strongly decreased NF-positive stainning (with a slight increase in 5-HT). In contrast, the FC-A treatment group exhibited a significant higher abundance of NF-positive signals (or an increased 5-HT signal) in the lesion site, which also suggests the reparative effect of FC-A on nerves. We also intend to refine our immunohistochemical methods in future experiments.
Minor Comments:
- Line 80 -84. To my knowledge the only manuscripts examining the effects of spastin in axon regeneration models includes the analysis in drosophila (i.e. ref 15 and 16) and a study in sciatic nerve that reported an index of functional recovery but did not perform any histology to assess axon regeneration phenotypes. The literature should be more accurately reflected in the introduction.
We appreciate the suggestions from the reviewer. In the revised version, we have provided further clarification on the novelty of spastin in the spinal cord injury repair process.
- Line 73: The meaning of the following statement needs to be clarified: "spastin has two major isoforms, namely M1 and M87, coded form different initial sites."
We have provided additional elaboration for this statement in the revised version.
- Line 216: Results indicated that GFP-spastin could be ubiquitinated, while inhibiting the 217 binding of 14-3-3/spastin promoted spastin ubiquitination (Figure 5G)." - Should be Fig 3G
Sorry about the mistake. We have made the corresponding changes in the revised version.
- Line 255: "Briefly, we established a neural injury model as previously described(31)" - the basics of the injury model need to be described in this manuscript.
In the revised version, we have provided further elaboration on the glutamate-induced neuronal injury model.
Reviewer #3 (Recommendations For The Authors):
Figure 1: A- Both legend and text fail to provide detail on this specific panel.
We have provided a more detailed and comprehensive description of the legend and results in this section.
B- Is the contribution of non-neuronal cells for co-IPs relevant? Co-IP with isolated neuronal extracts (instead of spinal cord tissue) should be performed.
We thank the review’s suggestion. To further elucidate their interaction within neurons, cortical neurons were cultured (Cultured in Neurobasal medium supplemented with 2%B27 and cytarabine was used to inhibit glial cell growth) and cells were lysed for co-IP experiments (Fig.1C), and the results demonstrated the interaction between 14-3-3 and spastin within neurons.
C- Both spastin and 14-3-3 appear to label the entire neuron with similar intensities throughout the entire cell which is rather unusual. Conditions of immunofluorescence should be improved and z-projections should be provided to support co-localization.
Thanks for the comment. Our dual-labeling experiments indicated that 14-3-3 exhibits a characteristic pattern of whole-cell distribution. Therefore, this result cannot confirm the interaction between 14-3-3 and spastin within neurons, but it does provide evidence regarding the intracellular distribution patterns of 14-3-3 and spastin. Consequently, we supplemented neuronal endogenous co-IP experiments to further demonstrate the direct interaction between 14-3-3 and spastin within neurons, and we have modified the wording in the revised version accordingly.
D- xx and yy axis information is either lacking or incomplete.
We have made the corrections to the figures.
E- It would be useful to show the conservation between the different 14-3-3 isoforms.
We appreciate the suggestions. We have included a conservation analysis of 14-3-3 to assist readers in better understanding these results (Fig.1F).
Figure 2:
D- The experiment using a general protein kinase inhibitor does not allow concluding that the specific phosphorylation of spastin is sufficient for binding to 14-3-3. An alternative phosphorylated protein might be involved in the process.
We appreciate the reviewer's consideration. We believe this serves as a prerequisite condition to demonstrate that "14-3-3 binding to spastin requires spastin phosphorylation." In fact, another project in our group has confirmed that CAMK II can mediate spastin phosphorylation, and the addition of staurosporine significantly reduces spastin phosphorylation levels (unpublished results). Here, we provide the western blot experiment showing the decrease in spastin phosphorylation under staurosporine treatment, with phosphorylation levels detected using the Pan Phospho antibody (Fig.2D).
H and I- Pseudo-replication. Only independent experiments should be plotted and not data on multiple cells obtained in the same experiment. Please indicate the number of independent experiments.
