Deciphering molecular heterogeneity and dynamics of human hippocampal neural stem cells at different ages and injury states
Curation statements for this article:-
Curated by eLife
eLife assessment
Using state-of-the-art single-nucleus RNA sequencing, Yao et al. investigate the transcriptomic features of neural stem cells (NSCs) in the human hippocampus to address how they vary across different age groups and stroke conditions. The authors report alterations in NSC subtype proportions and gene expression profiles after stroke. Although the study is valuable and the analysis is comprehensive, the significance is restricted by well-acknowledged technical limitations leading to incomplete evidence supporting some main conclusions.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
While accumulated publications support the existence of neurogenesis in the adult human hippocampus, the homeostasis and developmental potentials of neural stem cells (NSCs) under different contexts remain unclear. Based on our generated single-nucleus atlas of the human hippocampus across neonatal, adult, aging, and injury, we dissected the molecular heterogeneity and transcriptional dynamics of human hippocampal NSCs under different contexts. We further identified new specific neurogenic lineage markers that overcome the lack of specificity found in some well-known markers. Based on developmental trajectory and molecular signatures, we found that a subset of NSCs exhibit quiescent properties after birth, and most NSCs become deep quiescence during aging. Furthermore, certain deep quiescent NSCs are reactivated following stroke injury. Together, our findings provide valuable insights into the development, aging, and reactivation of the human hippocampal NSCs, and help to explain why adult hippocampal neurogenesis is infrequently observed in humans.
Article activity feed
-

-

-

-

Author Response
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
In this manuscript, Yao et al. explored the transcriptomic characteristics of neural stem cells (NSCs) in the human hippocampus and their changes under different conditions using single-nucleus RNA sequencing (snRNA-seq). They generated single-nucleus transcriptomic profiles of human hippocampal cells from neonatal, adult, and aging individuals, as well as from stroke patients. They focused on the cell groups related to neurogenesis, such as neural stem cells and their progeny. They revealed genes enriched in different NSC states and performed trajectory analysis to trace the transitions among NSC states and towards astroglial and neuronal lineages in silico. They also examined how NSCs are affected by aging and injury using …
Author Response
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
In this manuscript, Yao et al. explored the transcriptomic characteristics of neural stem cells (NSCs) in the human hippocampus and their changes under different conditions using single-nucleus RNA sequencing (snRNA-seq). They generated single-nucleus transcriptomic profiles of human hippocampal cells from neonatal, adult, and aging individuals, as well as from stroke patients. They focused on the cell groups related to neurogenesis, such as neural stem cells and their progeny. They revealed genes enriched in different NSC states and performed trajectory analysis to trace the transitions among NSC states and towards astroglial and neuronal lineages in silico. They also examined how NSCs are affected by aging and injury using their datasets and found differences in NSC numbers and gene expression patterns across age groups and injury conditions. One major issue of the manuscript is questionable cell type identification. For example, more than 50% of the cells in the astroglial lineage clusters are NSCs, which is extremely high and inconsistent with classic histology studies.
While the authors have made efforts to address previous critics, major concerns have not been adequately addressed, including a very limited sample size and with poor patient information. In addition, some analytical approaches are still questionable and the authors acknowledged that some they cannot address. Therefore, while the topic is interesting, some results are preliminary and some conclusions are not fully supported by the data presented.
We thank the reviewer for reevaluating our revised manuscript. We respect the reviewer’s comments and discuss the technical and conceptual limitations of this work. Here we provide the response to Reviewer #1 (Public Review) on these below.
Firstly, we appreciate the concerns raised by Reviewer 1 regarding the high proportion of NSCs within the astroglia lineage clusters. it is worth mentioning that distinguishing hippocampal qNSCs from astrocytes by transcription profiling poses a significant challenge in the field due to their high transcriptional similarity. From previous global UMAP analysis, AS1 (adult specific) can be separated from qNSCs, but AS2 (NSC-like astrocytes) cannot. Therefore, the data presented in Figure 2C to G aimed to further distinguish the qNSCs from AS2 by using gene set scores analysis. Based on different scores, we categorized qNSC/AS lineages into qNSC1, qNSC2 and AS2. Figure 2C presented the UMAP plot of qNSC/AS2 population from only neonatal sample. We apologize for not clarifying this in the figure legend. We have now clarified this information in the figure legend of Figure 2C. More importantly, we have added UMAP plots and quantifications for other groups in Figure 2-Supplement 2A and B, including adult, aging, and injure samples. This supplementary figure provides more complete information of the cell type composition and dynamic variations during aging and injury. Although the ratio of NSCs in the astroglia lineage clusters remains higher compared to classic histology studies, the trends indicate a reduction in qNSCs and an increase in astrocytes during aging and injury, which supports that cell type identification by using gene set score analysis is effective, although still not optimal. Combined methods to accurately distinguish between qNSCs and astrocytes are required in the future, and we also discuss this in the corresponding texts.
Secondly, we cannot adequately address the major concern regarding sample size raised by the reviewer due to the scarcity of stroke and neonatal human brain samples. We have collected additional details about the donors. Please refer to Figure 1-source data 1 for the updated information. Other information regarding the lifestyle parameters of these donors has not been sufficiently recorded by the hospital. Therefore, we cannot improve the patient information further.
Thirdly, regarding the questionable subpopulations of granule cells (GCs) that derive from neuroblasts in Figure 4A-4D, which are inconsistent with previous single-cell transcriptomic studies, we tried various strategies to confirm the identity of the two subpopulations of granule cells (GCs) derived from neuroblasts but didn’t get a clear answer. As a result, we can only provide an objective description of the differences in gene expression and developmental trajectory and speculate that these differences may be related to their degree of maturity but are not aligned on the same trajectory.
In the end, we have discussed the technical and conceptual limitations of this work and added a brief discussion about these limitations in the last paragraph of the main text. We hope the readers can interprate our data critically and objectively.
Reviewer #2 (Public Review):
In this manuscript, Yao et al. present a series of experiments aiming at generating a cellular atlas of the human hippocampus across aging, and how it may be affected by injury, in particular, stroke. Although the aim of the study is interesting and relevant for a larger audience, due to the ongoing controversy around the existence of adult hippocampal neurogenesis in humans, a number or technical weaknesses result in a poor support for many of the conclusions made from the results of these experiments.
In particular, a recent meta analysis of five previous studies applying similar techniques to human samples has identified different aspects of sample size as main determinants of the statistical power needed to make significant conclusions. Some of this aspects are the number of nuclei sequenced and subject stratification. These two aspects are of concern in Yao's study. First, the number of sequenced nuclei is lower than the calculated numbers of nuclei required for detecting rare cell types. However, Yao et al. report succeeding in detecting rare populations, including several types of neural stem cells in different proliferation states, which have been demonstrated to be extremely scarce by previous studies. It would be very interesting to read how the authors interpret these differences. Secondly, the number of donors included in some of the groups is extremely low (n=1) and the miscellaneous information provided about the donors is practically inexistent. As individual factors such as chronic conditions, medication, lifestyle parameters, etc... are considered determinant for the variability of adult hippocampal neurogenesis levels across individuals, this represents a series limitation of the current study. Overall, several technical weaknesses severely limit the relevance of this study and the ability of the authors to achieve their experimental aims.
After a first review round, the manuscript is still lacking a clear discussion of its several technical limitations, which will help the audience to grasp the relevance of the findings. In particular, detailed information about individual patients health status and relevant lifestyle parameters that may have affected it is lacking. The authors make the point themselves that the discrepancies among studies might be caused by health state differences across hippocampi, which subsequently lead to different degrees of hippocampal neurogenesis.". So, even in the authors own interpretation this is a serious limitation to the manuscript, that however out of the authors control, impacts on the quality of their findings.
Reviewer #2 (Recommendations For The Authors):
Please see public review. I do understand the authors point about incomplete patient data collection and low patient numbers and how the former is out of their control. Nevertheless, these are crucial parameters that impact negatively on the quality and relevance of several of their bold claims in the manuscript, especially given the low number of patients included. The current version still lacks a clear and honest discussion of the several technical and conceptual limitations of the authors work, as in some cases they are presented to the reviewers in the rebuttal letter, for the readership, so that they could critically evaluate the relevance of the authors' finding in a bigger perspective.
We thank the reviewer for reevaluating our revised manuscript. We respect the reviewer’s comm¬ents and discuss the technical and conceptual limitations of this work. Here we provide the response to Reviewer #2 (Public Review) on these below.
We understand the reviewer’s concern and have also noticed that according to the computational modeling conducted by Tosoni et al. (Neuron, 2023), at least 21 neuroblast cells (NBs) can be identified out of 30,000 granule cells (GCs) from a total of 180,000 dentate gyrus (DG) cells. In our dataset, we sequenced 24,671 GC nuclei and 92,966 total DG cell nuclei, which also includes neonatal samples. The number of nuclei we sequenced is 4.5 times higher than that of Wang et al. (Cell Research, 2022), who also detected NBs. Therefore, it is possible that we are able to detect NBs. Importantly, we have implemented strict quality control measures to support the reliability of our sequencing data. These measures include: 1. Immediate collection of tissue samples after postmortem (3-4 hrs) to ensure the quality of isolated nuclei. 2. Only nuclei expressing more than 200 genes but fewer than 5000-8600 genes (depending on the peak of enrichment genes) were considered. On average, each cell detected around 3000 genes. 3. The average proportion of mitochondrial genes in each sample was approximately 1.8%, with no sample exceeding 5%. We have shown that the number of cells captured from individual samples and the average number of genes detected per cell are sufficient, indicating overall good sequencing quality (Figure 1-supplement 1A,B andF, and Figure 1-source data 1). Additionally, we have further confirmed the presence of these cell types with low abundance by integrating immunofluorescence staining (Figure 4E, 5D and 6B), cell type-specific gene expression (Figure1 C and D), overall transcriptomic characteristics (Figure 1-supplement 1E), and developmental potential (Figure4 A-D, Figure 6E and F). We hope these evidences together could explain why we can identify the rare neurogenic populations.
Regarding the limited sample size and poor patient information, we cannot adequately address these two major concerns. Due to the scarcity of stroke or neonatal human samples, it was not feasible to collect a larger sample size within the expected timeframe. We have collected additional details about the donors. Please refer to Figure 1-source data 1 for the updated information. Other information regarding the lifestyle parameters of these donors has not been sufficiently recorded by the hospital. Therefore, we cannot improve the patient information further.
As per the reviewer’s recommendation, in the latest version, we have discussed the technical and conceptual limitations of this work and added a brief discussion about these limitations in the last paragraph of the main text. We hope the readers can interprate our data critically and objectively.
-

