The cation channel mechanisms of subthreshold inward depolarizing currents in the mice VTA dopaminergic neurons and their roles in the chronic-stress-induced depression-like behavior
Curation statements for this article:-
Curated by eLife
eLife assessment
This important study examined the mechanisms underlying reduced excitability of ventral tegmental area dopamine neurons in mice that underwent a chronic mild unpredictable stress treatment. The authors identify NALCN and TRPC6 channels as key mechanisms that regulate spontaneous firing of ventral tegmental area dopamine neurons and examined their roles in reduced firing in mice that underwent a chronic mild unpredictable stress treatment. The authors' conclusions on neurophysiological data are supported by multiple approaches and are convincing, although the relevance of the behavioral results to human depression remains unclear.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The slow-intrinsic-pacemaker dopaminergic (DA) neurons originating in the ventral tegmental area (VTA) are implicated in various mood- and emotion-related disorders, such as anxiety, fear, stress and depression. Abnormal activity of projection-specific VTA DA neurons is the key factor in the development of these disorders. Here, we describe the crucial role of the NALCN and TRPC6, non-selective cation channels in mediating the subthreshold inward depolarizing current and driving the firing of action potentials of VTA DA neurons in physiological conditions. Furthermore, we demonstrate that down-regulation of TRPC6 protein expression in the VTA DA neurons likely contributes to the reduced activity of projection-specific VTA DA neurons in chronic mild unpredictable stress (CMUS) depressive mice. In consistent with these, selective knockdown of TRPC6 channels in the VTA DA neurons conferred mice with depression-like behavior. This current study suggests down-regulation of TRPC6 expression/function is involved in reduced VTA DA neuron firing and chronic stress-induced depression-like behavior of mice.
Article activity feed
-

-

-

-

eLife assessment
This important study examined the mechanisms underlying reduced excitability of ventral tegmental area dopamine neurons in mice that underwent a chronic mild unpredictable stress treatment. The authors identify NALCN and TRPC6 channels as key mechanisms that regulate spontaneous firing of ventral tegmental area dopamine neurons and examined their roles in reduced firing in mice that underwent a chronic mild unpredictable stress treatment. The authors' conclusions on neurophysiological data are supported by multiple approaches and are convincing, although the relevance of the behavioral results to human depression remains unclear.
-

Reviewer #1 (Public Review):
Wang et al., present a paper aiming to identify NALCN and TRPC6 channels as key mechanisms regulating VTA dopaminergic neuron spontaneous firing and investigating whether these mechanisms are disrupted in a chronic unpredictable stress model mouse.
Major strengths:
This paper uses multiple approaches to investigate the role of NALCN and TRPC6 channels in VTA dopaminergic neurons.
-

Reviewer #2 (Public Review):
This paper describes the results of a set of complementary and convergent experiments aimed at describing roles for the non-selective cation channels NALCN and TRPC6 in mediating subthreshold inward depolarizing currents and action potential generation in VTA DA neurons under normal physiological conditions. In general, the authors have responded satisfactorily to reviewer comments, and the revised manuscript is improved.
-

Reviewer #3 (Public Review):
The authors of this study have examined which cation channels specifically confer to ventral tegmental area dopaminergic neurones their autonomic (spontaneous) firing properties. Having brought evidence for the key role played by NALCN and TRPC6 channels therein, the authors aimed at measuring whether these channels play some role in so-called depression-like (but see below) behaviors triggered by chronic exposure to different stressors. Following evidence for a down-regulation of TRPC6 protein expression in ventral tegmental area dopaminergic cells of stressed animals, the authors provide evidence through viral expression protocols for a causal link between such a down-regulation and so-called depression-like behaviors. The main strength of this study lies on a comprehensive bottom-up approach ranging from …
Reviewer #3 (Public Review):
The authors of this study have examined which cation channels specifically confer to ventral tegmental area dopaminergic neurones their autonomic (spontaneous) firing properties. Having brought evidence for the key role played by NALCN and TRPC6 channels therein, the authors aimed at measuring whether these channels play some role in so-called depression-like (but see below) behaviors triggered by chronic exposure to different stressors. Following evidence for a down-regulation of TRPC6 protein expression in ventral tegmental area dopaminergic cells of stressed animals, the authors provide evidence through viral expression protocols for a causal link between such a down-regulation and so-called depression-like behaviors. The main strength of this study lies on a comprehensive bottom-up approach ranging from patch-clamp recordings to behavioral tasks. These tasks mainly address anxiety-like behaviors and so-called depression-like behaviors (sucrose choice, forced swim test, tail suspension test). The results gathered by means of these procedures are clearcut.
-

Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
Comment 1: One of the only demonstrations of the expression and physiological significance of TRPCs in VTA DA neurons was published by (Rasmus et al., 2011; Klipec et al., 2016) which are not cited in this paper. In their study, TRPC4 expression was detected in a uniformly distributed subset of VTA DA neurons, and TRPC4 KO rats showed decreased VTA DA neuron tonic firing and deficits in cocaine reward and social behaviors. Update: The authors say they have added a discussion of these papers, but I do not see it in the updated manuscript.
We thank the reviewer for the suggestion. The discussion for this has been added (line 557-565).
Comment 2: The authors should report the results (exact data values) of female mice in the …
Author response:
The following is the authors’ response to the previous reviews.
Reviewer #1 (Public Review):
Comment 1: One of the only demonstrations of the expression and physiological significance of TRPCs in VTA DA neurons was published by (Rasmus et al., 2011; Klipec et al., 2016) which are not cited in this paper. In their study, TRPC4 expression was detected in a uniformly distributed subset of VTA DA neurons, and TRPC4 KO rats showed decreased VTA DA neuron tonic firing and deficits in cocaine reward and social behaviors. Update: The authors say they have added a discussion of these papers, but I do not see it in the updated manuscript.
We thank the reviewer for the suggestion. The discussion for this has been added (line 557-565).
Comment 2: The authors should report the results (exact data values) of female mice in the results text, or pool the male and female data if the sex differences are not significant.
We agree with reviewer. Some experiments were further redone with female and the data of male and female mice have been reported in the results of text.
Comment 3: The selectivity of drugs should be referred as "selective" rather than "specific".
Thanks, “specific” has been changed to “selective”.
Comment 4: Line 62: typo, "substantia nigra".
Thanks, “substantial nigra” has been changed to “substantia nigra” in line 65.
Comment 5: Line 77: some new studies suggest that NALCN might have voltage dependency
(rectification).
Thanks, description of NALCN voltage dependence has been corrected in line 81-83.
Comment 6: Line 175: change "less" to "fewer".
Thanks, “less” has been changed to “fewer”.
Comment 7: Line 299: choose one - "was not ... or" or "was neither ... nor".
Thanks, this error has been corrected.
Comment 8: In Figure 1Aii and Figure 3Bi, it was not specified in the results text or figure legend that C1-C5 represent individual cell until the legend for Figure 4.
Thanks, these description about gel have been added in the figure legends.
Reviewer #2 (Public Review):
Comment 1: From the previous review, we mentioned that " 'The HCN' as written in line 69 is a bit misleading, as HCN channels in the heart and brain are different members of a family of channels, although as written in the text, it seems that they are identical." This is still the case (now line 73).
We agreed with the reviewer’s comments. The introduction about HCN has been corrected (line 74-78).
Comment 2: The authors state in line 112 that "most of the experiments were also repeated in female mice" - this is true in the case of most electrophysiological experiments, although not behavioral experiments. Authors should amend the statement in line 112 and clarify in the Discussion section which findings are generalizable between sexes; e.g.:
a. Discussion of HCN contribution to VTA DA activity (beginning line 453) should clarify male mice.
b. Similarly, any discussion of behavioral findings should clarify male mice.
We agreed with the reviewer’s comments. The sexes of mice used have been noted in the results and discussion.
Comment 3: The authors' statement in lines 179-183 ("In contrast, fewer GABAergic neuronal markers (Glutamic acid decarboxylase, GAD1/2 and vesicular GABA transporter, VGAT) co-expressed with the DA neurons, which is consistent with previous studies that VTA DA neurons co-expressing GABAergic neuronal markers mainly project to the lateral habenula") is a little confusing - as stated, it seems that the authors are confirming DA/GABA coexpression in VTA-LHb neurons, which is not the case.
We agreed with the reviewer’s comments. We corrected this statement (line 182-186).
Comment 4: Additional information could be included in the Methods section description of Western Blotting procedures - e.g., what thickness of tissue and what size gauge were used to dissect VTA for these experiments?
Thanks. The description of tissue in Western Blotting procedures has been added.
Comment 5:
a. Grammatical errors in line 23 of Abstract (also lines 31-32)
b. "drove" should read "strove" in line 92
c. Grammatical errors in lines 401, 444, and 448
We thank the reviewer for pointing out grammatical errors and we corrected them.
Reviewer #3 (Public Review):
Comment 1: The main strength of this study lies on a comprehensive bottom-up approach ranging from patch-clamp recordings to behavioral tasks. These tasks mainly address anxiety-like behaviors and so-called depression-like behaviors (sucrose choice, forced swim test, tail suspension test). The results gathered by means of these procedures are clearcut. However, the reviewer believes that the authors should be more cautious when interpreting immobility responses to stress (forced swim, tail suspension) as "depression-like" responses. These stress models have been routinely used (and validated) in the past to detect the antidepressant properties of compounds under investigation, which by no means indicates that these are depression models. For readers interested by this debate, I suggest to read e.g. De Kloet and Molendijk (Biol. Pscyhiatry 2021).
We thank the reviewer for the suggestion. We will be more careful and rigorous in the selection of stress models in our subsequent research work.
Editor's note:
Should you choose to revise your manuscript, please include full statistical reporting including exact p-values wherever possible alongside the summary statistics (test statistic and df) and 95% confidence intervals. These should be reported for all key questions and not only when the p-value is less than 0.05.
We have added the full statistical reporting including exact p-values wherever possible alongside the summary statistics (test statistic and df) and 95% confidence intervals into the results and the figure legends of the revised manuscript.
-

