The zinc transporter Slc30a1 (ZnT1) in macrophages plays a protective role against attenuated Salmonella
Curation statements for this article:-
Curated by eLife
eLife assessment
Work described in this manuscript reveals the importance of the zinc transporter SLC30A1 in the antimicrobial function of macrophages, specifically against Salmonella. Cell-targeted deletion of the zinc transporter increased susceptibility of mice to systemic infection with Salmonella, leading to decreases in several cell functions such as nos2 expression. The authors argue that zinc homeostasis promotes macrophage cell function that is not conductive to the intracellular proliferation of Salmonella. This study provides novel and supportive evidence for a new pathway in nutritional immunity.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The zinc transporter Slc30a1 plays an essential role in maintaining cellular zinc homeostasis. Despite this, its functional role in macrophages remains largely unknown. Here, we examine the function of Slc30a1 in host defense using mice models infected with an attenuated stain of Salmonella enterica Typhimurium and primary macrophages infected with the attenuated Salmonella . Bulk transcriptome sequencing in primary macrophages identifies Slc30a1 as a candidate in response to Salmonella infection. Whole-mount immunofluorescence and confocal microscopy imaging of primary macrophage and spleen from Salmonella -infected Slc30a1 flag-EGFP mice demonstrate Slc30a1 expression is increased in infected macrophages with localization at the plasma membrane and in the cytosol. Lyz2 -Cre-driven Slc30a1 conditional knockout mice ( Slc30a1 fl/fl ;Lyz2-Cre ) exhibit increased susceptibility to Salmonella infection compared to control littermates. We demonstrate that Slc30a1-deficient macrophages are defective in intracellular killing, which correlated with reduced activation of nuclear factor kappa B and reduction in nitric oxide (NO) production. Notably, the model exhibits intracellular zinc accumulation, demonstrating that Slc30a1 is required for zinc export. We thus conclude that zinc export enables the efficient NO-mediated antibacterial activity of macrophages to control invading Salmonella .
Article activity feed
-

-

Author response:
Reviewer #1 (Public Review):
This is an important and very well conducted study providing novel evidence on the role of zinc homeostasis for the control of infection with the intracellular bacterium S. typhimurium also disentangling the underlying mechanisms and providing clear evidence on the importance of spatio-temporal distribution of (free) zinc within the cell.
We thank the reviewer for the positive comments.
- It would be important to provide more information on the genotype of mice.
As suggested by the reviewer, we have added the detailed genotype of Slc30a1flagEGFP/+ and Slc30a1fl/flLysMCre mice to the revised supplementary Figure supplement 10.
- It is rather unlikely that C57Bl6 mice survive up to two weeks after i.p. injection of 1x10E5 bacteria.
According to the reviewer comment, we have tested survival …
Author response:
Reviewer #1 (Public Review):
This is an important and very well conducted study providing novel evidence on the role of zinc homeostasis for the control of infection with the intracellular bacterium S. typhimurium also disentangling the underlying mechanisms and providing clear evidence on the importance of spatio-temporal distribution of (free) zinc within the cell.
We thank the reviewer for the positive comments.
- It would be important to provide more information on the genotype of mice.
As suggested by the reviewer, we have added the detailed genotype of Slc30a1flagEGFP/+ and Slc30a1fl/flLysMCre mice to the revised supplementary Figure supplement 10.
- It is rather unlikely that C57Bl6 mice survive up to two weeks after i.p. injection of 1x10E5 bacteria.
According to the reviewer comment, we have tested survival rate using a group of our experimental animals and C57BL/6 wild type.
The Salmonella stain is a gift from our friend, Professor Ge Bao-xue. We have sent this stain for genetic characterisation which we found 100% identity to Salmonella enterica Typhimurium with many strains originated from poultry. One of them is Salmonella enterica subsp. enterica serovar Typhimurium strain MeganVac1 (Accession: CP112994.1), a live attenuated stain. We hope that this would support the relationship between the high infectious dose and mice survive.