eLife assessment
Using state-of-the-art single-nucleus RNA sequencing, Yao et al. investigate the transcriptomic features of neural stem cells (NSCs) in the human hippocampus to address how they vary across different age groups and stroke conditions. The authors report alterations in NSC subtype proportions and gene expression profiles after stroke. Although the study is valuable and the analysis is comprehensive, the significance is restricted by well-acknowledged technical limitations leading to incomplete evidence supporting some main conclusions.
-

Reviewer #1 (Public Review):
In this manuscript, Yao et al. explored the transcriptomic characteristics of neural stem cells (NSCs) in the human hippocampus and their changes under different conditions using single-nucleus RNA sequencing (snRNA-seq). They generated single-nucleus transcriptomic profiles of human hippocampal cells from neonatal, adult, and aging individuals, as well as from stroke patients. They focused on the cell groups related to neurogenesis, such as neural stem cells and their progeny. They revealed genes enriched in different NSC states and performed trajectory analysis to trace the transitions among NSC states and towards astroglial and neuronal lineages in silico. They also examined how NSCs are affected by aging and injury using their datasets and found differences in NSC numbers and gene expression patterns …
Reviewer #1 (Public Review):
In this manuscript, Yao et al. explored the transcriptomic characteristics of neural stem cells (NSCs) in the human hippocampus and their changes under different conditions using single-nucleus RNA sequencing (snRNA-seq). They generated single-nucleus transcriptomic profiles of human hippocampal cells from neonatal, adult, and aging individuals, as well as from stroke patients. They focused on the cell groups related to neurogenesis, such as neural stem cells and their progeny. They revealed genes enriched in different NSC states and performed trajectory analysis to trace the transitions among NSC states and towards astroglial and neuronal lineages in silico. They also examined how NSCs are affected by aging and injury using their datasets and found differences in NSC numbers and gene expression patterns across age groups and injury conditions. One major issue of the manuscript is questionable cell type identification. For example, more than 50% of the cells in the astroglial lineage clusters are NSCs, which is extremely high and inconsistent with classic histology studies.
While the authors have made efforts to address previous critics, major concerns have not been adequately addressed, including a very limited sample size and patient information. In addition, some analytical approaches are still questionable and the authors acknowledge some issues they cannot address. Therefore, while the topic is interesting, some results are preliminary and some conclusions are not fully supported by the data presented.
-

Reviewer #2 (Public Review):
In this manuscript, Yao et al. present a series of experiments aiming at generating a cellular atlas of the human hippocampus across aging, and how it may be affected by injury, in particular, stroke. Although the aim of the study is interesting and relevant for a larger audience, due to the ongoing controversy around the existence of adult hippocampal neurogenesis in humans, a number or technical weaknesses result in a poor support for many of the conclusions made from the results of these experiments.
In particular, a recent meta analysis of five previous studies applying similar techniques to human samples has identified different aspects of sample size as main determinants of the statistical power needed to make significant conclusions. Some of this aspects are the number of nuclei sequenced and subject …Reviewer #2 (Public Review):
In this manuscript, Yao et al. present a series of experiments aiming at generating a cellular atlas of the human hippocampus across aging, and how it may be affected by injury, in particular, stroke. Although the aim of the study is interesting and relevant for a larger audience, due to the ongoing controversy around the existence of adult hippocampal neurogenesis in humans, a number or technical weaknesses result in a poor support for many of the conclusions made from the results of these experiments.
In particular, a recent meta analysis of five previous studies applying similar techniques to human samples has identified different aspects of sample size as main determinants of the statistical power needed to make significant conclusions. Some of this aspects are the number of nuclei sequenced and subject stratification. These two aspects are of concern in Yao's study. First, the number of sequenced nuclei is lower than the calculated numbers of nuclei required for detecting rare cell types. However, Yao et al. report succeeding in detecting rare populations, including several types of neural stem cells in different proliferation states, which have been demonstrated to be extremely scarce by previous studies. It would be very interesting to read how the authors interpret these differences. Secondly, the number of donors included in some of the groups is extremely low (n=1) and the miscellaneous information provided about the donors is practically inexistent. As individual factors such as chronic conditions, medication, lifestyle parameters, etc... are considered determinant for the variability of adult hippocampal neurogenesis levels across individuals, this represents a series limitation of the current study. Overall, several technical weaknesses severely limit the relevance of this study and the ability of the authors to achieve their experimental aims.After a first review round, the manuscript is still lacking a clear discussion of its several technical limitations, which will help the audience to grasp the relevance of the findings. In particular, detailed information about individual patients health status and relevant lifestyle parameters that may have affected it is lacking. The authors make the point themselves that the discrepancies among studies might be caused by health state differences across hippocampi, which subsequently lead to different degrees of hippocampal neurogenesis." So, even in the authors own interpretation this is a serious limitation to the manuscript, that however out of the authors control, impacts on the quality of their findings.
-