-

Author Response
Thank you for your letter and for the reviewers’ comments concerning our manuscript entitled “The cation channel mechanisms of subthreshold inward depolarizing currents in the VTA dopaminergic neurons and their roles in the depression-like behavior”. These comments are constructive and very helpful for improving our manuscript. We have studied comments carefully and have made provisional revision which we hope meet with approval. We also respond to the reviewer’s comments point by point as following.
Reviewer #1 (Public Review):
Comment 1:
The pharmacological tools used in this study are highly non-selective. Gd3+, used here to block NALCN is actually more commonly used to block TRP channels. 2-APB inhibits not only TRPC channels, but also TRPM and IP3 receptors while stimulating TRPV channels (Bon and Beech, 2013), …
Author Response
Thank you for your letter and for the reviewers’ comments concerning our manuscript entitled “The cation channel mechanisms of subthreshold inward depolarizing currents in the VTA dopaminergic neurons and their roles in the depression-like behavior”. These comments are constructive and very helpful for improving our manuscript. We have studied comments carefully and have made provisional revision which we hope meet with approval. We also respond to the reviewer’s comments point by point as following.
Reviewer #1 (Public Review):
Comment 1:
The pharmacological tools used in this study are highly non-selective. Gd3+, used here to block NALCN is actually more commonly used to block TRP channels. 2-APB inhibits not only TRPC channels, but also TRPM and IP3 receptors while stimulating TRPV channels (Bon and Beech, 2013), while FFA actually stimulates TRPC6 channels while inhibiting other TRPCs (Foster et al., 2009).
We agree with the reviewer that the substances mentioned are not specific. Although we performed shRNA experiments against NALCN and TRPC6, we also used more specific pharmacological modulators for these two channels, L703,606 (the antagonist of NALCN)[1] and larixyl acetate (a potent TRPC6 inhibitor)[2]. The results are shown in figure 3E, F and figure 4C, E.
Comment 2:
-The multimodal approach including shRNA knockdown experiments alleviates much of the concern about the non-specific pharmacological agents. Therefore, the author's claim that NALCN is involved in VTA dopaminergic neuron pacemaking is well-supported.
-However, the claim that TRPC6 is the key TRPC channel in VTA spontaneous firing is somewhat, but not completely supported. As with NALCN above, the pharmacology alone is much too non-specific to support the claim that TRPC6 is the TRP channel responsible for pacemaking. However, unlike the NALCN condition, there is an issue with interpreting the shRNA knockdown experiments. The issue is that TRPC channels often form heteromers with TRPC channels of other types (Goel, Sinkins and Schilling, 2002; Strübing et al., 2003). Therefore, it is possible that knocking down TRPC6 is interfering with the normal function of another TRPC channel, such as TRPC7 or TRPC4.
From our single-cell RNA-seq results, TRPC7 and TRPC4 are found not to be present broadly like TRPC6 in the VTA DA neurons. And in experiments using single cell PCR (sFig. 9A), only a very small proportion of TRPC6-positive DA cells (DAT+) expressed TRPC4 (sFig. 9Bi) or TRPC7 (sFig. 9Bii), in consistent with the results of single-cell RNA-seq (Fig.2). Therefore, it is possible that knocking down TRPC6 maybe not interfering with the normal function of another TRPC channel, such as TRPC7 or TRPC4.
Comment 3:
The claim that TRPC6 channels in the VTA are involved in the depressive-like symptoms of CMUS is supported.
- However, the connection between the mPFC-projecting VTA neurons, TRPC6 channels, and the chronic unpredictable stress model (CMUS) of depression is not well supported. In Figure 2, it appears that the mPFC-projecting VTA neurons have very low TRPC6 expression compared to VTA neurons projecting to other targets. However, in figure 6, the authors focus on the mPFC-projecting neurons in their CMUS model and show that it is these neurons that are no longer sensitive to pharmacological agents non-specifically blocking TRPC channels (2-APB, see above comment). Finally, in figure 7, the authors show that shRNA knockdown of TRPC6 channels (in all VTA dopaminergic neurons) results in depressive-like symptoms in CMUS mice. Due to the low expression of TRPC6 in mPFC-projecting VTA neurons, the author's claims of "broad and strong expression of TRPC6 channels across VTA DA neurons" is not fully supported. Because of the messy pharmacological tools used, it cannot be clamed that TRPC6 in the mPFC-projecting VTA neurons is altered after CMUS. And because the knockdown experiments are not specific to mPFC-projecting VTA neurons, it cannot be claimed that reducing TRPC6 in these specific neurons is causing depressive symptoms.
The reason we focused on the mPFC-projecting VTA DA neurons is that this pathway is indicated in depressive-like behaviors of the CMUS model[3-5]. Although mPFC-projecting VTA DA neurons seem have lower level of TRPC6, we reason they are still functional there. However, we do agree with the reviewer that the statement “broad and strong expression of TRPC6 channels across VTA DA neurons" is not fully supported. We have changed the statements based on the reviewer suggestion. Furthermore, we did selectively knockdown TRPC6 in the mPFC-projecting VTA DA neurons, and then studied the behavior (Fig.8).
Comment 4:
It is important to note that the experiments presented in Figure 1 have all been previously performed in VTA dopaminergic neurons (Khaliq and Bean, 2010) including showing that low calcium increases VTA neuron spontaneous firing frequency and that replacement of sodium with NMDG hyperpolarizes the membrane potential.
We agree with reviewer that similar experiments have been performed previously [6] for the flow of our manuscript and for general readers.
Comment 5:
-The authors explanation for the increase in firing frequency in 0 calcium conditions is that calcium-activated potassium channels would no longer be activated. However, there is a highly relevant finding that low calcium enhances the NALCN conductance through the calcium sensing receptor from Dejian Ren's lab (Lu et al., 2010) which is not cited in this paper. This increase in NALCN conductance with low calcium has been shown in SNc dopaminergic neurons (Philippart and Khaliq, 2018), and is likely a factor contributing to the low-calcium-mediated increase in spontaneous VTA neuron firing.
We agree with the reviewer and thanks for the suggestions. A discussion for this has been added.
Comment 6:
-One of the only demonstrations of the expression and physiological significance of TRPCs in VTA DA neurons was published by (Rasmus et al., 2011; Klipec et al., 2016) which are not cited in this paper. In their study, TRPC4 expression was detected in a uniformly distributed subset of VTA DA neurons, and TRPC4 KO rats showed decreased VTA DA neuron tonic firing and deficits in cocaine reward and social behaviors.
We thank the reviewer for the suggestion. The references and a discussion for this has been added.
Comment 7:
- Out of all seven TRPCs, TRPC5 is the only one reported to have basal/constitutive activity in heterologous expression systems (Schaefer et al., 2000; Jeon et al., 2012). Others TRPCs such as TRPC6 are typically activated by Gq-coupled GPCRs. Why would TRPC6 be spontaneously/constitutively active in VTA DA neurons?
In a complex neuronal environment where VTA DA neurons are located, multiple modulatory factors including the GPCRs could be dynamically active, this could lead to the activation of TRP channels including TRPC6.
Comment 8:
A new paper from the group of Myoung Kyu Park (Hahn et al., 2023) shows in great detail the interactions between NALCN and TRPC3 channels in pacemaking of SNc DA neurons.
The reference mentioned has been added. We thank the reviewer.
Reviewer #2 (Public Review):
Comment 1:
These results do not show that TRPC6 mediates stress effects on depression-like behavior. As stated by the authors in the first sentence of the final paragraph, "downregulation of TRPC6 proteins was correlated with reduced firing activity of the VTA DA neurons, the depression-like behaviors, and that knocking down of TRPC6 in the VTA DA neurons confer the mice with depression behaviors." Therefore, the results show associations between TRPC6 downregulation and stress effects on behavior, occlusion of the effects of one by the other on some outcome measures, and cell manipulation effects that resemble stress effects. There is no experiment that shows reversal of stress effects with cell/circuit-specific TRPC6 manipulations. Please adjust the title, abstract and interpretation accordingly.
We agree with the reviewer’s suggestion. The title was changed to ‘’The cation channel mechanisms of subthreshold inward depolarizing currents in the VTA dopaminergic neurons and their roles in the chronic stress-induced depression-like behavior” and the abstract and interpretation were also adjusted accordingly.
Comment 2:
Statistical tests and results are unclear throughout. For all analyses, please report specific tests used, factors/groups, test statistic and p-value for all data analyses reported. In some cases, the chosen test is not appropriate. For example, in Figure 6E, it is not clear how an experiment with 2 factors (stress and drug) can be analyzed with a 1-way RM ANOVA. The potential impact of inappropriate statistical tests on results makes it difficult to assess the accuracy of data interpretation.
We have redone the statistical analysis as suggested by the reviewer and added specific tests used, factors/groups, test statistic and p-value for all data analyses into the figure legends of the revised manuscript.
Comment 3:
Why were only male mice used? Please justify and discuss in the manuscript. Also, change the title to reflect this.
Although most similar previous studies used male mice or rats[7, 8], we do agree with the reviewer that the female animals should also be tested, in consideration possible role of sex hormones, as such we repeated some key experiments on female mice (sFig.1.6.8. and 13).
Comment 4:
Number of recorded cells is very low in Figure 1. Where in VTA did recordings occur? Given the heterogeneity in this brain region, this n may be insufficient. Additional information (e.g., location within VTA, criteria used to identify neurons) should be included. Report the number of mice (i.e., n = 6 cells from X mice) in all figures.
Yes indeed, the number here is not high. More experiments were performed to increase the N/n number. And the location of recorded cells in VTA and the number of used mice is now shown in all figures; criteria to identify neurons is stated in the Methods-Identification of DA neurons and electrophysiological recordings. At the end of electrophysiological recordings, the recorded VTA neurons were collected for single-cell PCR. VTA DA neurons were identified by single-cell PCR for the presence of TH and DAT.
Comment 5:
Authors refer to VTA DA neurons as those that are DAT+ in line 276, although TH expression is considered the standard of DAergic identity, and studies (e.g., Lammel et al, 2008) have shown that a subset of VTA DA neurons have low levels of DAT expression. Authors should reword/clarify that these are DAT-expressing VTA DA neurons.
The study published by Lammel[9] in 2015 has shown the low dopamine specificity of transgene expression in ventral midbrain of TH-Cre mice; on the other hand, DAT-Cre mice exhibit dopamine-specific Cre expression patterns, although DAT-Cre mice are likely to suffer from their own limitations (for example, low DAT expression in mesocortical DA neurons may make it difficult to target this subpopulation, see Lammel et al., 2008[10]).Hence, in our study, the DAT was used as criteria to identify DAT neurons. Of course, TH and DAT were all tested in single-cell PCR to identify whether the recorded cells were DA neurons.
Comment 6:
Neuronal subtype proportions should be quantified and reported (Fig. 1Aii).
Neuronal subtype proportions are now quantified and reported in Fig. 1Aii.
Comment 7:
In addition to reporting projection specificity of neurons expressing specific channels, it would be ideal to report these data according to spatial location in VTA.
The spatial location of recorded cells in VTA are now shown in all figures.
Comment 8:
The authors state that there are a small number of Glut neurons in VTA, then they state that a "significant proportion" of VTA neurons are glutamatergic.
Thanks, “a significant proportion of neurons” has been changed to “less than half of sequenced DA neurons”.
Comment 9:
It is an overstatement that VTA DA neurons are the key determinant of abnormal behaviors in affective disorders.
Thanks, we have amended the statement to that “Dopaminergic (DA) neurons in the ventral tegmental area (VTA) play an important role in mood, reward and emotion-related behaviors”.
Reviewer #3 (Public Review):
Comment 1:
The authors of this study have examined which cation channels specifically confer to ventral tegmental area dopaminergic neurons their autonomic (spontaneous) firing properties. Having brought evidence for the key role played by NALCN and TRPC6 channels therein, the authors aimed at measuring whether these channels play some role in so-called depression-like (but see below) behaviors triggered by chronic exposure to different stressors. Following evidence for a down-regulation of TRPC6 protein expression in ventral tegmental area dopaminergic cells of stressed animals, the authors provide evidence through viral expression protocols for a causal link between such a down-regulation and so-called depression-like behaviors. The main strength of this study lies on a comprehensive bottom-up approach ranging from patch-clamp recordings to behavioral tasks. However, the interpretation of the results gathered from these behavioral tasks might also be considered one main weakness of the abovementioned approach. Thus, the authors make a confusion (widely observed in numerous publications) with regard to the use of paradigms (forced swim test, tail suspension test) initially aimed (and hence validated) at detecting the antidepressant effects of drugs and which by no means provide clues on "depression" in their subjects. Indeed, in their hands, the authors report that stress elicits changes in these tests which are opposed to those theoretically seen after antidepressant medication. However, these results do not imply that these changes reflect "depression" but rather that the individuals under scrutiny simply show different responses from those seen in nonstressed animals. These limits are even more valid in nonstressed animals injected with TRPC6 shRNAs (how can 5-min tests be compared to a complex and chronic pathological state such as depression?). With regard to anxiety, as investigated with the elevated plus-maze and the open field, the data, as reported, do not allow to check the author's interpretation as anxiety indices are either not correctly provided (e.g. absolute open arm data instead of percents of open arm visits without mention of closed arm behaviors) or subjected to possible biases (lack of distinction between central and peripheral components of the apparatus).
We agree with the reviewer that behavior tests we used here is debatable whether they represent a real depression state, and this is an open question that could be discussed from different respective. Since these testes (forced swimming and tail suspension), as the reviewer noted, were “widely observed in numerous publications”, we used these seemly only options to reflect a “depression-like” state. One could argue that since these testes were initially used for testing antidepressants (“validated”), with decreased immobility time as indications of anti-depressive effects, why not an increased immobility time reflect a “depression-like” state. As for anxiety tests, the data concerning the elevated plus-maze are also changed based on the reviewer’s suggestion.
Recommendations for the authors: please note that you control which, if any, revisions, to undertake
Reviewer #1 (Recommendations For The Authors):
Recommendation 1 for improving the paper:
-The paper needs extensive editing for both overall structural clarity and for the high number of typos and grammatical errors.
We thank the reviewer’s suggestion. The revised manuscript has been edited extensively.
Recommendation 2 for improving the paper:
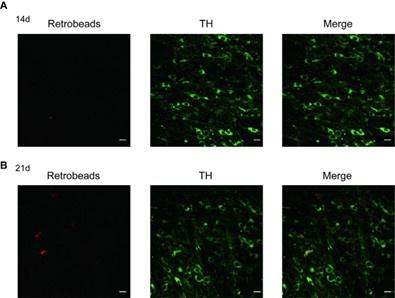
-Retrobeads are often toxic to cells and build up with increasing time. It is surprising that the authors wait 14-21 days for retrobead expression in their target cells. It is also a problem that the mPFC projecting cells have a longer time with the retrobeads than the other projection-targeting cells because the toxicity could be more extensive with the longer wait time thus confounding the results. The authors should repeat some mPFC experiments at the 14 day time point to confirm that the longer time with the beads is not influencing the differential effects in these cells.
According to the methods published by Stephan Lammel and Jochen Roeper, “For sufficient labeling, survival periods for retrograde tracer transport depended on respective injection areas: DS and NAc lateral shell, 7 days; NAc core, NAc medial shell, and BLA, 14 days; and mPFC, 21 days[10]”, we did the experiments related to mPFC projecting cells at the 21 day time point. Consistent with the mentioned above, the labeled mPFC projecting cells at 14 day time point, is not sufficient, compared with this at 21 day time point, which is shown as followings.
Author response image 1.
Confocal images showing the anatomical distribution of mPFC-projecting DA neurons labelled with retrobeads (red) in the VTA after DAT-immunofluorescence (green) staining at different day time point (A, 14d; B, 21d) after retrobeads injection; Scale bars=10 μm.
Recommendation 3 for improving the paper:
-The experiment with FFA in Figure 4E seems weird. Why is there no baseline before the FFA application? And why is the baseline trending downward immediately? The authors should explain why this example experiment is presented differently from all the others.
We apologize for this part that this example time-course is not typical. Since the FFA is not specific antagonist for TRPC6 and actually stimulates TRPC6 channels, we repeated the experiments with a more specific pharmacological modulator for TRPC6, larixyl acetate (LA), and the results are shown in Figure 4C and 4F.
Recommendation 4 for improving the paper:
-It would be much more useful to see exact p values in the text, as it aids in interpreting the 'insignificance' of specific comparisons. Specifically, in Figure 5F, the 2-APB looks like it is having a small effect, and the already low firing rate (due to the TRPC6 knockdown) makes a big effect less likely. It would be useful to know what the actual p value is here (and everywhere).
OK. We now report all P values in the figure legends of the revised version.
Recommendation 5 for improving the paper:
-In the results, it should be explained that the "RMP" of VTA DA neurons was obtained by treating the cells with TTX.
A sentence indicating the presence of TTX when measuring “RMP” is added in the Results part of the revised version.
Recommendation 6 for improving the paper:
-The spacing of the panels in the figures is somewhat odd. The figures could be more compact.
Thanks, we have re-arranged all figures.
Recommendation 7 for improving the paper:
The paper is difficult to read because of significant grammatical errors. Here are some examples by line number, but this list is not at all exhaustive.
We thank the reviewer for pointing out grammatical errors and we corrected them.
Reviewer #2 (Recommendations For The Authors):
Recommendation 1 for improving the paper:
Fix typos: e.g., change HCH to HCN, change EMP to EPM, "these finding", "compact par" should read "pars compacta", "substantial" in line 475 should read "substantia", Incomplete sentences on line 73 and line 107, etc. Also, what is meant by "autonomic" firing activity? What is meant by "expression files"? Change "depression behaviors" to depression-like behaviors. "The HCN" as written in line 69 is a bit misleading, as HCN channels in the heart and brain are different members of a family of channels, although as written in the text, it seems that they are identical. In Figure 2, rearrange order of brain regions (e.g., from "BLA-VTA" to "VTA-BLA"), because as written, it seems that the focus is on projections into the VTA from each brain region, rather than VTA neurons that project to each respective region.
We thank the reviewer for pointing out these errors and we corrected them. Autonomic firing activity has been changed to spontaneous firing activity. Expression files has been changed to expression levels. All the “depression behavior” have been changed to depression-like behaviors. In the Figure 2, all “xx-VTA” have been changed to “VTA-xx”.
Reviewer #3 (Recommendations For The Authors):
Recommendation 1 for improving the paper:
Methodology: as opposed to sFig. 8 where the order through which mice were repeatedly tested is precise, such a key information is lacking in Fig. 6 as well as in the Methods section (for example, when such traumatic stress as forced swimming is performed with regard to the other tests?). Relevant to this point is the possible bias triggered by such chronological testing as exposure to the forced swim test likely affects the behaviors recorded in the other tests. Furthermore, the way this test is conducted is appealing as it is mentioned that the water depth was set to 10 cms which is quite low given that immobility scores might be affected by the ability of mice to stand on their tails.
With regard to the elevated plus-maze, data are erroneously provided. Absolute values regarding open arm behaviors should be provided as percentages of the number of visits (or time spent therein) over the total (open + closed) number of arm visits. Indeed, closed arm visits should also be provided. This variable, also considered an index of locomotor activity, would allow the reader to exclude any effect of locomotion on the exploration in the open field.
As they stand, data in the open field seem to indicate parallel changes at the center(center time) and the periphery (total distance), hence suggesting locomotor effects rather than anxiogenic effects. Data related to the center and the periphery should be clearly distinguished. Lastly, the number of weeks allowed for the mice to recover from surgeries aimed at delivering viruses are not mentioned. This is important as it could have affected the amplitude of the sensitivity to the stressors.
We thank the reviewer for the suggestion. The lack information in Figure 6 and the Methods is now supplied. We apologize for the wrong number of “10 cm” in the forced swimming test, this has been corrected. The data concerning the elevated plus-maze are also changed based on the reviewer’s suggestion. For a possible role of locomotor effect, we tested the mice on the rota-rod test. From the result, there is no difference in locomotor activity between control and depressed-like mice (sFig.10G, sFig.12I and sFig.13G). We modified the experimental procedure timeline in Figure 6 and in the method- AAV for gene knockdown or overexpression and viral construct and injection, we added “Mice were singly housed with enough food and water to recover for 4-5 weeks after injection of virus, before behavior tests and electrophysiological recordings.” to report the number of weeks allowed for the mice to recover from surgeries aimed at delivering virus.
Recommendation 2 for improving the paper:
Results/conclusions: as yet mentioned, the authors make a confusion in the interpretation of their tail suspension tests and forced swimming tests. I acknowledge that such a confusion is frequent but it is important to note that the tests used by the authors were INITIALLY aimed at detecting the antidepressant effects of drugs under investigation. However, it is not because a test reveals such antidepressant properties that they also provide indices of depression. The authors will surely agree that it is unlikely that a 5-min test provides a model of a chronic pathology accounted for by a complex intrication between genetics and environmental factors. I would propose the authors to read for example Molendijk and De Kloet (Eur J Neurosci 2022). I think that the authors should just neutrally mention their results without any interpretation related to depression. On the other hand, what could have been interesting is to test whether the so-called "depressive-like" responses recorded in the study were sensitive to chronic antidepressant treatments. This would have allowed the authors to further suggest some relevance (if any) with depression-like pathologies.
As we discussed above, we again agree with the reviewer’s concern. However, if as stated by the reviewer that “However, it is not because a test reveals such antidepressant properties that they also provide indices of depression”, then the experiments suggested by the reviewer “….. to test whether the so-called "depressive-like" responses recorded in the study were sensitive to chronic antidepressant treatments”
Recommendation 3 for improving the paper:
A close examination of the responses to CMUS or chronic restraint suggests that indeed two populations of animals were detected, possibly sensitive and resilient to these stressors. Did the authors try to examine this possibility?
Based on the results of behavior test in CMUS and CRS, animals might be divided into two populations of animals highly-sensitive and moderately-sensitive ones.
Recommendation 4 for improving the paper:
There are some text changes that need to be performed:
Page 2 line 46: ref 4 uses a social stress model which brings no clearcut evidence for it being a "depression" model. Indeed, this model can also be suggested to be a model of chronic anxiety (Kalueff et al., Science 2006; Chaouloff, Cell tissue Res 2013), hence indicating that VTA dopaminergic neurons might also be involved in anxiety.
page 11, line 329: the references supporting the hypothesis that VTA DA neurons are linked to depression cannot be found in the reference list (10-15 do not correspond to the appropriate references).
page 11, line 3341: reference 47 does not fit with the authors' assertion as it did not include any behavior.
Fig. S8: body weight data are likely provided as changes rather than absolute values (e.g. 8 g)
We agreed with the reviewer’s comments. The line 46“……such as depression states” has been changed to “such as depression- or anxiety-related states”. And we corrected the references in line 329 and 341. Finally, the body weight has been changed to the change in body weight.
References:
Um, K.B., et al., TRPC3 and NALCN channels drive pacemaking in substantia nigra dopaminergic neurons. Elife, 2021. 10.
Urban, N., et al., Identification and Validation of Larixyl Acetate as a Potent TRPC6 Inhibitor. Mol Pharmacol, 2016. 89(1): p. 197-213.
Zhong, P., et al., HCN2 channels in the ventral tegmental area regulate behavioral responses to chronic stress. Elife, 2018. 7.
Liu, D., et al., Brain-derived neurotrophic factor-mediated projection-specific regulation of depressive-like and nociceptive behaviors in the mesolimbic reward circuitry. Pain, 2018. 159(1): p. 175.
Walsh, J.J. and M.H. Han, The Heterogeneity of Ventral Tegmental Area Neurons: Projection Functions in a Mood-Related Context. Neuroscience, 2014. 282: p. 101-108.
Khaliq, Z.M. and B.P. Bean, Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci, 2010. 30(21): p. 7401-13.
Li, L., et al., Selective targeting of M-type potassium K(v) 7.4 channels demonstrates their key role in the regulation of dopaminergic neuronal excitability and depression-like behaviour. Br J Pharmacol, 2017. 174(23): p. 4277-4294.
Friedman, A.K., et al., Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science, 2014. 344(6181): p. 313-9.
Lammel, S., et al., Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron, 2015. 85(2): p. 429-38.
Lammel, S., et al., Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron, 2008. 57(5): p. 760-73.
-