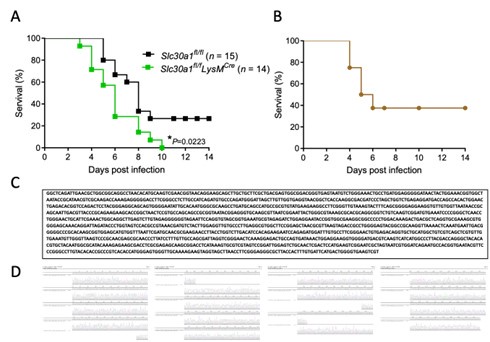
Author response image 1.
(A) Survival rate of Slc30a1fl/fl and Slc30a1fl/flLysMCre (n = 14-15/group) and (B) Survival rate of C57BL/6 wild type (n = 8) after Salmonella infection for two weeks. (C) A fulllength sequence (1,478 bases) of 16S rDNA genes sequences of Salmonella stain and (D) the sequencing electropherogram.
- To be sure that macrophages Slc30A1 fl/fl LysMcre mice really have an impaired clearance of bacteria it would be important to rule out an effect of Slc30A1 deletion of bacterial phagocytosis and containment (f.e. evaluation of bacterial numbers after 30 min of infection).
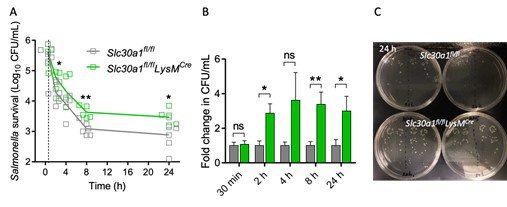
As the reviewer advised, we have repeated the experiment and measured the bacterial numbers after 30 min of infection (dashed line in A). The results show that there is no statistical difference in the bacterial numbers after 30 min between Slc30a1fl/flLysMCre and Slc30a1fl/fl BMDMs. Therefore, the reduction of bacterial numbers after 24 hours occurs due to the impairment of intracellular pathogen-killing capacity as the reviewer pointed out.
Author respnse image 2.
(A) Time course of the intracellular pathogen-killing capacity of Salmonellainfected Slc30a1fl/flLysMCre and Slc30a1fl/fl BMDMs measured in colony-forming units per ml (n = 5). (B) Fold change in Salmonella survival (CFU/mL) at different time points from A. (C) Representative images of Salmonella colonies on solid agar medium at 24 hours. Data are represented as mean ± SEM. P values were determined using 2-tailed unpaired Student’s t-test. *P<0.05, **P<0.01, and ns, not significant.
- Does the addition of zinc to macrophages negatively affect iNOS transcription as previously observed for the divalent metal iron and is a similar mechanism also employed (CEBPß/NF-IL6 modulation) (Dlaska M et al. J Immunol 1999)?
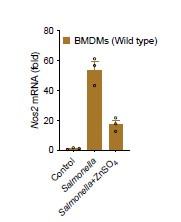
The reviewer has raised an important point here since free zinc also play a role in multiple levels of cellular signaling components (Kembe et al., 2015). Dlaska and colleague reported that NF-IL6, a protein responsible for iNOS transcription is negatively regulated by iron perturbation under IFNg/LPS stimulation in macrophages (Dlaska and Weiss, 1999). As the reviewer suggested, our results showed that zinc supplementation decreases the iNOS expression in macrophages after Salmonella infection, suggesting that free zinc might play a role in iNOS regulation.
However, in Slc30a1fl/flLysMCre macrophages, despite increase intracellular free zinc, lacking Slc30a1 also induces Mt1, a zinc reservoir which might negatively affect NO production (Schwarz et al., 1995) or alternatively inhibits iNOS through NF-kB pathway (Cong et al., 2016) as reported by previous studies. Therefore, we couldn’t rule out the possibility that defects in Salmonella clearance due to iNOS/NO inhibition may be caused by a complex combination of excess free zinc and overexpression of the zinc reservoir. To prove this hypothesis, further studies using the specific target, for example Mtfl/fliNOSfl/flLysMCre model might be needed to investigate the precision mechanism.
Author response image 3.