-

Author Response
We appreciate your consideration of our manuscript entitled “Deciphering molecular heterogeneity and dynamics of neural stem cells in human hippocampal development, aging, and injury” (eLife-RP-RA-2023-89507). We thank all the reviewers for their valuable and thoughtful comments and suggestions. We have carefully considered all the comments and revised our manuscript (eLife-VOR-RA2023-89507) accordingly. You can find our point-by-point responses here. In the revised manuscript, we have addressed most of the issues and concerns raised by the reviewers. We hope that the changes will better illustrate the quality of our sn-RNA data and the criteria of the cell type identification. However, due to the scarcity of stroke and neonatal human brain samples, we cannot strengthen our findings and conclusions by increasing this …
Author Response
We appreciate your consideration of our manuscript entitled “Deciphering molecular heterogeneity and dynamics of neural stem cells in human hippocampal development, aging, and injury” (eLife-RP-RA-2023-89507). We thank all the reviewers for their valuable and thoughtful comments and suggestions. We have carefully considered all the comments and revised our manuscript (eLife-VOR-RA2023-89507) accordingly. You can find our point-by-point responses here. In the revised manuscript, we have addressed most of the issues and concerns raised by the reviewers. We hope that the changes will better illustrate the quality of our sn-RNA data and the criteria of the cell type identification. However, due to the scarcity of stroke and neonatal human brain samples, we cannot strengthen our findings and conclusions by increasing this type of hippocampal tissue for analysis within the expected timeframe. With these improvements and limitations, we would like to ask whether we could get a better judgment from the reviewers.
Reviewer #1 (Public Review):
In this manuscript, Yao et al. explored the transcriptomic characteristics of neural stem cells (NSCs) in the human hippocampus and their changes under different conditions using single-nucleus RNA sequencing (snRNA-seq). They generated single-nucleus transcriptomic profiles of human hippocampal cells from neonatal, adult, and aging individuals, as well as from stroke patients. They focused on the cell groups related to neurogenesis, such as neural stem cells and their progeny. They revealed genes enriched in different NSC states and performed trajectory analysis to trace the transitions among NSC states and towards astroglia and neuronal lineages in silico. They also examined how NSCs are affected by aging and injury using their datasets and found differences in NSC numbers and gene expression patterns across age groups and injury conditions. One major issue of the manuscript is questionable cell type identification. For example, in Figure 2C, more than 50% of the cells in the astroglia lineage clusters are NSCs, which is extremely high and inconsistent with classic histology studies.
We appreciate the concerns raised by Reviewer 1 regarding the cell type identification. We suggest that the identification of the 16 main cell types in our study is accurate, as supported by the differential gene expression and the similarity of transcriptional profiles across species (Figure 1B to D, Figure Supplement 1C to E, and Figure 2A and B).
While we appreciate the reviewer for bringing up the concern regarding the high proportion of NSCs within the astroglia lineage clusters, it is worth mentioning that distinguishing hippocampal qNSCs from astrocytes by transcription profiling poses a significant challenge in the field due to their high transcriptional similarity. From previous global UMAP analysis, AS1 (adult specific) can be separated from qNSCs, but AS2 (NSC-like astrocytes) cannot. Therefore, the data presented in Figure 2C to G aimed to further distinguish the qNSCs from AS2 by using gene set scores analysis. Based on different scores, we categorized qNSC/AS lineages into qNSC1, qNSC2 and AS2. Figure 2C presented the UMAP plot of qNSC/AS2 population from only neonatal sample. We apologize for not clarifying this in the figure legend. We have now clarified this information in the figure legend of Figure 2C. More importantly, we have added UMAP plots and quantifications for other groups in Figure2Supplement 2A and B, including adult, aging, and injure samples. This supplementary figure provides more complete information of the cell type composition and dynamic variations during aging and injury. Although the ratio of NSCs in the astroglia lineage clusters remains higher compared to classic histology studies, the trends indicate a reduction in qNSCs and an increase in astrocytes during aging and injury, which supports that cell type identification by using gene set score analysis is effective, although still not optimal. Combined methods to accurately distinguish between qNSCs and astrocytes are required in the future, and we also discuss this in the corresponding texts.
Major comments:
In Figure 1E, the authors should provide supporting quality control of their snRNAseq dataset in the corresponding supplementary figures. Specifically, they should show that the average number of genes and transcripts detected in each cluster are similar across different conditions. This would rule out the possibility that the stem cell gene enrichment is an artifact of increased global gene expression.
Thanks for the suggestion. We have provided the supporting quality control of our snRNA-seq dataset in Figure1-Supplement 1A, B and F. The detailed data presented in Figure 1-Supplement 1A and Figure 1-source data 1 show that more than 2000 genes per cell were detected in all donor samples and mitochondrial genes accounted for less than 5%, suggesting that most cells were viable before freezing and underwent minimal RNA degradation. The hippocampi were dissected and collected from donors with a short post-mortem interval of about 3-4 hours to ensure low levels of RNA degradation and cellular apoptosis rates in the collected samples. For subsequent transcriptome analysis, we removed cells with fewer than 200 genes or more than 8600 genes (potentially indicating cell debris and doublets) and those with more than 20% of transcripts generated from mitochondrial genes, as shown in Figure 1-Supplement 1A and B. Figure 1-Supplement 1F provides evidence supporting that the average number of genes detected in each neurogenic cell type (AS2/qNSC, pNSC, aNSC, NB and GC) is similar across different conditions. This suggests that the enrichment of stem cell genes is not simply an artifact of increased global gene expression.
In Figure 2A, the authors performed a cross-species comparative analysis of neurogenic cell clusters by integrating their datasets with published datasets from mice, pigs, and macaques. They assigned cell types to the clusters based on their similarity to the same cell group across species. However, they did not address why a previous study by Franjic et al. (Neuron 2022) using the same method and analysis did not detect any neurogenic clusters in human hippocampal and entorhinal cells. This discrepancy could have implications for the validity of their approach and the interpretation of their results. The authors should provide possible explanations for the different outcomes.
We appreciate the valuable feedback provided by the reviewer. In our dataset, we sequenced 24,671 GC nuclei and 92,966 total DG cell nuclei, which also includes neonatal samples. The number of nuclei we sequenced is 4.5 times higher than that of Wang et al. (Cell Research, 2022), who also detected NBs. Thus, it is reasonable to conclude that we were able to detect NBs. Moreover, the presence of these rare cell types has been demonstrated in our study through immunostaining techniques, which provides further evidence. In addition, we downloaded the snRNAseq data from Franjic et al. (Neuron 2022) and mapped the dataset onto our snRNAseq dataset using the “multimodal reference mapping” method. Based on the mapping analysis, astrocytes, qNSCs, and aNSCs were identified in Franjic’s data with varying correlation efficiencies, but neuroblasts or immature neurons could not be detected (Figure 6-figure supplement 11 A to G). Therefore, we speculated that the discrepancies between our study and Franjic’s might be caused by health state differences across hippocampi, which subsequently lead to different degrees of hippocampal neurogenesis and immature neuron maintenance.