eLife assessment
This important study examined the mechanisms underlying reduced excitability of ventral tegmental area dopamine neurons in mice that underwent a chronic mild unpredictable stress treatment. The authors identify NALCN and TRPC6 channels as key mechanisms that regulate spontaneous firing of ventral tegmental area dopamine neurons and examined their roles in reduced firing in mice that underwent a chronic mild unpredictable stress treatment. The authors' conclusions on neurophysiological data are supported by multiple approaches and convincing, although the relevance of the behavioral results to human depression remains unclear.
-

Reviewer #1 (Public Review):
Wang et al., present a paper aiming to identify NALCN and TRPC6 channels as key mechanisms regulating VTA dopaminergic neuron spontaneous firing and investigating whether these mechanisms are disrupted in a chronic unpredictable stress model mouse.
Major strengths:
This paper uses multiple approaches to investigate the role of NALCN and TRPC6 channels in VTA dopaminergic neurons.
Major weaknesses:
In this revision, the authors have addressed the concerns about non-selective pharmacological tools.Are the author's claims supported by the data?
The multimodal approach including shRNA knockdown experiments alleviates much of the concern about the non-specific pharmacological agents. Therefore, the author's claim that NALCN is involved in VTA dopaminergic neuron pacemaking is well-supported.
The claim that TRPC6 …
Reviewer #1 (Public Review):
Wang et al., present a paper aiming to identify NALCN and TRPC6 channels as key mechanisms regulating VTA dopaminergic neuron spontaneous firing and investigating whether these mechanisms are disrupted in a chronic unpredictable stress model mouse.
Major strengths:
This paper uses multiple approaches to investigate the role of NALCN and TRPC6 channels in VTA dopaminergic neurons.
Major weaknesses:
In this revision, the authors have addressed the concerns about non-selective pharmacological tools.Are the author's claims supported by the data?
The multimodal approach including shRNA knockdown experiments alleviates much of the concern about the non-specific pharmacological agents. Therefore, the author's claim that NALCN is involved in VTA dopaminergic neuron pacemaking is well-supported.
The claim that TRPC6 channels in the VTA are involved in the depressive-like symptoms of CMUS is supported.
Impact:
It is important to compare pacemaking mechanisms in VTA and SNc neurons and this paper convincingly shows that NALCN contributes to VTA pacemaking, as it is known to contribute to SNc pacemaking. It also shows that TRPC6 channels in VTA dopamine neurons contribute to the depressive-like symptoms associated with CMUS.
Additional context:
One of the only demonstrations of the expression and physiological significance of TRPCs in VTA DA neurons was published by (Rasmus et al., 2011; Klipec et al., 2016) which are not cited in this paper. In their study, TRPC4 expression was detected in a uniformly distributed subset of VTA DA neurons, and TRPC4 KO rats showed decreased VTA DA neuron tonic firing and deficits in cocaine reward and social behaviors.
Update: The authors say they have added a discussion of these papers, but I do not see it in the updated manuscript.
-

Reviewer #2 (Public Review):
This paper describes the results of a set of complementary and convergent experiments aimed at describing roles for the non-selective cation channels NALCN and TRPC6 in mediating subthreshold inward depolarizing currents and action potential generation in VTA DA neurons under normal physiological conditions. In general, the authors have responded satisfactorily to reviewer comments, and the revised manuscript is improved. The manuscript could still benefit from additional revision, including the following:
1. From the previous review, we mentioned that " 'The HCN' as written in line 69 is a bit misleading, as HCN channels in the heart and brain are different members of a family of channels, although as written in the text, it seems that they are identical." This is still the case (now line 73).
2. The …
Reviewer #2 (Public Review):
This paper describes the results of a set of complementary and convergent experiments aimed at describing roles for the non-selective cation channels NALCN and TRPC6 in mediating subthreshold inward depolarizing currents and action potential generation in VTA DA neurons under normal physiological conditions. In general, the authors have responded satisfactorily to reviewer comments, and the revised manuscript is improved. The manuscript could still benefit from additional revision, including the following:
1. From the previous review, we mentioned that " 'The HCN' as written in line 69 is a bit misleading, as HCN channels in the heart and brain are different members of a family of channels, although as written in the text, it seems that they are identical." This is still the case (now line 73).
2. The authors state in line 112 that "most of the experiments were also repeated in female mice" - this is true in the case of most electrophysiological experiments, although not behavioral experiments. Authors should amend the statement in line 112 and clarify in the Discussion section which findings are generalizable between sexes; e.g.:
a. Discussion of HCN contribution to VTA DA activity (beginning line 453) should clarify male mice.
b. Similarly, any discussion of behavioral findings should clarify male mice.3. The authors' statement in lines 179-183 ("In contrast, fewer GABAergic neuronal markers (Glutamic acid decarboxylase, GAD1/2 and vesicular GABA transporter, VGAT) co-expressed with the DA neurons, which is consistent with previous studies that VTA DA neurons co-expressing GABAergic neuronal markers mainly project to the lateral habenula") is a little confusing - as stated, it seems that the authors are confirming DA/GABA coexpression in VTA-LHb neurons, which is not the case.
4. Additional information could be included in the Methods section description of Western Blotting procedures - e.g., what thickness of tissue and what size gauge were used to dissect VTA for these experiments?
-

Reviewer #3 (Public Review):
The authors of this study have examined which cation channels specifically confer to ventral tegmental area dopaminergic neurones their autonomic (spontaneous) firing properties. Having brought evidence for the key role played by NALCN and TRPC6 channels therein, the authors aimed at measuring whether these channels play some role in so-called depression-like (but see below) behaviors triggered by chronic exposure to different stressors. Following evidence for a down-regulation of TRPC6 protein expression in ventral tegmental area dopaminergic cells of stressed animals, the authors provide evidence through viral expression protocols for a causal link between such a down-regulation and so-called depression-like behaviors. The main strength of this study lies on a comprehensive bottom-up approach ranging from …
Reviewer #3 (Public Review):
The authors of this study have examined which cation channels specifically confer to ventral tegmental area dopaminergic neurones their autonomic (spontaneous) firing properties. Having brought evidence for the key role played by NALCN and TRPC6 channels therein, the authors aimed at measuring whether these channels play some role in so-called depression-like (but see below) behaviors triggered by chronic exposure to different stressors. Following evidence for a down-regulation of TRPC6 protein expression in ventral tegmental area dopaminergic cells of stressed animals, the authors provide evidence through viral expression protocols for a causal link between such a down-regulation and so-called depression-like behaviors. The main strength of this study lies on a comprehensive bottom-up approach ranging from patch-clamp recordings to behavioral tasks. These tasks mainly address anxiety-like behaviors and so-called depression-like behaviors (sucrose choice, forced swim test, tail suspension test). The results gathered by means of these procedures are clearcut. However, the reviewer believes that the authors should be more cautious when interpreting immobility responses to stress (forced swim, tail suspension) as "depression-like" responses. These stress models have been routinely used (and validated) in the past to detect the antidepressant properties of compounds under investigation, which by no means indicates that these are depression models. For readers interested by this debate, I suggest to read e.g. De Kloet and Molendijk (Biol. Pscyhiatry 2021).
-