RT-qPCR analysis of mRNA encoding Nos2 in BMDMs after infected with Salmonella and Salmonella plus ZnSO4 (20 μM) for 4 h.
Reference:
Dlaska M, Weiss G. 1999. Central role of transcription factor NF-IL6 for cytokine and ironmediated regulation of murine inducible nitric oxide synthase expression. The Journal of Immunology. 162:6171-6177, PMID: 10229861
Kambe T, Tsuji T, Hashimoto A, Itsumura N. 2015. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiological Reviews. 95:749-784. https://doi: 10.1152/physrev.00035.2014, PMID: 26084690
Schwarz MA, Lazo JS, Yalowich JC, Allen WP, Whitmore M, Bergonia HA, Tzeng E, Billiar TR, Robbins PD, Lancaster JR Jr, et al. 1995. Metallothionein protects against the cytotoxic and DNA-damaging effects of nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 92: 4452-4456. https://doi: 10.1073/pnas.92.10.4452, PMID: 7538671
Cong W, Niu C, Lv L, Ni M, Ruan D, Chi L, Wang Y, Yu Q, Zhan K, Xuan Y, Wang Y, Tan Y, Wei T, Cai L, Jin L. 2016. Metallothionein prevents age-associated cardiomyopathy via inhibiting NF-κB pathway activation and associated nitrative damage to 2-OGD. Antioxidants & Redox Signaling. 25: 936-952. https://doi: 10.1089/ars.2016.6648, PMID: 27477335
- How does Zinc or TPEN supplementation to bacteria in LB medium affect the log growth of Salmonella?
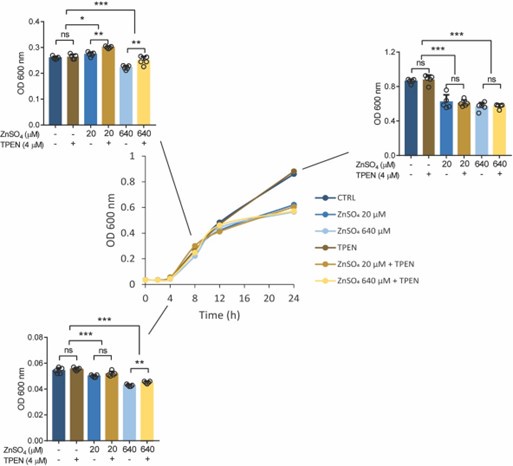
We found that zinc supplementation at both low (20 µM) and high (640 µM) concentrations negatively effects Salmonella growth, especially during log phase and stationary phase in the broth culture medium, but not TPEN (20 µM) supplementation. These indicates that high zinc conditions occur at cellular levels such as within phagosomes (Botella et al., 2011) can limit bacterial growth.
Author response image 4.
Growth curve (optical density, OD 600 nm) of Salmonella in LB medium at different concentrations of ZnSO4 and/or TPEN. Bar graph indicating Salmonella growth at specific time points. Each value was expressed as mean of triplicates for each testing and data were determined using 2-tailed unpaired Student’s t-test. *P<0.05, **P<0.01, ***P<0.001 and ns, not significant.
Reference:
Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charrière GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. 2011. Mycobacterial p(1)-type ATPases mediate resistance to zinc poisoning in human macrophages. Cell Host Microbe. 10:248-59. https://doi: 10.1016/j.chom.2011.08.006, PMID: 21925112
Reviewer #2 (Public Review):
This paper explores the importance of zinc metabolism in host defense against the intracellular pathogen Salmonella Typhimurium. Using conditional mice with a deletion of the Slc30a1 zinc exporter, the authors show a critical role for zinc homeostasis in the pathogenesis of Salmonella. Specifically, mice deficient in Slc30a1 gene in LysM+ myeloid cells are hypersusceptible to Salmonella infection, and their macrophages show alter phenotypes in response to Salmonella. The study adds important new information on the role metal homeostasis plays in microbe host interactions. Despite the strengths, the manuscript has some weaknesses. The authors conclude that lack of slc30a1 in macrophages impairs nos2-dependent anti-Salmonella activity. However, this idea is not tested experimentally. In addition, the research presented on Mt1 is preliminary. The text related to Figure 7 could be deleted without affecting the overall impact of the findings.