In Figure 2C-2J, the authors examined the astroglia lineage clusters to identify NSC subpopulations and their gene features. However, they did not use consistent cell types for the analysis. Some comparisons involved quiescent NSCs (qNSCs) and differentiated astrocytes, while others involved primed NSCs (pNSCs), and active NSCs (aNSCs). This could introduce bias and affect the results. The authors should consistently include all astroglia cell clusters in their analysis, such as q, p, a NSCs and astrocytes.
We understand the concerns raised by the reviewer, and we use different cell types as the starting points for the developmental trajectory for specific reasons. pNSCs represent an intermediate state between quiescence and activation. During embryonic development, pNSCs demonstrate the greatest similarity to RGLs. Subsequently, pNSCs progressively exit the cell cycle and transition into qNSCs during the postnatal stage. These qNSCs have the ability to re-enter the cell cycle upon activation by stimuli. Based on this knowledge, we have set the pNSC population as the root of the developmental trajectory in the neonatal sample, which aligns more closely with the actual developmental process. However, setting qNSCs as the root of the NSC developmental trajectory in the adult injury sample is more fit to the process of adult neurogenesis.
In addition, the authors’ identification of qNSCs, pNSCs and aNSCs is very questionable in Figure 2. For instance, qNSC2 cells in Figure 2G express MBP, PLP1, and MOBP, which are markers of mature oligodendrocytes. They receive low scores in RGL gene module scoring in Figure 2E, even lower than those of astrocytes. These cells are likely misclassified mature oligodendrocytes. In Figure 2H-I, the authors did not present the DEGs in pNSCs and aNSCs, the GO terms of these clusters are very similar. To confirm their results, the authors should either use histology or cite literature that supports the differentiation of pNSCs and aNSCs by these genes.
We appreciate the reviewer’s observation regarding the high expression of oligodendrocyte (OL) genes in the qNSC2 population, and we acknowledge that we currently do not have a clear explanation for this finding. However, despite the expression of OL genes in qNSC2, when we conducted a transcriptional similarity analysis comparing qNSC2 to other cell populations, we still observed a higher similarity between qNSC2 and qNSC1, as well as between qNSC2 and astrocytes, rather than oligodendrocytes. Therefore, qNSC2 are not misclassified mature oligodendrocytes (Figure 2-figure supplement 2C).
Regarding pNSCs and aNSCs, both cell types share similar molecular characteristics, with a key distinction in their proliferation abilities. Notably, aNSCs primarily reside in the S/G2/M phase and highly express the cell cycle-related gene CCND2, reflecting active mitosis. Since its capacity to differentiate into neuroblast/immature granule cells, aNSCs also express a small subset of genes associated with neuronal differentiation, including STMN2, SOX11, and SOX4 (Figure 1C, D, and Figure 2J). As per the reviewer’s request, we have presented the DEGs in pNSCs and aNSCs (Figure 2-figure supplement 2D, Figure 2-source data 2). The results of GO analysis reveal that pNSC is more associated with the Wnt signaling pathway, axonogenesis, and Hippo signaling, while aNSC is more associated with G2/M transition of mitotic cell cycle, neuron projection development, axon development, and dendritic spine organization (Figure2-figure supplement 2E, Figure 2-source data 2).
As Figure 2C illustrates, the authors isolated qNSCs and differentiated astrocytes from the astroglia lineage clusters to identify DEGs. However, more than 50% of the cells in the astroglia lineage clusters are NSCs, which is extremely high and inconsistent with classic histology studies. This could be due to cluster misclassification or over-representation of neonatal NSCs in the NSC cluster. The authors should stratify their data by age groups and provide corresponding UMAP plots and quantification. They should also compare DEGs between NSCs and astrocytes within each age group in all of the analyses, as neonatal, adult, and aging NSCs may have different properties and outputs.
While we appreciate the reviewer for bringing up the concern regarding the high proportion of NSCs within the astroglia lineage clusters, it is worth mentioning that distinguishing hippocampal qNSCs from astrocytes by transcription profiling poses a significant challenge in the field due to their high transcriptional similarity. From previous global UMAP analysis, AS1 (adult specific) can be separated from qNSCs, but AS2 (NSC-like astrocytes) cannot. Therefore, the data presented in Figure 2C to G aimed to further distinguish the qNSCs from AS2 by using gene set scores analysis. Based on different scores, we categorized qNSC/AS lineages into qNSC1, qNSC2 and AS2. Figure 2C presented the UMAP plot of qNSC/AS2 population from only neonatal sample. We apologize for not clarifying this in the figure legend. We have now clarified this information in the figure legend of Figure 2C. More importantly, we have added UMAP plots and quantifications for other groups in Figure2-Supplement 2A and B, including adult, aging, and injure samples. This supplementary figure provides more complete information of the cell type composition and dynamic variations during aging and injury. Although the ratio of NSCs in the astroglia lineage clusters remains higher compared to classic histology studies, the trends indicate a reduction in qNSCs and an increase in astrocytes during aging and injury, which supports that cell type identification by using gene set score analysis is effective, although still not optimal. Combined methods to accurately distinguish between qNSCs and astrocytes are required in the future, and we also discuss this in the corresponding texts. (The same question has been answered in the first part of this letter.)
In Figure 3, the authors discuss the important issues of shared gene expression between interneurons and NB/im-GCs. In the published work (Zhou et al. Nature 2022; Wang et al. Cell Research 2022), however, NBs and im-GCs are not located in the interneuron cluster. This needs to be stated to avoid confusion. Specifically, this suggests the limitation of using a few preselected markers for cell type identification. The author should also examine whether these shared markers are indeed expressed in human interneurons by immunostaining as one application of these markers will be in histology for the field.
Thanks for the reviewer’s comments. We agree that single nucleus transcriptome analysis is capable of effectively distinguishing between immature neurons and interneurons. In our UMAP plot, the NBs and im-GCs are not located in the interneuron cluster, either. When we compared the granule cell lineage which contains NB/immature GC and the interneuron population at the whole transcriptome level between our dataset and published mouse (Hochgerner et al. 2018), macaque and human (Franjic et al. 2022) transcriptome datasets, we found high transcriptomic congruence across different datasets (Figure 3-figure supplement 3A). Specifically, our identified human GABA-INs very highly resembled the well-annotated interneurons in different species (similarity scores > 0.95) (Figure 3-figure supplement 3A). The point we want to convey here is that many markers previously used to identify immature neurons are also expressed in interneurons. Therefore, when using these markers for staining and identification purposes, there is a possibility of mistaking an interneuron for an immature neuron. Hence, when selecting markers, we need to be aware of this and exclude genes that are highly expressed in interneurons as markers for immature neurons. To support our view, we conducted co-immunostainings of DCX (a traditional neuroblast marker) and SST (a typical interneuron marker). Our results demonstrate that SST-positive interneurons are indeed capable of being stained by the traditional neuroblast marker DCX in primates. Please see Figure 3-figure supplement 4A-C.