-

Author Response
Reviewer #1 (Public Review):
Comment 1:
The pharmacological tools used in this study are highly non-selective. Gd3+, used here to block NALCN is actually more commonly used to block TRP channels. 2-APB inhibits not only TRPC channels, but also TRPM and IP3 receptors while stimulating TRPV channels (Bon and Beech, 2013), while FFA actually stimulates TRPC6 channels while inhibiting other TRPCs (Foster et al., 2009).
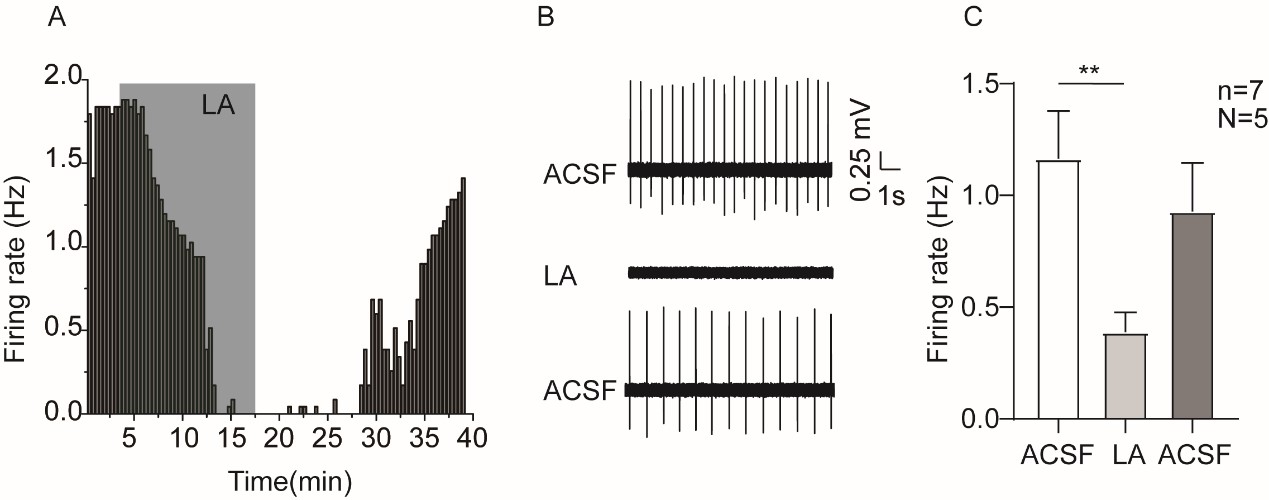
We agree with the reviewer that the substances mentioned are not specific. Although we performed shRNA experiments against NALCN and TRPC6, we do plan to use more specific pharmacological modulators for these two channels; for this, L703,606 (the antagonist of NALCN) [1] and larixyl acetate (a potent TRPC6 inhibitor) [2] will be used. Actually, we have completed experiments of using larixyl acetate and …
Author Response
Reviewer #1 (Public Review):
Comment 1:
The pharmacological tools used in this study are highly non-selective. Gd3+, used here to block NALCN is actually more commonly used to block TRP channels. 2-APB inhibits not only TRPC channels, but also TRPM and IP3 receptors while stimulating TRPV channels (Bon and Beech, 2013), while FFA actually stimulates TRPC6 channels while inhibiting other TRPCs (Foster et al., 2009).
We agree with the reviewer that the substances mentioned are not specific. Although we performed shRNA experiments against NALCN and TRPC6, we do plan to use more specific pharmacological modulators for these two channels; for this, L703,606 (the antagonist of NALCN) [1] and larixyl acetate (a potent TRPC6 inhibitor) [2] will be used. Actually, we have completed experiments of using larixyl acetate and the results are shown in Author response image 1.
Author response image 1.
Example time-course (A), traces (B) and the summaried data (C) for the effect of larixyl acetate (LA), the antagonist of TRPC6 channel, on the spontaneous firing activity of VTA DA neurons. Paired-sample T test, ** P < 0.01. n is number of neurons recorded and N is number of mice used
Comment 2:
The multimodal approach including shRNA knockdown experiments alleviates much of the concern about the non-specific pharmacological agents. Therefore, the author's claim that NALCN is involved in VTA dopaminergic neuron pacemaking is well-supported.
However, the claim that TRPC6 is the key TRPC channel in VTA spontaneous firing is somewhat, but not completely supported. As with NALCN above, the pharmacology alone is much too non-specific to support the claim that TRPC6 is the TRP channel responsible for pacemaking. However, unlike the NALCN condition, there is an issue with interpreting the shRNA knockdown experiments. The issue is that TRPC channels often form heteromers with TRPC channels of other types (Goel, Sinkins and Schilling, 2002; Strübing et al., 2003). Therefore, it is possible that knocking down TRPC6 is interfering with the normal function of another TRPC channel, such as TRPC7 or TRPC4.
According with your advice, we plan to perform single-cell qPCR experiments to check the expression level of other TRPC channels, after selective knockdown of TRPC6 in VTA DAT+ neurons, results will be shown later in the revised version. From our single-cell RNA-seq results, TRPC7 and TRPC4 are found not to be present broadly like TRPC6 in the VTA DA neurons, therefore it is possible that knocking down TRPC6 maybe not interfering with the normal function of another TRPC channel, such as TRPC7 or TRPC4.
Comment 3:
The claim that TRPC6 channels in the VTA are involved in the depressive-like symptoms of CMUS is supported.
However, the connection between the mPFC-projecting VTA neurons, TRPC6 channels, and the chronic unpredictable stress model (CMUS) of depression is not well supported. In Figure 2, it appears that the mPFC-projecting VTA neurons have very low TRPC6 expression compared to VTA neurons projecting to other targets. However, in figure 6, the authors focus on the mPFC-projecting neurons in their CMUS model and show that it is these neurons that are no longer sensitive to pharmacological agents non-specifically blocking TRPC channels (2-APB, see above comment). Finally, in figure 7, the authors show that shRNA knockdown of TRPC6 channels (in all VTA dopaminergic neurons) results in depressive-like symptoms in CMUS mice. Due to the low expression of TRPC6 in mPFC-projecting VTA neurons, the author's claims of "broad and strong expression of TRPC6 channels across VTA DA neurons" is not fully supported. Because of the messy pharmacological tools used, it cannot be clamed that TRPC6 in the mPFC-projecting VTA neurons is altered after CMUS. And because the knockdown experiments are not specific to mPFC-projecting VTA neurons, it cannot be claimed that reducing TRPC6 in these specific neurons is causing depressive symptoms.
The reason we focused on the mPFC-projecting VTA DA neurons is that this pathway is indicated in depressive-like behaviors of the CMUS model[3-5]. Although mPFC-projecting VTA DA neurons seem have lower level of TRPC6, we reason they are still functional there. However, we do agree with the reviewer that the statement “broad and strong expression of TRPC6 channels across VTA DA neurons" is not fully supported. We have changed the statements based on the reviewer suggestion. Furthermore, we also plan to selectively knockdown TRPC6 in the mPFC-projecting VTA DA neurons, and then study the behavior.
Comment 4:
It is important to note that the experiments presented in Figure 1 have all been previously performed in VTA dopaminergic neurons (Khaliq and Bean, 2010) including showing that low calcium increases VTA neuron spontaneous firing frequency and that replacement of sodium with NMDG hyperpolarizes the membrane potential.
We agree with reviewer that similar experiments have been performed previously [6]for the flow of our manuscript and for general readers.
Comment 5:
The authors explanation for the increase in firing frequency in 0 calcium conditions is that calcium-activated potassium channels would no longer be activated. However, there is a highly relevant finding that low calcium enhances the NALCN conductance through the calcium sensing receptor from Dejian Ren's lab (Lu et al., 2010) which is not cited in this paper. This increase in NALCN conductance with low calcium has been shown in SNc dopaminergic neurons (Philippart and Khaliq, 2018), and is likely a factor contributing to the low-calcium-mediated increase in spontaneous VTA neuron firing.
We agree with the reviewer and thanks for the suggestions. A discussion for this has been added.
Comment 6:
One of the only demonstrations of the expression and physiological significance of TRPCs in VTA DA neurons was published by (Rasmus et al., 2011; Klipec et al., 2016) which are not cited in this paper. In their study, TRPC4 expression was detected in a uniformly distributed subset of VTA DA neurons, and TRPC4 KO rats showed decreased VTA DA neuron tonic firing and deficits in cocaine reward and social behaviors.
We thank the reviewer for the suggestion.The references and a discussion for this has been added.
Comment 7:
Out of all seven TRPCs, TRPC5 is the only one reported to have basal/constitutive activity in heterologous expression systems (Schaefer et al., 2000; Jeon et al., 2012). Others TRPCs such as TRPC6 are typically activated by Gq-coupled GPCRs. Why would TRPC6 be spontaneously/constitutively active in VTA DA neurons?
In a complex neuronal environment where VTA DA neurons are located, multiple modulatory factors including the GPCRs could be dynamically active, this could lead to the activation of TRP channels including TRPC6.
Comment 8:
A new paper from the group of Myoung Kyu Park (Hahn et al., 2023) shows in great detail the interactions between NALCN and TRPC3 channels in pacemaking of SNc DA neurons.
The reference mentioned has been added. We thank the reviewer.
Reviewer #2 (Public Review):
Comment 1:
These results do not show that TRPC6 mediates stress effects on depression-like behavior. As stated by the authors in the first sentence of the final paragraph, "downregulation of TRPC6 proteins was correlated with reduced firing activity of the VTA DA neurons, the depression-like behaviors, and that knocking down of TRPC6 in the VTA DA neurons confer the mice with depression behaviors." Therefore, the results show associations between TRPC6 downregulation and stress effects on behavior, occlusion of the effects of one by the other on some outcome measures, and cell manipulation effects that resemble stress effects. There is no experiment that shows reversal of stress effects with cell/circuit-specific TRPC6 manipulations. Please adjust the title, abstract and interpretation accordingly.
We agree with the reviewer’s suggestion. The title was changed to ‘’The cation channel mechanisms of subthreshold inward depolarizing currents in the VTA dopaminergic neurons and their roles in the chronic stress-induced depression-like behavior” and the abstract and interpretation were also adjusted accordingly.
Comment 2:
Statistical tests and results are unclear throughout. For all analyses, please report specific tests used, factors/groups, test statistic and p-value for all data analyses reported. In some cases, the chosen test is not appropriate. For example, in Figure 6E, it is not clear how an experiment with 2 factors (stress and drug) can be analyzed with a 1-way RM ANOVA. The potential impact of inappropriate statistical tests on results makes it difficult to assess the accuracy of data interpretation.
We have redone the statistical analysis as suggested by the reviewer and added specific tests used, factors/groups, test statistic and p-value for all data analyses into the revised manuscript.
Comment 3:
Why were only male mice used? Please justify and discuss in the manuscript. Also, change the title to reflect this.
Although most similar previous studies used male mice or rats[7, 8], we do agree with the reviewer that the female animals should also be tested, in consideration possible role of sex hormones, as such we plan to repeat some key experiments on female mice.
Comment 4:
Number of recorded cells is very low in Figure 1. Where in VTA did recordings occur? Given the heterogeneity in this brain region, this n may be insufficient. Additional information (e.g., location within VTA, criteria used to identify neurons) should be included. Report the number of mice (i.e., n = 6 cells from X mice) in all figures.
Yes indeed, the number here is not high. More experiments will be performed to increase the N/n number. And the location of recorded cells in VTA and the number of used mice are now shown in all figures; criteria to identify neurons is stated in the Methods- Identification of DA neurons and electrophysiological recordings. At the end of electrophysiological recordings, the recorded VTA neurons were collected for single-cell PCR. VTA DA neurons were identified by single-cell PCR for the presence of TH and DAT.
Comment 5:
Authors refer to VTA DA neurons as those that are DAT+ in line 276, although TH expression is considered the standard of DAergic identity, and studies (e.g., Lammel et al, 2008) have shown that a subset of VTA DA neurons have low levels of DAT expression. Authors should reword/clarify that these are DAT-expressing VTA DA neurons.
The study published by Lammel[9] in 2015 has shown the low dopamine specificity of transgene expression in ventral midbrain of TH-Cre mice; on the other hand, DAT-Cre mice exhibit dopamine-specific Cre expression patterns, although DAT-Cre mice are likely to suffer from their own limitations (for example, low DAT expression in mesocortical DA neurons may make it difficult to target this subpopulation, see Lammel et al., 2008[10]). Hence, in our study, the DAT was used as criteria to identify DAT neurons. Of course, TH and DAT were all tested in single-cell PCR to identify whether the recorded cells were DA neurons.
Comment 6:
Neuronal subtype proportions should be quantified and reported (Fig. 1Aii).
Neuronal subtype proportions are now quantified and reported in Fig. 1Aii.
Comment 7:
In addition to reporting projection specificity of neurons expressing specific channels, it would be ideal to report these data according to spatial location in VTA.
The spatial location of recorded cells in VTA are now shown in all figures.
Comment 8:
The authors state that there are a small number of Glut neurons in VTA, then they state that a "significant proportion" of VTA neurons are glutamatergic.
Thanks, “a significant proportion of neurons” has been changed to “ less than half of sequenced DA neurons”.
Comment 9:
It is an overstatement that VTA DA neurons are the key determinant of abnormal behaviors in affective disorders.
Thanks, we have amended the statement to that “Dopaminergic (DA) neurons in the ventral tegmental area (VTA) play an important role in mood, reward and emotion-related behaviors”.
Reviewer #3 (Public Review):
Comment 1:
The authors of this study have examined which cation channels specifically confer to ventral tegmental area dopaminergic neurons their autonomic (spontaneous) firing properties. Having brought evidence for the key role played by NALCN and TRPC6 channels therein, the authors aimed at measuring whether these channels play some role in so-called depression-like (but see below) behaviors triggered by chronic exposure to different stressors. Following evidence for a down-regulation of TRPC6 protein expression in ventral tegmental area dopaminergic cells of stressed animals, the authors provide evidence through viral expression protocols for a causal link between such a down-regulation and so-called depression-like behaviors. The main strength of this study lies on a comprehensive bottom-up approach ranging from patch-clamp recordings to behavioral tasks. However, the interpretation of the results gathered from these behavioral tasks might also be considered one main weakness of the abovementioned approach. Thus, the authors make a confusion (widely observed in numerous publications) with regard to the use of paradigms (forced swim test, tail suspension test) initially aimed (and hence validated) at detecting the antidepressant effects of drugs and which by no means provide clues on "depression" in their subjects. Indeed, in their hands, the authors report that stress elicits changes in these tests which are opposed to those theoretically seen after antidepressant medication. However, these results do not imply that these changes reflect "depression" but rather that the individuals under scrutiny simply show different responses from those seen in nonstressed animals. These limits are even more valid in nonstressed animals injected with TRPC6 shRNAs (how can 5-min tests be compared to a complex and chronic pathological state such as depression?). With regard to anxiety, as investigated with the elevated plus-maze and the open field, the data, as reported, do not allow to check the author's interpretation as anxiety indices are either not correctly provided (e.g. absolute open arm data instead of percents of open arm visits without mention of closed arm behaviors) or subjected to possible biases (lack of distinction between central and peripheral components of the apparatus).
We agree with the reviewer that behavior tests we used here is debatable whether they represent a real depression state, and this is an open question that could be discussed from different respective. Since these testes (forced swimming and tail suspension), as the reviewer noted, were “widely observed in numerous publications”, we used these seemly only options to reflect a “depression-like” state. One could argue that since these testes were initially used for testing antidepressants (“validated”), with decreased immobility time as indications of anti-depressive effects, why not an increased immobility time reflect a “depression-like” state. As for anxiety tests, both absolute time in open and closed arms are now provided.
-