We thank the reviewer for his/her positive comments and constructive suggestions.
Reviewer #3 (Public Review):
Na-Phatthalung et al observed that transcripts of the zinc transporter Slc30a1 was upregulated in Salmonella-infected murine macrophages and in human primary macrophages therefore they sought to determine if, and how, Slc30a1 could contribute to the control of bacterial pathogens. Using a reporter mouse the authors show that Slc30a1 expression increases in a subset of peritoneal and splenic macrophages of Salmonella-infected animals. Specific deletion of Slc30a1 in LysM+ cells resulted in a significantly higher susceptibility of mice to Salmonella infection which, counter to the authors conclusions, is not explained by the small differences in the bacterial burden observed in vivo and in vitro. Although loss of Slc30a1 resulted in reduced iNOS levels in activated macrophages, the study lacks experiments that mechanistically link loss of NO-mediated bactericidal activity to Salmonella survival in Slc30a1 deficient cells. The additional deletion of Mt1, another zinc binding protein, resulted in even lower nitrite levels of activated macrophages but only modest effects on Salmonella survival. By combining genetic approaches with molecular techniques that measure variables in macrophage activation and the labile zinc pool, Na-Phattalung et al successfully demonstrate that Slc30a1 and metallothionein 1 regulate zinc homeostasis in order to modulate effective immune responses to Salmonella infection. The authors have done a lot of work and the information that Slc30a1 expression in macrophages contributes to control of Salmonella infection in mice is a new finding that will be of interest to the field. Whether the mechanism by which SLC30A1 controls bacterial replication and/or lethality of infection involves nitric oxide production by macrophages remains to be shown.
We very much appreciate the reviewer’s detailed evaluation and suggestions. The manuscript has been revised thoroughly according to the reviewer’s advice.
-

eLife assessment
Work described in this manuscript reveals the importance of the zinc transporter SLC30A1 in the antimicrobial function of macrophages, specifically against Salmonella. Cell-targeted deletion of the zinc transporter increased susceptibility of mice to systemic infection with Salmonella, leading to decreases in several cell functions such as nos2 expression. The authors argue that zinc homeostasis promotes macrophage cell function that is not conductive to the intracellular proliferation of Salmonella. This study provides novel and supportive evidence for a new pathway in nutritional immunity.
-

Reviewer #1 (Public Review):
This is an important and very well conducted study providing novel evidence on the role of zinc homeostasis for the control of infection with the intracellular bacterium S. typhimurium also disentangling the underlying mechanisms and providing clear evidence on the importance of spatio-temporal distribution of (free) zinc within the cell.
Comments:
It would be important to provide more information on the genotype of mice. It is rather unlikely that C57Bl6 mice survive up to two weeks after i.p. injection of 1x10E5 bacteria.
To be sure that macrophages Slc30A1 fl/fl LysMcre mice really have an impaired clearance of bacteria it would be important to rule out an effect of Slc30A1 deletion of bacterial phagocytosis and containment (f.e. evaluation of bacterial numbers after 30 min of infection).
Does the …
Reviewer #1 (Public Review):
This is an important and very well conducted study providing novel evidence on the role of zinc homeostasis for the control of infection with the intracellular bacterium S. typhimurium also disentangling the underlying mechanisms and providing clear evidence on the importance of spatio-temporal distribution of (free) zinc within the cell.
Comments:
It would be important to provide more information on the genotype of mice. It is rather unlikely that C57Bl6 mice survive up to two weeks after i.p. injection of 1x10E5 bacteria.
To be sure that macrophages Slc30A1 fl/fl LysMcre mice really have an impaired clearance of bacteria it would be important to rule out an effect of Slc30A1 deletion of bacterial phagocytosis and containment (f.e. evaluation of bacterial numbers after 30 min of infection).
Does the addition of zinc to macrophages negatively affect iNOS transcription as previously observed for the divalent metal iron and is a similar mechanism also employed (CEBPß/NF-IL6 modulation) (Dlaska M et al. J Immunol 1999)?