In Figure 4, the authors' classification of cell subpopulations in the neuronal lineage is not convincing. They claim to have identified two subpopulations of granule cells (GCs) that derive from neuroblasts in Figure 4A-4D. However, this is inconsistent with previous single-cell transcriptomic studies of human hippocampus, which only identified one GC cluster. The differentially expressed genes (DEGs) that they used to distinguish the two GC subpopulations are not supported by prior research. This could be a result of over-classification or technical bias. CALB1 marks mature neurons whereas CALB2 marks immature neurons. However, in Figure 4F, it suggests that CALB1 is expressed in cells that have similar pseudotime scores as CALB2, both of which reside in an intermediate position during the differentiation trajectory. This does not match the known expression patterns of these markers in GCs. The authors should explain this discrepancy and provide additional evidence to support their claims. In addition, for Figure 4F, the authors should address how the different cell fate groups correspond to cell clusters.
We appreciate the concerns raised by the reviewer. Unfortunately, despite trying various strategies to confirm the identity of the two subpopulations of granule cells (GCs) derived from neuroblasts, we were unable to find a clear answer. As a result, we can only provide an objective description of the differences in gene expression and developmental trajectory and speculate that these differences may be related to their degree of maturity but are not aligned on the same trajectory.
Regarding the expression of CALB1 and CALB2, the original Figure 4F did not provide precise positional information for these genes due to the compression of a large amount of gene information. In order to address this, we conducted a separate trajectory analysis specifically for CALB1 and CALB2 (Figure4-figure supplement 6B). The results of this analysis are in line with previous literature reports: CALB2 was found to be enriched in immature neurons, while CALB1 exhibited a delayed expression pattern and was enriched in mature neurons.
The authors compared NSCs in different age groups in Figure 5, but their analysis in Figure S5A-D only included neonatal and aging stages, omitting adult stages. They should perform cross-age analyses with all three stages for consistency.
Thank you for the reviewer's comments. We have now included the differentially expressed genes (DEGs) of the neurogenic lineage in the adult stage. Please see Figure5-supplyment 8.
In Figure 6E, the authors should separate the data by age and calculate the proportion of the re-clustered cell groups, as they did in Figure 6B. In the re-clustered groups, how do the aNSCs and reactive astrocytes change with age?
Thanks for the reviewer's comments. We have removed the previous Figure 6B and recalculated the proportions of the re-clustered cell groups, including reactive astrocytes (AS). The changes in the proportions of qNSC1, qNSC2, pNSC, aNSCs, and reactive astrocytes with age are now shown in Figure 6E of the updated version. We observed that the proportion of aNSCs decreases with age but increases after injury. Reactive astrocytes primarily appear in the injury group, while their proportion is very low in the other groups.
In Figure 6E-H, the authors assert that the aNSC group in stroke injury can produce oligodendrocytes in vivo based on trajectory analysis, which is a bold claim and lacks literature support. Their evidence is insufficient, as it relies on a single in vitro study.
Thanks for the reviewer's comments. We have provided more references to support our claim (e.g., El Waly, Cayre, and Durbec 2018; Parras et al. 2004; Enric Llorens-Bobadilla et al. 2015b; Koutsoudaki et al. 2016). These studies have indicated that under injury conditions, neural stem cells have potentials to differentiate into oligodendrocytes.
In Figure S8 and the Discussion section, they compared their dataset with Zhou et al. (Nature 2022), a published snRNA-seq dataset of the human hippocampus across the lifespan. The authors speculated that the new neurons identified in the EdU in vitro culture analysis in Zhou et al. might be related to epilepsy, but they did not provide any evidence for this claim. To partially validate their speculation, the authors should conduct the same integrative analysis with Ayhan et al. (Neuron 2021), which examined snRNA-seq data from epileptic patient hippocampi, to demonstrate that they could detect the injury-induced aNSC population and injury-associated genes. Furthermore, they should also conduct the same integrative analysis with the other two published human hippocampal datasets, namely Franjic et al. (Neuron 2022) and Wang et al. (Cell Research 2022).
Thanks for the reviewer's comments. As the reviewer’s request, we down loaded the snRNA-seq data from Zhou et al. (Nature 2022), Wang et al (Cell Research, 2022a), Franjic et al. (Neuron 2022) and Ayhan et al. (Neuron 2021) for integrative analysis. Except for the dataset from Zhou et al. (Nature 2022), which utilized machine learning and made it difficult to extract cell type information for fitting with our own data, the datasets from the other three laboratories were successfully mapped onto our dataset. Different levels of correlation were observed, confirming the presence of astrocytes, qNSCs, aNSCs, and NBs (Figure 6-figure supplement 11 E to G).
There are a few minor concerns that the authors could improve upon. In Fig. 5D, HOPX immunostaining pattern doesn't not look like NSCs. In Figure 5B and 6B, the same data were presented twice. And proper statistical tests are missing in Figure 6B.
Thanks for the reviewer's comments. We have added the arrowheads to indicate the typical immunostaining of HOPX immunostaining, which clearly shows its nuclear localization. This observation is consistent with previous reports on the subcellular distribution of HOPX protein. In the updated version, Figure 5B and 6D are distinct and not repetitive. The inclusion of the proportions of reactive astrocytes in Figure 6D provides valuable information about their distribution within the different groups. Unfortunately, statistical tests cannot be conducted for the neonatal and injury samples since only one sample is available in each case.
# Reviewer 2
Major points:
- The number of sequenced nuclei is lower than the calculated numbers of nuclei required for detecting rare cell types according to a recent meta-analysis of five similar datasets (Tosoni et al., Neuron, 2023). However, Yao et al report succeeding in detecting rare populations, including several types of neural stem cells in different proliferation states, which have been demonstrated to be extremely scarce by previous studies. It would be very interesting to read how the authors interpret these differences.
We appreciate the valuable comments from the reviewer. We understand the reviewer’s concern and have also noticed that according to the computational modeling conducted by Tosoni et al. (Neuron, 2023), at least 21 neuroblast cells (NBs) can be identified out of 30,000 granule cells (GCs) from a total of 180,000 dentate gyrus (DG) cells. In our dataset, we sequenced 24,671 GC nuclei and 92,966 total DG cell nuclei, which also includes neonatal samples. The number of nuclei we sequenced is 4.5 times higher than that of Wang et al. (Cell Research, 2022), who also detected NBs. Therefore, it is reasonable to conclude that we were able to detect NBs. Moreover, the presence of these rare cell types has been demonstrated in our study through immunostaining techniques, which provides further evidence. we have implemented strict quality control measures to support the reliability of our sequencing data. These measures include: 1. Immediate collection of tissue samples after postmortem (3-4 hrs) to ensure the quality of isolated nuclei. 2. Only nuclei expressing more than 200 genes but fewer than 5000-8600 genes (depending on the peak of enrichment genes) were considered. On average, each cell detected around 3000 genes. 3. The average proportion of mitochondrial genes in each sample was approximately 1.8%, with no sample exceeding 5%. The related supplementary information has been included in Figure 1-supplement 1A, B and F, and Figure 1source data 1.