We appreciate the reviewer's correction. We now have included the mean value of three independent experiments and we have made relevant revisions to the statistical charts.
Figure 3:
The rationale for the hypothesis that spastin S233D transfection might upregulate the expression of spastin relative to WT and spastin S233A is unclear.
We appreciate the reviewer's consideration. We have supplemented the relevant results, as depicted in the Fig.3G, which demonstrates that 14-3-3 can enhance the protein levels of spastin, and phosphorylated spastin (S233D) exhibits a significantly increased protein level compared to wild-type spastin. These findings indicate that 14-3-3 not only inhibits the degradation of spastin but also increases its protein levels.
I- pseudo-replication. Please plot and do statistical analysis of independent experiments.
Thank you for the reviewer's corrections. We have made the necessary revisions.
Figure 4: E-J: I- pseudo-replication. Please plot and do statistical analysis of independent experiments.
Thank you for the reviewer's corrections. We have made the necessary revisions.
Figure 5:
B- Please show individual data points.
Thank you for the reviewer's corrections. We have made the necessary revisions.
D- Longitudinal images of spinal cords where spastazoline was used cannot correspond to contusion as there is a very sharp discontinuity between the rostral and caudal spinal cord tissue. A full transection seems to have occurred. Alternatively, technical problems with tissue collection/preservation might have occurred.
Thank you for the reviewer's consideration. The sharp discontinuity observed in the spastazoline group is not due to modeling issues but rather a result of the drug's effects on the injury site. This is primarily because spastin plays a crucial role not only in neuronal development but also in mitosis. Since the highly active proliferation of stromal cells at the injury site, . spastazoline may inhibit the proliferation of injury site-related stormal cells, thereby impeding the wound healing process following spinal cord injury, resulting in the observed discontinuous injury gap. We have made the corresponding revision accordingly.
E- Images do not have the quality to allow analysis. 5HT staining should not be considered as a clear axonal labeling is not seen. This is also the case for neurofilament staining.
We appreciate the concerns. While we did not present whole nerve fibers, we therefore employed NF and 5-HT immunoreactive fluorescence intensity as an indicator to assess the regeneration of nerve fibers as previously described, but not axons per square millimeter (Baltan S, et, al. J Neurosci. 2011 Mar 16;31(11):3990-9; Iwai M, et, al. Stroke. 2010 May;41(5):1032-7; Wang Y, et, al. Elife. 2018 Sep 12;7:e39016; Altmann C, et, al. Mol Neurodegeneration. 2016 Oct 22;11(1):69).
Our results showed that in the spinal cord injury group, there was strongly decreased NF-positive stainning (with a slight increase in 5-HT). In contrast, our FC-A treatment group exhibited a significant higher abundance of NF-positive signals (or an increased 5-HT signal) in the lesion site, which also suggests the reparative effect of FC-A on nerves. We also intend to refine our immunohistochemical methods in future experiments.
F- Images do not allow analysis. Higher magnifications are needed.
Thank you for the reviewer's consideration. We have now included higher-magnification images (Fig.5M) to address this concern.
Figure 7:
Same issues as in Figure 5.
A- Images do not have the quality to allow analysis. 5HT staining should not be considered as a clear axonal labeling is not seen.
B- Images do not have the quality to allow analysis. Neurofilament staining should not be considered as clear axonal labeling is not seen. MBP staining does not have a pattern consistent with myelin staining
We appreciate the concerns. While we did not present whole nerve fibers, we therefore employed NF and 5-HT immunoreactive fluorescence intensity as an indicator to assess the regeneration of nerve fibers as previously described, but not axons per square millimeter (Baltan S, et, al. J Neurosci. 2011 Mar 16;31(11):3990-9; Iwai M, et, al. Stroke. 2010 May;41(5):1032-7; Wang Y, et, al. Elife. 2018 Sep 12;7:e39016; Altmann C, et, al. Mol Neurodegeneration. 2016 Oct 22;11(1):69). In this study, sagittal slices were used. MBP covers the axonal surface, indicating its co-localization with the axons. However, as we did not present intact nerve fibers, so we were unable to show the typical myelin staining of MBP.
-

