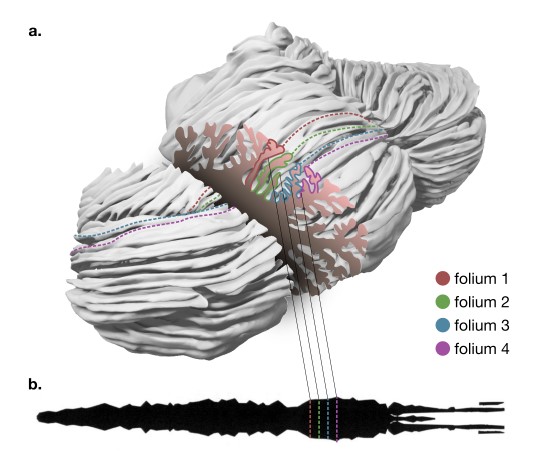
Diversity and evolution of cerebellar folding in mammals
Curation statements for this article:-
Curated by eLife
eLife assessment
This important study gives novel insight into the folding diversity of the cerebellum compared to the cerebrum among 56 mammalian species. Based on impressive data, the analyses performed for anatomical descriptions and phylogenetic comparisons are solid, although some issues need to be addressed regarding the choice of statistical models, and the sample size versus the number of explanatory variables. This study will be of interest to neuroscientists, evolutionary and developmental biologists, and physicists interested in biomechanics, as these observations provide a basis for models of brain folding mechanisms.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The process of brain folding is thought to play an important role in the development and organisation of the cerebrum and the cerebellum. The study of cerebellar folding is challenging due to the small size and abundance of its folia. In consequence, little is known about its anatomical diversity and evolution. We constituted an open collection of histological data from 56 mammalian species and manually segmented the cerebrum and the cerebellum. We developed methods to measure the geometry of cerebellar folia and to estimate the thickness of the molecular layer. We used phylogenetic comparative methods to study the diversity and evolution of cerebellar folding and its relationship with the anatomy of the cerebrum. Our results show that the evolution of cerebellar and cerebral anatomy follows a stabilising selection process. We observed two groups of phenotypes changing concertedly through evolution: a group of ‘diverse’ phenotypes – varying over several orders of magnitude together with body size, and a group of ‘stable’ phenotypes varying over less than 1 order of magnitude across species. Our analyses confirmed the strong correlation between cerebral and cerebellar volumes across species, and showed in addition that large cerebella are disproportionately more folded than smaller ones. Compared with the extreme variations in cerebellar surface area, folial anatomy and molecular layer thickness varied only slightly, showing a much smaller increase in the larger cerebella. We discuss how these findings could provide new insights into the diversity and evolution of cerebellar folding, the mechanisms of cerebellar and cerebral folding, and their potential influence on the organisation of the brain across species.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
- Although I found the introduction well written, I think it lacks some information or needs to develop more on some ideas (e.g., differences between the cerebellum and cerebral cortex, and folding patterns of both structures). For example, after stating that "Many aspects of the organization of the cerebellum and cerebrum are, however, very different" (1st paragraph), I think the authors need to develop more on what these differences are. Perhaps just rearranging some of the text/paragraphs will help make it better for a broad audience (e.g., authors could move the next paragraph up, i.e., "While the cx is unique to mammals (...)").
We have added additional context to the introduction and developed the differences between cerebral and cerebellar cortex, also re-arranging the text as …
Author Response
Reviewer #1 (Public Review):
- Although I found the introduction well written, I think it lacks some information or needs to develop more on some ideas (e.g., differences between the cerebellum and cerebral cortex, and folding patterns of both structures). For example, after stating that "Many aspects of the organization of the cerebellum and cerebrum are, however, very different" (1st paragraph), I think the authors need to develop more on what these differences are. Perhaps just rearranging some of the text/paragraphs will help make it better for a broad audience (e.g., authors could move the next paragraph up, i.e., "While the cx is unique to mammals (...)").
We have added additional context to the introduction and developed the differences between cerebral and cerebellar cortex, also re-arranging the text as suggested.
- Given that the authors compare the folding patterns between the cerebrum and cerebellum, another point that could be mentioned in the introduction is the fact that the cerebellum is convoluted in every mammalian species (and non-mammalian spp as well) while the cerebrum tends to be convoluted in species with larger brains. Why is that so? Do we know about it (check Van Essen et al., 2018)? I think this is an important point to raise in the introduction and to bring it back into the discussion with the results.
We now mention in the introduction the fact that the cerebellum is folded in mammals, birds and some fishes, and provide references to the relevant literature. We have also expanded our discussion about the reasons for cortical folding in the discussion, which now contains a subsection addressing the subject (this includes references to the work of Van Essen).
- In the results, first paragraph, what do the authors mean by the volume of the medial cerebellum? This needs clarification.
We have modified the relevant section in the results, and made the definition of the medial cerebellum more clear indicating that we refer to the vermal region of the cerebellum.
- In the results: When the authors mention 'frequency of cerebellar folding', do they mean the degree of folding in the cerebellum? At least in non-mammalian species, many studies have tried to compare the 'degree or frequency of folding' in the cerebellum by different proxies/measurements (see Iwaniuk et al., 2006; Yopak et al., 2007; Lisney et al., 2007; Yopak et al., 2016; Cunha et al., 2022). Perhaps change the phrase in the second paragraph of the result to: "There are no comparative analyses of the frequency of cerebellar folding in mammals, to our knowledge".