- The information regarding the donors including in this study is very scarce. Factors such as chronic conditions, medication, lifestyle parameters, inflammatory levels should be provided.
Thanks for the reviewer's comments. We have incorporated additional details about the donors. However, we would like to clarify that information regarding lifestyle parameters has not been collected. Please refer to Figure 1-source data 1 for the updated information.
- The number of donors included per group is insufficient: neonatal group n=1; adult group n=2; stroke n=1. Although the scarcity and value of each human brain sample is a factor to be considered, the authors must explain why and how the results obtained from individuals can be extrapolated to the population at these low numbers, especially considering that the rate of adult hippocampal neurogenesis is assumed to be very variable across individuals (Tosoni et al., Neuron, 2023).
Thanks for the reviewer's comments. We acknowledge these limitations and understand that the inclusion of a larger number of donors would strengthen the statistical power and generalizability of our findings. However, due to the scarcity of stroke or neonatal human samples, it was not feasible to collect a larger sample size within the expected timeframe. To explain why and how we could identify the rare neurogenic populations, we have shown that the number of cells captured from individual samples and the average number of genes detected per cell are sufficient, indicating overall good sequencing quality (Figure 1-supplement 1A and B, and Figure 1-source data 1). Additionally, we have further confirmed the presence of these cell types with low abundance by integrating immunofluorescence staining (Figure 4E and Figure 6F), cell type-specific gene expression (Figure1 C and D), overall transcriptomic characteristics (Figure 1-supplement 1E), and developmental potential (Figure4 A-D, Figure 6A-D).
- The definition of primed NSCs (pNSCs) is poor and questionable. "Primed" may be interpreted as a loaded term and the authors only make an effort to follow them into their neurogenic trajectory while figure 4A suggest that they also, if not preferentially judging on the directionality of the RNA velocity vectors, generate astrocytes and quiescent NSCs.
Thanks for the reviewer's comments. We apologize for not clearly explaining the definition of pNSC in our study. We have now included an explanation in the text and added supplementary information to highlight the features of pNSC and aNSC (Figure 2H to J, Figure2-figure supplement 2D and E). The results of GO analysis reveal that pNSC is more associated with the Wnt signaling pathway, axonogenesis, and Hippo signaling, while aNSC is more associated with G2/M transition of mitotic cell cycle, neuron projection development, axon development, and dendritic spine organization (Figure2-figure supplement 2E, Figure 2-source data 2). The pNSCs referred to in this study represent an intermediate state between quiescence and activation. During embryonic development, pNSCs exhibit the greatest similarity to RGLs. Subsequently, pNSCs gradually exit the cell cycle and transition into qNSCs during the postnatal development (Figure 2J). Thus, in Figure 4A, for the neonatal sample analysis, some pNSCs are shown to enter the neurogenic trajectory, while others exit the cell cycle and transition into qNSCs or become astrocytes (AS2) during postnatal development, indicating a bidirectional trajectory.
- The experimental definition of quiescent NSCs (qNSC1) is poor and questionable. The qNSC1 cluster is defined by the expression of HOXP (page 6), which the authors indicate is a"quiescence NSC gene". However, at least in mice, HOXP collages with BrdU in proliferative NSCs (Deqiang Li et al, Stem Cell Res. 2015).
Thank you for providing the information about the study conducted by Deqiang Li et al (Stem Cell Res. 2015). We have carefully reviewed their findings. They propose that Hopx is specifically expressed in RGL cells, which are predominantly in a quiescent state. Additionally, they observed that Hopx-positive cells are long-term BrdU-label retaining cells, and Hopx-null NSCs show enhanced neurogenesis, as evidenced by an increased number of BrdU-positive cells. These results suggest that high expression of Hopx in NSCs indicates their quiescence. Furthermore, other studies have provided further support for using high expression of the HOPX gene as a marker to identify quiescent NSCs (Jaehoon Shin et al., Cell Stem Cell 2015; Daniel A. Berg et al., Cell 2019)
- The term quiescent is never defined in the text, and the reader is forced to assume that they refer to the absence of active proliferation genes, most commonly MKI67. Is that what the authors intended? this should be clarified.
Thanks for the reviewer's comments. We apologize for not clearly explaining the definition of qNSC in our study. We have now included an explanation in the text. qNSCs exhibit reversible cell cycle arrest and display a low rate of metabolic activity. However, they still possess a latent capacity to generate neurons and glia when they receive activation signals. They express genes such as GFAP, ALDH1L1, ID4, and HOPX (Figure 2B). The absence or low expression of active proliferation genes is one feature of qNSCs. The main difference lies in the state of the cell cycle and metabolism.
- They find cell clusters that express the proliferation marker MKI67. however, previous studies have indicated the difficulty of snRNA-seq techniques to detect proliferation marker transcripts, specially MKI67 even in hippocampal samples from human infants (for example see the snRNAseq studies from Wang and from Zhou cited by the authors and previously mentioned meta-analysis).
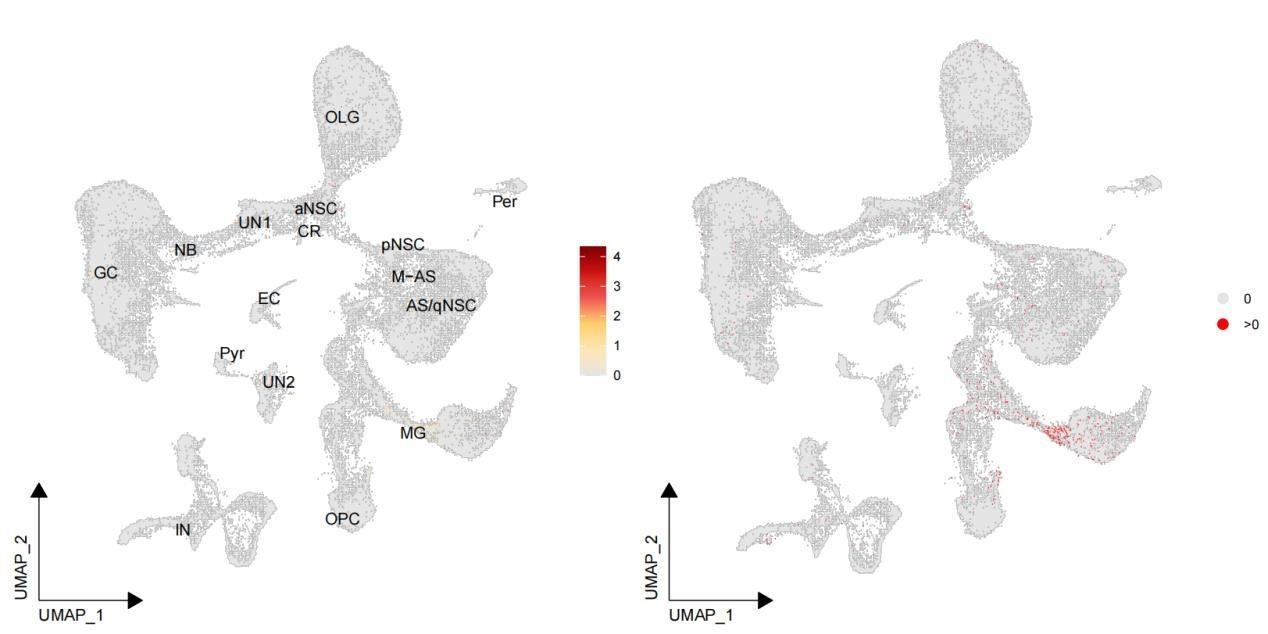
Thanks for the reviewer's comments. We could detect MKI67 in our snRNA-seq data, albeit with a very low number of cells (not clustered) expressing it. Here, we are providing the feature plot in Author response image 1 to illustrate the expression of MKI67. In our Figure 5C, we compared the expression level of MKI67 in neurogenic lineage among neonatal, adult and aged groups, and observed its high expression in neonatal rather than adult and aged groups. But the fraction of cells expressed MIK67 is still very low. We apologize for the confusion. We did not claim that we identified specific cell clusters expressing MKI67 in our study.
Author response image 1.
- The authors observe declining numbers of proliferating cells with aging and interpret this as evidence of declining neurogenesis. However, they also observe sustained neuroblast numbers in the aged brains they analyzed. Wouldn't these neuroblast support neurogenesis? This is unclear and should be discussed.
Thanks for the reviewer's question. We will revise the inaccurate description to clarify that the number of proliferating NPCs, rather than immature neurons, is dramatically reduced with aging. This is because, compared to rodents, immature neurons in primates are indeed retained for a longer period and possess the potential to further develop into mature neurons (Kohler, S.J., et al., PNAS, 2011). We have discussed this in the corresponding texts (Figure 5).
- The authors indicate that they find DCX transcript expression in interneurons. This is a potentially interesting observation. However, the authors should be very clear to state that in most studies that use DCX as a marker of immature granule cells, DCX's expression is detected by immunohistochemistry. Therefore, the fact that DCX transcripts may be present in other immature neurons does not necessarily disqualify its use as a protein maker of immature granule cells. This clarification will help to prevent misinterpretations of the data presented by the authors.
Thanks for the reviewer's suggestion. We have clarified that we observed DCX transcripts present in interneurons in addition to immature neurons by snRNAseq. In this revised version, we conducted co-immunostainings of DCX (a traditional neuroblast marker) and SST (a typical interneuron marker). Our results demonstrate that SST-positive interneurons are indeed capable of being stained by the traditional neuroblast marker DCX in primates. Please see Figure 3-figure supplement 4A-C. The similar result has also been reported by Franjic et al. (Neuron 2022).
-