eLife assessment
This valuable study examined the mechanisms underlying reduced excitability of ventral tegmental area dopamine neurons in mice that underwent a chronic mild unpredictable stress treatment. The authors identify NALCN and TRPC6 channels as key mechanisms that regulate spontaneous firing of ventral tegmental area dopamine neurons and examined their roles in reduced firing in mice that underwent a chronic mild unpredictable stress treatment. The evidence supporting the authors' conclusions are solid yet the reviewers pointed out some limitations in the study including statistics, specificity in pharmacological and gene knockdown experiments, and the relevance to depressions.
-

Reviewer #1 (Public Review):
Wang et al., present a paper aiming to identify NALCN and TRPC6 channels as key mechanisms regulating VTA dopaminergic neuron spontaneous firing and investigating whether these mechanisms are disrupted in a chronic unpredictable stress model mouse.
Major strengths:
-This paper uses multiple approaches to investigate the role of NALCN and TRPC6 channels in VTA dopaminergic neurons.
Major weaknesses:
-The pharmacological tools used in this study are highly non-selective. Gd3+, used here to block NALCN is actually more commonly used to block TRP channels. 2-APB inhibits not only TRPC channels, but also TRPM and IP3 receptors while stimulating TRPV channels (Bon and Beech, 2013), while FFA actually stimulates TRPC6 channels while inhibiting other TRPCs (Foster et al., 2009).
Are the author's claims supported by …
Reviewer #1 (Public Review):
Wang et al., present a paper aiming to identify NALCN and TRPC6 channels as key mechanisms regulating VTA dopaminergic neuron spontaneous firing and investigating whether these mechanisms are disrupted in a chronic unpredictable stress model mouse.
Major strengths:
-This paper uses multiple approaches to investigate the role of NALCN and TRPC6 channels in VTA dopaminergic neurons.
Major weaknesses:
-The pharmacological tools used in this study are highly non-selective. Gd3+, used here to block NALCN is actually more commonly used to block TRP channels. 2-APB inhibits not only TRPC channels, but also TRPM and IP3 receptors while stimulating TRPV channels (Bon and Beech, 2013), while FFA actually stimulates TRPC6 channels while inhibiting other TRPCs (Foster et al., 2009).
Are the author's claims supported by the data?
-The multimodal approach including shRNA knockdown experiments alleviates much of the concern about the non-specific pharmacological agents. Therefore, the author's claim that NALCN is involved in VTA dopaminergic neuron pacemaking is well-supported.
-However, the claim that TRPC6 is the key TRPC channel in VTA spontaneous firing is somewhat, but not completely supported. As with NALCN above, the pharmacology alone is much too non-specific to support the claim that TRPC6 is the TRP channel responsible for pacemaking. However, unlike the NALCN condition, there is an issue with interpreting the shRNA knockdown experiments. The issue is that TRPC channels often form heteromers with TRPC channels of other types (Goel, Sinkins and Schilling, 2002; Strübing et al., 2003). Therefore, it is possible that knocking down TRPC6 is interfering with the normal function of another TRPC channel, such as TRPC7 or TRPC4.
-The claim that TRPC6 channels in the VTA are involved in the depressive-like symptoms of CMUS is supported.
- However, the connection between the mPFC-projecting VTA neurons, TRPC6 channels, and the chronic unpredictable stress model (CMUS) of depression is not well supported. In Figure 2, it appears that the mPFC-projecting VTA neurons have very low TRPC6 expression compared to VTA neurons projecting to other targets. However, in figure 6, the authors focus on the mPFC-projecting neurons in their CMUS model and show that it is these neurons that are no longer sensitive to pharmacological agents non-specifically blocking TRPC channels (2-APB, see above comment). Finally, in figure 7, the authors show that shRNA knockdown of TRPC6 channels (in all VTA dopaminergic neurons) results in depressive-like symptoms in CMUS mice. Due to the low expression of TRPC6 in mPFC-projecting VTA neurons, the author's claims of "broad and strong expression of TRPC6 channels across VTA DA neurons" is not fully supported. Because of the messy pharmacological tools used, it cannot be clamed that TRPC6 in the mPFC-projecting VTA neurons is altered after CMUS. And because the knockdown experiments are not specific to mPFC-projecting VTA neurons, it cannot be claimed that reducing TRPC6 in these specific neurons is causing depressive symptoms.
Impact:
It is valuable to compare pacemaking mechanisms in VTA and SNc neurons and this paper convincingly shows that NALCN contributes to VTA pacemaking, as it is known to contribute to SNc pacemaking. It also shows that TRPC6 channels in VTA dopamine neurons contribute to the depressive-like symptoms associated with CMUS.
It is important to note that the experiments presented in Figure 1 have all been previously performed in VTA dopaminergic neurons (Khaliq and Bean, 2010) including showing that low calcium increases VTA neuron spontaneous firing frequency and that replacement of sodium with NMDG hyperpolarizes the membrane potential.
Additional context:
-The authors explanation for the increase in firing frequency in 0 calcium conditions is that calcium-activated potassium channels would no longer be activated. However, there is a highly relevant finding that low calcium enhances the NALCN conductance through the calcium sensing receptor from Dejian Ren's lab (Lu et al., 2010) which is not cited in this paper. This increase in NALCN conductance with low calcium has been shown in SNc dopaminergic neurons (Philippart and Khaliq, 2018), and is likely a factor contributing to the low-calcium-mediated increase in spontaneous VTA neuron firing.
-One of the only demonstrations of the expression and physiological significance of TRPCs in VTA DA neurons was published by (Rasmus et al., 2011; Klipec et al., 2016) which are not cited in this paper. In their study, TRPC4 expression was detected in a uniformly distributed subset of VTA DA neurons, and TRPC4 KO rats showed decreased VTA DA neuron tonic firing and deficits in cocaine reward and social behaviors.
- Out of all seven TRPCs, TRPC5 is the only one reported to have basal/constitutive activity in heterologous expression systems (Schaefer et al., 2000; Jeon et al., 2012). Others TRPCs such as TRPC6 are typically activated by Gq-coupled GPCRs. Why would TRPC6 be spontaneously/constitutively active in VTA DA neurons?
-A new paper from the group of Myoung Kyu Park (Hahn et al., 2023) shows in great detail the interactions between NALCN and TRPC3 channels in pacemaking of SNc DA neurons.
References
Bon, R.S. and Beech, D.J. (2013) 'In pursuit of small molecule chemistry for calcium-permeable non-selective TRPC channels -- mirage or pot of gold?', British Journal of Pharmacology, 170(3), pp. 459-474. Available at: https://doi.org/10.1111/bph.12274.
Foster, R.R. et al. (2009) 'Flufenamic acid is a tool for investigating TRPC6-mediated calcium signalling in human conditionally immortalised podocytes and HEK293 cells', Cell Calcium, 45(4), pp. 384-390. Available at: https://doi.org/10.1016/j.ceca.2009.01.003.
Goel, M., Sinkins, W.G. and Schilling, W.P. (2002) 'Selective association of TRPC channel subunits in rat brain synaptosomes', The Journal of Biological Chemistry, 277(50), pp. 48303-48310. Available at: https://doi.org/10.1074/jbc.M207882200.
Hahn, S. et al. (2023) 'Proximal dendritic localization of NALCN channels underlies tonic and burst firing in nigral dopaminergic neurons', The Journal of Physiology, 601(1), pp. 171-193. Available at: https://doi.org/10.1113/JP283716.
Jeon, J.-P. et al. (2012) 'Selective Gαi subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels', The Journal of Biological Chemistry, 287(21), pp. 17029-17039. Available at: https://doi.org/10.1074/jbc.M111.326553.
Khaliq, Z.M. and Bean, B.P. (2010) 'Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances', The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(21), pp. 7401-7413. Available at: https://doi.org/10.1523/JNEUROSCI.0143-10.2010.
Klipec, W.D. et al. (2016) 'Loss of the trpc4 gene is associated with a reduction in cocaine self-administration and reduced spontaneous ventral tegmental area dopamine neuronal activity, without deficits in learning for natural rewards', Behavioural Brain Research, 306, pp. 117-127. Available at: https://doi.org/10.1016/j.bbr.2016.03.027.
Lu, B. et al. (2010) 'Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex', Neuron, 68(3), pp. 488-499. Available at: https://doi.org/10.1016/j.neuron.2010.09.014.
Philippart, F. and Khaliq, Z.M. (2018) 'Gi/o protein-coupled receptors in dopamine neurons inhibit the sodium leak channel NALCN', eLife, 7. Available at: https://doi.org/10.7554/eLife.40984.
Rasmus, K. et al. (2011) 'Sociability is decreased following deletion of the trpc4 gene', Nature Precedings, pp. 1-1. Available at: https://doi.org/10.1038/npre.2011.6367.1.
Schaefer, M. et al. (2000) 'Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5', The Journal of Biological Chemistry, 275(23), pp. 17517-17526. Available at: https://doi.org/10.1074/jbc.275.23.17517.
Strübing, C. et al. (2003) 'Formation of novel TRPC channels by complex subunit interactions in embryonic brain', The Journal of Biological Chemistry, 278(40), pp. 39014-39019. Available at: https://doi.org/10.1074/jbc.M306705200.
-