eLife assessment
The finding that Fusicoccin (FC-A) promotes locomotor recovery after spinal cord injury is useful, and the idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. However, the main methods, data, and analyses are inadequate to support the primary claim of the manuscript that a 14-3-3-Spastin complex is necessary for the observed FC-A effects.
-

Reviewer #1 (Public Review):
The present work establishes 14-3-3 proteins as binding partners of spastin and suggests that this binding is positively regulated by phosphorylation of spastin. The authors show evidence that 14-3-3 - spastin binding prevents spastin ubiquitination and final proteasomal degradation, thus increasing the availability of spastin. The authors measured microtubule severing activity in cell lines and axon regeneration and outgrowth as a prompt to spastin activity. By using drugs and peptides that separately inhibit 14-3-3 binding or spastin activity, they show that both proteins are necessary for axon regeneration in cell culture and in vivo models in rats.
The following is an account of the major strengths and weaknesses of the methods and results.Major strengths
-The authors performed pulldown assays on spinal …Reviewer #1 (Public Review):
The present work establishes 14-3-3 proteins as binding partners of spastin and suggests that this binding is positively regulated by phosphorylation of spastin. The authors show evidence that 14-3-3 - spastin binding prevents spastin ubiquitination and final proteasomal degradation, thus increasing the availability of spastin. The authors measured microtubule severing activity in cell lines and axon regeneration and outgrowth as a prompt to spastin activity. By using drugs and peptides that separately inhibit 14-3-3 binding or spastin activity, they show that both proteins are necessary for axon regeneration in cell culture and in vivo models in rats.
The following is an account of the major strengths and weaknesses of the methods and results.Major strengths
-The authors performed pulldown assays on spinal cord lysates using GST-spastin, then analyzed pulldowns via mass spectrometry and found 3 peptides common to various forms of 14-3-3 proteins. In co-expression experiments in cell lines, recombinant spastin co-precipitated with all 6 forms of 14-3-3 tested. The authors could also co-immunoprecipitate spastin-14-3-3 complexes from spinal cord samples and from primary neuronal cultures.
-By protein truncation experiments they found that the Microtubule Binding Domain of spastin contained the binding capability to 14-3-3. This domain contained a putative phosphorylation site, and substitutions that cannot be phosphorylated cannot bind to 14-3-3.
-Overexpression of GFP-spastin shows a turn-over of about 12 hours when protein synthesis is inhibited by cycloheximide. When 14-3-3 is co-overexpressed, GFP-spastin does not show a decrease by 12 hours. When S233A is expressed, a turn-over of 9 hours is observed, suggesting that phosphorylation increases the stability of the protein. In support of that notion, the phospho-mimetic S233D makes it more stable, lasting as much as the over-expression of 14-3-3.
-By combining FCA with Spastazoline, authors claim that FCA increased regeneration is due to increased spastin activity in various models of neurite outgrowth and regeneration in cell culture and in vivo, the authors show impressive results on the positive effect of FCA in regeneration, and that this is abolished when spastin is inhibited.Major weaknesses
1- The present manuscript suggests that 14-3-3 and spastin work in the same pathway to promote regeneration. Although the manuscript contains valuable evidence in support for a role of 14-3-3 and spasting in regeneration, the conclusive evidence is difficult to generate, and is missing in the present manuscript. For example, there are simpler explanations for the combined effect of FC-A and spastazoline. The FC-A mechanism of action can be very broad, since it will increase the binding of all 14-3-3 proteins with presumably all their substrates, hence the pathways affected can rise to the hundreds. The fact that spastazoline abolishes FC-A effect, may not be because of their direct interaction, but because spastin is a necessary component of the execution of the regeneration machinery further downstream, in line with the fact that spastazoline alone prevented outgrowth and regeneration, and in agreement with previous work showing that normal spastin activity is necessary for regeneration.
With this in mind, I consider the title and most major conclusions of the manuscript related to these two proteins acting together for the observed effects are overstated.2- Authors show that S233D increases MT severing activity, and explain that it is related to increased binding to 14-3-3. An alternative explanation is that phosphorylation at S233 by itself could increase MT severing activity. The authors could test if purified spastin S233D alone could have more potent enzymatic activity.
3- The interpretation of the authors cannot explain how Spastin can engage in MT severing while bound to 14-3-3 using its Microtubule Binding Domain.
4- Also, the term "microtubule dynamics", which is present in the title and in other major conclusions, is overstated. Although authors show, in cell lines, changes in microtubule content, it is far from evidence for changes in "MT dynamics" in the settings of interest (i.e. injured axons).
5- In the same lines, the manuscript lacks evidence for the changes of MT content and/dynamics as a function of the proposed 14-3-3 - Spastin pathway.
-

Reviewer #2 (Public Review):
Summary: The idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. In this manuscript Liu et al. explore a 14-3-3-Spastin complex and its role in axon regeneration.
Strengths: Some of the effects of FC-A on locomotor recovery after spinal cord contusion look interesting
Weaknesses: The manuscript falls short of establishing that a 14-3-3-Spastin complex is important for any FC-A-dependent effects and there are several issues with data quality that make it difficult to interpret the results. Importantly, the effects of the spastin inhibitor has a major impact on neurite outgrowth suggesting that cells simply cannot grow in the presence of the inhibitor and raising serious questions about any selectivity for FC-A - …
Reviewer #2 (Public Review):
Summary: The idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. In this manuscript Liu et al. explore a 14-3-3-Spastin complex and its role in axon regeneration.
Strengths: Some of the effects of FC-A on locomotor recovery after spinal cord contusion look interesting
Weaknesses: The manuscript falls short of establishing that a 14-3-3-Spastin complex is important for any FC-A-dependent effects and there are several issues with data quality that make it difficult to interpret the results. Importantly, the effects of the spastin inhibitor has a major impact on neurite outgrowth suggesting that cells simply cannot grow in the presence of the inhibitor and raising serious questions about any selectivity for FC-A - dependent growth. Aspects of the histology following spinal cord injury were not convincing.
-