eLife assessment
Using state-of-the-art single-nucleus RNA sequencing, Yao et al. investigate the transcriptomic features of neural stem cells (NSCs) in the human hippocampus to address how they vary across different age groups and stroke conditions. The authors report alterations in NSC subtype proportions and gene expression profiles after stroke and an exemplary gene elevated in NSCs and reactive astrocytes in stroke patients. Although the study is valuable, the significance is restricted by technical limitations and the incomplete evidence supporting the main conclusions.
-

Reviewer #1 (Public Review):
In this manuscript, Yao et al. explored the transcriptomic characteristics of neural stem cells (NSCs) in the human hippocampus and their changes under different conditions using single-nucleus RNA sequencing (snRNA-seq). They generated single-nucleus transcriptomic profiles of human hippocampal cells from neonatal, adult, and aging individuals, as well as from stroke patients. They focused on the cell groups related to neurogenesis, such as neural stem cells and their progeny. They revealed genes enriched in different NSC states and performed trajectory analysis to trace the transitions among NSC states and towards astroglial and neuronal lineages in silico. They also examined how NSCs are affected by aging and injury using their datasets and found differences in NSC numbers and gene expression patterns …
Reviewer #1 (Public Review):
In this manuscript, Yao et al. explored the transcriptomic characteristics of neural stem cells (NSCs) in the human hippocampus and their changes under different conditions using single-nucleus RNA sequencing (snRNA-seq). They generated single-nucleus transcriptomic profiles of human hippocampal cells from neonatal, adult, and aging individuals, as well as from stroke patients. They focused on the cell groups related to neurogenesis, such as neural stem cells and their progeny. They revealed genes enriched in different NSC states and performed trajectory analysis to trace the transitions among NSC states and towards astroglial and neuronal lineages in silico. They also examined how NSCs are affected by aging and injury using their datasets and found differences in NSC numbers and gene expression patterns across age groups and injury conditions. One major issue of the manuscript is questionable cell type identification. For example, more than 50% of the cells in the astroglial lineage clusters are NSCs, which is extremely high and inconsistent with classic histology studies.
While the authors have made efforts to address previous critics, major concerns have not been adequately addressed, including a very limited sample size and with poor patient information. In addition, some analytical approaches are still questionable and the authors acknowledged that some they cannot address. Therefore, while the topic is interesting, some results are preliminary and some conclusions are not fully supported by the data presented.
-

Reviewer #2 (Public Review):
In this manuscript, Yao et al. present a series of experiments aiming at generating a cellular atlas of the human hippocampus across aging, and how it may be affected by injury, in particular, stroke. Although the aim of the study is interesting and relevant for a larger audience, due to the ongoing controversy around the existence of adult hippocampal neurogenesis in humans, a number or technical weaknesses result in a poor support for many of the conclusions made from the results of these experiments.
In particular, a recent meta analysis of five previous studies applying similar techniques to human samples has identified different aspects of sample size as main determinants of the statistical power needed to make significant conclusions. Some of this aspects are the number of nuclei sequenced and subject …Reviewer #2 (Public Review):
In this manuscript, Yao et al. present a series of experiments aiming at generating a cellular atlas of the human hippocampus across aging, and how it may be affected by injury, in particular, stroke. Although the aim of the study is interesting and relevant for a larger audience, due to the ongoing controversy around the existence of adult hippocampal neurogenesis in humans, a number or technical weaknesses result in a poor support for many of the conclusions made from the results of these experiments.
In particular, a recent meta analysis of five previous studies applying similar techniques to human samples has identified different aspects of sample size as main determinants of the statistical power needed to make significant conclusions. Some of this aspects are the number of nuclei sequenced and subject stratification. These two aspects are of concern in Yao's study. First, the number of sequenced nuclei is lower than the calculated numbers of nuclei required for detecting rare cell types. However, Yao et al. report succeeding in detecting rare populations, including several types of neural stem cells in different proliferation states, which have been demonstrated to be extremely scarce by previous studies. It would be very interesting to read how the authors interpret these differences. Secondly, the number of donors included in some of the groups is extremely low (n=1) and the miscellaneous information provided about the donors is practically inexistent. As individual factors such as chronic conditions, medication, lifestyle parameters, etc... are considered determinant for the variability of adult hippocampal neurogenesis levels across individuals, this represents a series limitation of the current study. Overall, several technical weaknesses severely limit the relevance of this study and the ability of the authors to achieve their experimental aims.After a first review round, the manuscript is still lacking a clear discussion of its several technical limitations, which will help the audience to grasp the relevance of the findings. In particular, detailed information about individual patients health status and relevant lifestyle parameters that may have affected it is lacking. The authors make the point themselves that the discrepancies among studies might be caused by health state differences across hippocampi, which subsequently lead to different degrees of hippocampal neurogenesis.". So, even in the authors own interpretation this is a serious limitation to the manuscript, that however out of the authors control, impacts on the quality of their findings.
-