How does Zinc or TPEN supplementation to bacteria in LB medium affect the log growth of Salmonella?
-

Reviewer #2 (Public Review):
This paper explores the importance of zinc metabolism in host defense against the intracellular pathogen Salmonella Typhimurium. Using conditional mice with a deletion of the Slc30a1 zinc exporter, the authors show a critical role for zinc homeostasis in the pathogenesis of Salmonella. Specifically, mice deficient in Slc30a1 gene in LysM+ myeloid cells are hypersusceptible to Salmonella infection, and their macrophages show alter phenotypes in response to Salmonella. The study adds important new information on the role metal homeostasis plays in microbe host interactions. Despite the strengths, the manuscript has some weaknesses. The authors conclude that lack of slc30a1 in macrophages impairs nos2-dependent anti-Salmonella activity. However, this idea is not tested experimentally. In addition, the research …
Reviewer #2 (Public Review):
This paper explores the importance of zinc metabolism in host defense against the intracellular pathogen Salmonella Typhimurium. Using conditional mice with a deletion of the Slc30a1 zinc exporter, the authors show a critical role for zinc homeostasis in the pathogenesis of Salmonella. Specifically, mice deficient in Slc30a1 gene in LysM+ myeloid cells are hypersusceptible to Salmonella infection, and their macrophages show alter phenotypes in response to Salmonella. The study adds important new information on the role metal homeostasis plays in microbe host interactions. Despite the strengths, the manuscript has some weaknesses. The authors conclude that lack of slc30a1 in macrophages impairs nos2-dependent anti-Salmonella activity. However, this idea is not tested experimentally. In addition, the research presented on Mt1 is preliminary. The text related to Figure 7 could be deleted without affecting the overall impact of the findings.
-

Reviewer #3 (Public Review):
Na-Phatthalung et al observed that transcripts of the zinc transporter Slc30a1 was upregulated in Salmonella-infected murine macrophages and in human primary macrophages therefore they sought to determine if, and how, Slc30a1 could contribute to the control of bacterial pathogens. Using a reporter mouse the authors show that Slc30a1 expression increases in a subset of peritoneal and splenic macrophages of Salmonella-infected animals. Specific deletion of Slc30a1 in LysM+ cells resulted in a significantly higher susceptibility of mice to Salmonella infection which, counter to the authors conclusions, is not explained by the small differences in the bacterial burden observed in vivo and in vitro. Although loss of Slc30a1 resulted in reduced iNOS levels in activated macrophages, the study lacks experiments that …
Reviewer #3 (Public Review):
Na-Phatthalung et al observed that transcripts of the zinc transporter Slc30a1 was upregulated in Salmonella-infected murine macrophages and in human primary macrophages therefore they sought to determine if, and how, Slc30a1 could contribute to the control of bacterial pathogens. Using a reporter mouse the authors show that Slc30a1 expression increases in a subset of peritoneal and splenic macrophages of Salmonella-infected animals. Specific deletion of Slc30a1 in LysM+ cells resulted in a significantly higher susceptibility of mice to Salmonella infection which, counter to the authors conclusions, is not explained by the small differences in the bacterial burden observed in vivo and in vitro. Although loss of Slc30a1 resulted in reduced iNOS levels in activated macrophages, the study lacks experiments that mechanistically link loss of NO-mediated bactericidal activity to Salmonella survival in Slc30a1 deficient cells. The additional deletion of Mt1, another zinc binding protein, resulted in even lower nitrite levels of activated macrophages but only modest effects on Salmonella survival. By combining genetic approaches with molecular techniques that measure variables in macrophage activation and the labile zinc pool, Na-Phattalung et al successfully demonstrate that Slc30a1 and metallothionein 1 regulate zinc homeostasis in order to modulate effective immune responses to Salmonella infection. The authors have done a lot of work and the information that Slc30a1 expression in macrophages contributes to control of Salmonella infection in mice is a new finding that will be of interest to the field. Whether the mechanism by which SLC30A1 controls bacterial replication and/or lethality of infection involves nitric oxide production by macrophages remains to be shown.
-