Reviewer #3 (Public Review):
Summary:
The current manuscript shows that 14-3-3 are binding partners of spastin, preventing its degradation. It is additionally shown, using complementary methods, that both 14-3-3 and spastin are necessary for axon regeneration in vitro and in vivo. While interesting in vitro and vivo data is provided, some of the claims of the authors are not convincingly supported.Major strengths:
Very interesting effect of FC-A in functional recovery after spinal cord injury.Major Weaknesses:
Some of the in vitro data, including colocalizations, and analysis of microtubule severing fall short to support the claims of the authors.
The in vivo selectivity of FC-A towards spastin is not adequately supported by the data presented.
There are aspects of the spinal cord injury site histology that are unclear. -

-

eLife assessment
The finding that Fusicoccin promotes locomotor recovery after spinal cord injury is useful, and the idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. However, the main methods, data, and analyses are inadequate to support the primary claim of the manuscript that a 14-3-3-Spastin complex is necessary for the observed Fusicoccin effects.
-

Reviewer #1 (Public Review):
The present work establishes 14-3-3 proteins as binding partners of Spastin and suggests that this binding is positively regulated by phosphorylation of Spastin. The authors show evidence that 14-3-3 - Spastin binding prevents Spastin ubiquitination and final proteasomal degradation, thus increasing the availability of Spastin. The authors measured microtubule severing activity in cell lines and axon regeneration and outgrowth as a prompt to Spastin activity. By using drugs and peptides that separately inhibit 14-3-3 binding or Spastin activity, they show that both proteins are necessary for axon regeneration in cell culture and in vivo models in rats.
The following is an account of the major strengths and weaknesses of the methods and results.
Major strengths
-The authors performed pulldown assays on spinal …Reviewer #1 (Public Review):
The present work establishes 14-3-3 proteins as binding partners of Spastin and suggests that this binding is positively regulated by phosphorylation of Spastin. The authors show evidence that 14-3-3 - Spastin binding prevents Spastin ubiquitination and final proteasomal degradation, thus increasing the availability of Spastin. The authors measured microtubule severing activity in cell lines and axon regeneration and outgrowth as a prompt to Spastin activity. By using drugs and peptides that separately inhibit 14-3-3 binding or Spastin activity, they show that both proteins are necessary for axon regeneration in cell culture and in vivo models in rats.
The following is an account of the major strengths and weaknesses of the methods and results.
Major strengths
-The authors performed pulldown assays on spinal cord lysates using GST-spastin, then analyzed pulldowns via mass spectrometry and found 3 peptides common to various forms of 14-3-3 proteins. In co-expression experiments in cell lines, recombinant Spastin co-precipitated with all 6 forms of 14-3-3 tested.
-By protein truncation experiments they found that the Microtubule Binding Domain of Spastin contained the binding capability to 14-3-3. This domain contained a putative phosphorylation site, and substitutions that cannot be phosphorylated cannot bind to Spastin.
-Spastin overexpression increased neurite growth and branching, and so did the phospho null spastin. On the other hand, the phospho mimetic prevents all kinds of neurite development.
-Overexpression of GFP-Spastin shows a turn-over of about 12 hours when protein synthesis is inhibited by cycloheximide. When 14-3-3 is co-overexpressed, GFP-Spastin does not show a decrease by 12 hours. When S233A is expressed, a turn-over of 9 hours is observed, indicating that the ability to be phosphorylated increases the stability of the protein.
-In support of that notion, the phospho-mimetic S233D makes it more stable, lasting as much as the over-expression of 14-3-3.
-Authors show that Spastin can be ubiquitinated, and that in the presence of ubiquitin, Spastin-MT severing activity is inhibited.
-By combining FCA with Spastazoline, the authors claim that FCA increased regeneration is due to increased Spastin Activity in various models of neurite outgrowth and regeneration in cell culture and in vivo, the authors show impressive results on the positive effect of FCA in regeneration, and that this is abolished when Spastin is inhibited.Major weaknesses
-However convincing the pull-downs of the expressed proteins, the evidence would be stronger if a co-immunoprecipitation of the endogenous proteins were included.
-To better establish the impact of Spastin phosphorylation in the interaction, there is no indication that the phosphomimetic (S233D) can better bind Spastin, and this result is contradicting to the conclusion of the authors that Spastin-14-3-3 interaction is necessary for (or increases) Spastin function
-To fully support the authors' suggestion that 14-3-3 and Spastin work in the same pathway to promote regeneration, I believe that some key observations are missing.
1-There is no evidence showing that 14-3-3 overexpression increases the total levels of Spastin, not only its turnover.
2- There is no indication that increasing the ubiquitination of Spastin decreases its levels. To suggest that proteasomal activity is affecting the levels of a protein, one would expect that proteasomal inhibition (with bortezomib or epoxomycin), would increase its levels.
3- Authors show that S233D increases MT severing activity, and explain that it is related to increased binding to 14-3-3. An alternative explanation is that phosphorylation at S233 by itself could increase MT severing activity. The authors could test if purified Spastin S233D alone could have more potent enzymatic activity.
-Finally, I consider that there are simpler explanations for the combined effect of FC-A and spastazoline. FC-A mechanism of action can be very broad, since it will increase the binding of all 14-3-3 proteins with presumably all their substrates, hence the pathways affected can rise to the hundreds. The fact that spastazoline abolishes FC-A effect, may not be because of their direct interaction, but because Spastin is a necessary component of the execution of the regeneration machinery further downstream, in line with the fact that spastizoline alone prevented outgrowth and regeneration, and in agreement with previous work showing that normal Spastin activity is necessary for regeneration.In summary, the evidence of the interaction of 14-3-3 and Spastin is solid, but it is weak with respect to showing evidence for the binding of endogenous proteins in neurons. Another strength of the manuscript is the important recovery of function after spinal cord injury after stimulation of 14-3-3 interactions. Although it is experimentally difficult to demonstrate that the effect of FC-A is due to the prevention of Spastin ubiquitination, the effect itself is very robust and remarkable in vivo.
-