-

Author Response
We are very grateful to the editors and reviewers for their valuable comments of our manuscript. We carefully consider all the comments and will provide a revised manuscript with our point-by-point responses as soon as possible. In the meantime, we will try our best to carry out additional experiments to bolster our conclusions. Here, we would like to respond provisionally to the public reviews.
We appreciate the concerns raised by Reviewer 1 regarding the identification of cell types in our study. Specifically, they noted that the high proportion of NSCs within the astroglial lineage clusters is inconsistent with classic histology studies. We apologize for not clearly specifying in the text and figure legend that the data presented in Figure 2C were obtained from neonatal samples, which may explain the higher presence …
Author Response
We are very grateful to the editors and reviewers for their valuable comments of our manuscript. We carefully consider all the comments and will provide a revised manuscript with our point-by-point responses as soon as possible. In the meantime, we will try our best to carry out additional experiments to bolster our conclusions. Here, we would like to respond provisionally to the public reviews.
We appreciate the concerns raised by Reviewer 1 regarding the identification of cell types in our study. Specifically, they noted that the high proportion of NSCs within the astroglial lineage clusters is inconsistent with classic histology studies. We apologize for not clearly specifying in the text and figure legend that the data presented in Figure 2C were obtained from neonatal samples, which may explain the higher presence of NSCs. To rectify this issue, we will revise the text to ensure clarity regarding the age group from which the data in Figure 2C were obtained. Additionally, we commit to providing additional UMAP plots and quantitative analysis separately for different age groups to support our findings. This will allow a more accurate representation of the cell type composition, taking into consideration any potential variations that may occur with age.
We appreciate Reviewer 2's acknowledgment that the finding of our study is interesting and relevant to a broader audience. However, he raised two major concerns that could weaken the conclusions drawn from our study. First, the reviewer noted that the number of sequenced nuclei in our study is lower than the calculated number required for detecting rare cell types. We noticed that according to the computational modeling conducted by Tosoni et al. (Neuron, 2023), at least 21 neuroblast cells (NBs) can be identified out of 30,000 granule cells (GCs) from a total of 180,000 dentate gyrus (DG) cells. In our dataset, we sequenced 24,671 GC nuclei and 92,966 total DG cell nuclei, which also includes neonatal samples. The number of nuclei we sequenced is 4.5 times higher than that of Wang et al. (Cell Research, 2022), who also detected NBs. Therefore, it is reasonable to conclude that we were able to detect NBs. Moreover, the presence of these rare cell types has been demonstrated in our study through immunostaining techniques, which provides further evidence. Secondly, Reviewer 2 raised concerns about the low number of donors included in some of the groups, with only one donor (n=1) being represented in certain cases. We acknowledge these limitations and understand that the inclusion of a larger number of donors would strengthen the statistical power and generalizability of our findings. However, due to the scarcity of stroke or neonatal human samples, it is not feasible to collect a larger sample size within the expected timeframe. Although one sample is not enough to show the precise changes in cells and molecular mechanisms caused by stroke, it can provide a typical example to demonstrate our hypothesis that neural stem cells could be activated under conditions of injury. The latter is what we really want to address in the manuscript. Regrading to the donor’s information, we will provide more details about the donors, including any clinical characteristics available, to enhance the transparency of our study. Importantly, we have implemented strict quality control measures to support the reliability of our sequencing data. These measures include: 1) Immediate collection of tissue samples after postmortem (3-4 hrs) to ensure the quality of isolated nuclei. 2) Only nuclei expressing more than 200 genes but fewer than 5000-8600 genes (depending on the peak of enrichment genes) were considered. On average, each cell detected around 3000 genes. 3) The average proportion of mitochondrial genes in each sample was approximately 1.8%, with no sample exceeding 5%.
-

eLife assessment
Using state-of-the-art single-nucleus RNA sequencing, Yao et al. investigate the transcriptomic features of neural stem cells (NSCs) in the human hippocampus to address how they vary across different age groups and stroke conditions. The authors report alterations in NSC subtype proportions and gene expression profiles after stroke and an exemplary gene elevated in NSCs and reactive astrocytes in stroke patients. Although the aim of the study is valuable, the significance is restricted by technical limitations and the incomplete evidence supporting the main conclusions.
-

Reviewer #1 (Public Review):
In this manuscript, Yao et al. explored the transcriptomic characteristics of neural stem cells (NSCs) in the human hippocampus and their changes under different conditions using single-nucleus RNA sequencing (snRNA-seq). They generated single-nucleus transcriptomic profiles of human hippocampal cells from neonatal, adult, and aging individuals, as well as from stroke patients. They focused on the cell groups related to neurogenesis, such as neural stem cells and their progeny. They revealed genes enriched in different NSC states and performed trajectory analysis to trace the transitions among NSC states and towards astroglial and neuronal lineages in silico. They also examined how NSCs are affected by aging and injury using their datasets and found differences in NSC numbers and gene expression patterns …
Reviewer #1 (Public Review):
In this manuscript, Yao et al. explored the transcriptomic characteristics of neural stem cells (NSCs) in the human hippocampus and their changes under different conditions using single-nucleus RNA sequencing (snRNA-seq). They generated single-nucleus transcriptomic profiles of human hippocampal cells from neonatal, adult, and aging individuals, as well as from stroke patients. They focused on the cell groups related to neurogenesis, such as neural stem cells and their progeny. They revealed genes enriched in different NSC states and performed trajectory analysis to trace the transitions among NSC states and towards astroglial and neuronal lineages in silico. They also examined how NSCs are affected by aging and injury using their datasets and found differences in NSC numbers and gene expression patterns across age groups and injury conditions. One major issue of the manuscript is questionable cell type identification. For example, in Figure 2C, more than 50% of the cells in the astroglial lineage clusters are NSCs, which is extremely high and inconsistent with classic histology studies.
-

Reviewer #2 (Public Review):
In this manuscript, Yao et al. present a series of experiments aiming at generating a cellular atlas of the human hippocampus across aging, and how it may be affected by injury, in particular, stroke. Although the aim of the study is interesting and relevant for a larger audience, due to the ongoing controversy around the existence of adult hippocampal neurogenesis in humans, a number or technical weaknesses result in poor support for many of the conclusions made from the results of these experiments.
In particular, a recent meta-analysis of five previous studies applying similar techniques to human samples has identified different aspects of sample size as main determinants of the statistical power needed to make significant conclusions. Some of these aspects are the number of nuclei sequenced and subject …
Reviewer #2 (Public Review):
In this manuscript, Yao et al. present a series of experiments aiming at generating a cellular atlas of the human hippocampus across aging, and how it may be affected by injury, in particular, stroke. Although the aim of the study is interesting and relevant for a larger audience, due to the ongoing controversy around the existence of adult hippocampal neurogenesis in humans, a number or technical weaknesses result in poor support for many of the conclusions made from the results of these experiments.
In particular, a recent meta-analysis of five previous studies applying similar techniques to human samples has identified different aspects of sample size as main determinants of the statistical power needed to make significant conclusions. Some of these aspects are the number of nuclei sequenced and subject stratification. These two aspects are of concern in Yao's study. First, the number of sequenced nuclei is lower than the calculated number of nuclei required for detecting rare cell types. However, Yao et al. report succeeding in detecting rare populations, including several types of neural stem cells in different proliferation states, which have been demonstrated to be extremely scarce by previous studies. It would be very interesting to read how the authors interpret these differences. Secondly, the number of donors included in some of the groups is extremely low (n=1) and the miscellaneous information provided about the donors is practically inexistent. As individual factors such as chronic conditions, medication, lifestyle parameters, etc... are considered determinant for the variability of adult hippocampal neurogenesis levels across individuals, this represents a series limitation of the current study. Overall, several technical weaknesses severely limit the relevance of this study and the ability of the authors to achieve their experimental aims.
-