Reviewer #2 (Public Review):
This paper describes the results of a set of complementary and convergent experiments aimed at describing roles for the non-selective cation channels NALCN and TRPC6 in mediating subthreshold inward depolarizing currents and action potential generation in VTA DA neurons under normal physiological conditions. That said, some datasets are underpowered, and general flaws in statistical reporting make assessment difficult. There is also a lack of clarity at various points throughout the manuscript, as well as overinterpretation of the data generated in these experiments. Specific comments follow:
1. These results do not show that TRPC6 mediates stress effects on depression-like behavior. As stated by the authors in the first sentence of the final paragraph, "downregulation of TRPC6 proteins was correlated with …
Reviewer #2 (Public Review):
This paper describes the results of a set of complementary and convergent experiments aimed at describing roles for the non-selective cation channels NALCN and TRPC6 in mediating subthreshold inward depolarizing currents and action potential generation in VTA DA neurons under normal physiological conditions. That said, some datasets are underpowered, and general flaws in statistical reporting make assessment difficult. There is also a lack of clarity at various points throughout the manuscript, as well as overinterpretation of the data generated in these experiments. Specific comments follow:
1. These results do not show that TRPC6 mediates stress effects on depression-like behavior. As stated by the authors in the first sentence of the final paragraph, "downregulation of TRPC6 proteins was correlated with reduced firing activity of the VTA DA neurons, the depression-like behaviors, and that knocking down of TRPC6 in the VTA DA neurons confer the mice with depression behaviors." Therefore, the results show associations between TRPC6 downregulation and stress effects on behavior, occlusion of the effects of one by the other on some outcome measures, and cell manipulation effects that resemble stress effects. There is no experiment that shows reversal of stress effects with cell/circuit-specific TRPC6 manipulations. Please adjust the title, abstract and interpretation accordingly.
2. Statistical tests and results are unclear throughout. For all analyses, please report specific tests used, factors/groups, test statistic and p-value for all data analyses reported. In some cases, the chosen test is not appropriate. For example, in Figure 6E, it is not clear how an experiment with 2 factors (stress and drug) can be analyzed with a 1-way RM ANOVA. The potential impact of inappropriate statistical tests on results makes it difficult to assess the accuracy of data interpretation.
3. Why were only male mice used? Please justify and discuss in the manuscript. Also, change the title to reflect this.
4. Number of recorded cells is very low in Figure 1. Where in VTA did recordings occur? Given the heterogeneity in this brain region, this n may be insufficient. Additional information (e.g., location within VTA, criteria used to identify neurons) should be included. Report the number of mice (i.e., n = 6 cells from X mice) in all figures.
5. Authors refer to VTA DA neurons as those that are DAT+ in line 276, although TH expression is considered the standard of DAergic identity, and studies (e.g., Lammel et al, 2008) have shown that a subset of VTA DA neurons have low levels of DAT expression. Authors should reword/clarify that these are DAT-expressing VTA DA neurons.
6. Neuronal subtype proportions should be quantified and reported (Fig. 1Aii).
7. In addition to reporting projection specificity of neurons expressing specific channels, it would be ideal to report these data according to spatial location in VTA.
8. The authors state that there are a small number of Glut neurons in VTA, then they state that a "significant proportion" of VTA neurons are glutamatergic.
9. It is an overstatement that VTA DA neurons are the key determinant of abnormal behaviors in affective disorders. -

Reviewer #3 (Public Review):
The authors of this study have examined which cation channels specifically confer to ventral tegmental area dopaminergic neurons their autonomic (spontaneous) firing properties. Having brought evidence for the key role played by NALCN and TRPC6 channels therein, the authors aimed at measuring whether these channels play some role in so-called depression-like (but see below) behaviors triggered by chronic exposure to different stressors. Following evidence for a down-regulation of TRPC6 protein expression in ventral tegmental area dopaminergic cells of stressed animals, the authors provide evidence through viral expression protocols for a causal link between such a down-regulation and so-called depression-like behaviors. The main strength of this study lies on a comprehensive bottom-up approach ranging from …
Reviewer #3 (Public Review):
The authors of this study have examined which cation channels specifically confer to ventral tegmental area dopaminergic neurons their autonomic (spontaneous) firing properties. Having brought evidence for the key role played by NALCN and TRPC6 channels therein, the authors aimed at measuring whether these channels play some role in so-called depression-like (but see below) behaviors triggered by chronic exposure to different stressors. Following evidence for a down-regulation of TRPC6 protein expression in ventral tegmental area dopaminergic cells of stressed animals, the authors provide evidence through viral expression protocols for a causal link between such a down-regulation and so-called depression-like behaviors. The main strength of this study lies on a comprehensive bottom-up approach ranging from patch-clamp recordings to behavioral tasks. However, the interpretation of the results gathered from these behavioral tasks might also be considered one main weakness of the abovementioned approach. Thus, the authors make a confusion (widely observed in numerous publications) with regard to the use of paradigms (forced swim test, tail suspension test) initially aimed (and hence validated) at detecting the antidepressant effects of drugs and which by no means provide clues on "depression" in their subjects. Indeed, in their hands, the authors report that stress elicits changes in these tests which are opposed to those theoretically seen after antidepressant medication. However, these results do not imply that these changes reflect "depression" but rather that the individuals under scrutiny simply show different responses from those seen in nonstressed animals. These limits are even more valid in nonstressed animals injected with TRPC6 shRNAs (how can 5-min tests be compared to a complex and chronic pathological state such as depression?). With regard to anxiety, as investigated with the elevated plus-maze and the open field, the data, as reported, do not allow to check the author's interpretation as anxiety indices are either not correctly provided (e.g. absolute open arm data instead of percents of open arm visits without mention of closed arm behaviors) or subjected to possible biases (lack of distinction between central and peripheral components of the apparatus).
-