Reviewer #2 (Public Review):
Summary:
The idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. In this manuscript, Liu et al. explore a 14-3-3-Spastin complex and its role in axon regeneration.Strengths:
Some of the effects of FC-A on locomotor recovery after spinal cord contusion look interesting.Weaknesses:
The manuscript falls short of establishing that a 14-3-3-Spastin complex is important for any FC-A-dependent effects and there are several issues with data quality that make it difficult to interpret the results. Importantly, the effects of the Spastin inhibitor have a major impact on neurite outgrowth suggesting that cells simply cannot grow in the presence of the inhibitor and raising serious questions about any selectivity for FC-A - …Reviewer #2 (Public Review):
Summary:
The idea of harnessing small molecules that may affect protein-protein interactions to promote axon regeneration is interesting and worthy of study. In this manuscript, Liu et al. explore a 14-3-3-Spastin complex and its role in axon regeneration.Strengths:
Some of the effects of FC-A on locomotor recovery after spinal cord contusion look interesting.Weaknesses:
The manuscript falls short of establishing that a 14-3-3-Spastin complex is important for any FC-A-dependent effects and there are several issues with data quality that make it difficult to interpret the results. Importantly, the effects of the Spastin inhibitor have a major impact on neurite outgrowth suggesting that cells simply cannot grow in the presence of the inhibitor and raising serious questions about any selectivity for FC-A - dependent growth. Aspects of the histology following spinal cord injury were not convincing. -

Reviewer #3 (Public Review):
Summary:
The current manuscript claims that 14-3-3 interacts with Spastin and that the 14-3-3/spastin interaction is important to regulate axon regeneration after spinal cord injury.Strengths:
In its present form, this reviewer identified no clear strengths for this manuscript.Weaknesses:
In general, most of the figures lack sufficient quality to allow analyses and support the author's claims (detailed below). The legends also fail to provide enough information on the figures which makes it hard to interpret some of them. Most of the quantifications were done based on pseudo-replication. The number of independent experiments (that should be defined as n) is not shown. The overall quality of the written text is also low and typos are too many to list. The original nature of the spinal cord injury-related …Reviewer #3 (Public Review):
Summary:
The current manuscript claims that 14-3-3 interacts with Spastin and that the 14-3-3/spastin interaction is important to regulate axon regeneration after spinal cord injury.Strengths:
In its present form, this reviewer identified no clear strengths for this manuscript.Weaknesses:
In general, most of the figures lack sufficient quality to allow analyses and support the author's claims (detailed below). The legends also fail to provide enough information on the figures which makes it hard to interpret some of them. Most of the quantifications were done based on pseudo-replication. The number of independent experiments (that should be defined as n) is not shown. The overall quality of the written text is also low and typos are too many to list. The original nature of the spinal cord injury-related experiments is unclear as the role of 14-3-3 (and Spastin) in axon regeneration has been extensively explored in the past. -