We have modified the subsection in the methods referring to the measurement of folial width and folial perimeter to make the difference more clear. The folding indices that have been used previously (which we cite) are based on Zilles’s gyrification index. This index provides only a global idea of degree of folding, but it’s unable to distinguish a cortex with profuse shallow folds from one with a few deep ones. An example of this is now illustrated in Fig. 3d, where we also show how that problem is solved by the use of our two measurements (folial width and perimeter). The problem is also discussed in the section about the measurement of folding in the discussion section:
“Previous studies of cerebellar folding have relied either on a qualitative visual score (Yopak et al. 2007, Lisney et al. 2008) or a “gyrification index” based on the method introduced by Zilles et al. (1988, 1989) for the study of cerebral folding (Iwaniuk et al. 2006, Cunha et al. 2020, 2021). Zilles’s gyrification index is the ratio between the length of the outer contour of the cortex and the length of an idealised envelope meant to reflect the length of the cortex if it were not folded. For instance, a completely lissencephalic cortex would have a gyrification index close to 1, while a human cerebral cortex typically has a gyrification index of ~2.5 (Zilles et al. 1988). This method has certain limitations, as highlighted by various researchers (Germanaud et al. 2012, 2014, Rabiei et al. 2018, Schaer et al. 2008, Toro et al. 2008, Heuer et al. 2019). One important drawback is that the gyrification index produces the same value for contours with wide variations in folding frequency and amplitude, as illustrated in Fig. 3d. In reality, folding frequency (inverse of folding wavelength) and folding amplitude represent two distinct dimensions of folding that cannot be adequately captured by a single number confusing both dimensions. To address this issue we introduced 2 measurements of folding: folial width and folial perimeter. These measurements can be directly linked to folding frequency and amplitude, and are comparable to the folding depth and folding wavelength we introduced previously for cerebral 3D meshes (Heuer et al. 2019). By using these measurements, we can differentiate folding patterns that could be confused when using a single value such as the gyrification index (Fig. 3d). Additionally, these two dimensions of folding are important, because they can be related to the predictions made by biomechanical models of cortical folding, as we will discuss now.”
- Sultan and Braitenberg (1993) measured cerebella that were sagittally sectioned (instead of coronal), right? Do you think this difference in the plane of the section could be one of the reasons explaining different results on folial width between studies? Why does the foliation index calculated by Sultan and Braitenberg (1993) not provide information about folding frequency?
The measurement of foliation should be similar as far as enough folds are sectioned perpendicular to their main axis. This will be the case for folds in the medial cerebellum (vermis) sectioned sagittally, and for folds in the lateral cerebellum sectioned coronally. The foliation index of Sultan and Braitenberg does not provide a similar account of folding frequency as we do because they only measure groups of folia (what some called lamellae), whereas we measure individual folia. It is not easy to understand exactly how Sultan and Braitenberg proceeded from their paper. We contacted Prof. Fahad Sultan (we acknowledge his help in our manuscript). Author response image 1 provides a more clear description of their procedure:
Author response image 1.
As Author response image 1 shows, each of the structures that they call a fold is composed of several folia, and so their measurements are not comparable with ours which measure individual folia (a). The flattened representation (b) is made by stacking the lengths of the fold axes (dashed lines), separating them by the total length of each fold (the solid lines), which each may contain several folia.
- Another point that needs to be clarified is the log transformation of the data. Did the authors use log-transformed data for all types of analyses done in the study? Write this information in the material and methods.
Yes, we used the log10 transformation for all our measurements. This is now mentioned in the methods section, and again in the section concerning allometry. We are including a link to all our code to facilitate exact replication of our entire method, including this transformation.
- The discussion needs to be expanded. The focus of the paper is on the folding pattern of the cerebellum (among different mammalian species) and its relationship with the anatomy of the cerebrum. Therefore, the discussion on this topic needs to be better developed, in my opinion (especially given the interesting results of this paper). For example, with the findings of this study, what can we say about how the folding of the cerebellum is determined across mammals? The authors found that the folial width, folial perimeter, and thickness of the molecular layer increase at a relatively slow rate across the species studied. Does this mean that these parameters have little influence on the cerebellar folding pattern? What mostly defines the folding patterns of the cerebellum given the results? Is it the interaction between section length and area? Can the authors explain why size does not seem to be a "limiting factor" for the folding of the cerebellum (for example, even relatively small cerebella are folded)? Is that because the 'white matter' core of the cerebellum is relatively small (thus more stress on it)?
We have expanded the discussion as suggested, with subsections detailing the measuring of folding, the modelling of folding for the cerebrum and the cerebellum, and the role that cerebellar folding may play in its function. We refer to the literature on cortical folding modelling, and we discuss our results in terms of the factors that this research has highlighted as critical for folding. From the discussion subsection on models of cortical folding:
“The folding of the cerebral cortex has been the focus of intense research, both from the perspective of neurobiology (Borrell 2018, Fernández and Borrell 2023) and physics (Toro and Burnod 2005, Tallinen et al. 2014, Kroenke and Bayly 2018). Current biomechanical models suggest that cortical folding should result from a buckling instability triggered by the growth of the cortical grey matter on top of the white matter core. In such systems, the growing layer should first expand without folding, increasing the stress in the core. But this configuration is unstable, and if growth continues stress is released through cortical folding. The wavelength of folding depends on cortical thickness, and folding models such as the one by Tallinen et al. (2014) predict a neocortical folding wavelength which corresponds well with the one observed in real cortices. Tallinen et al. (2014) provided a prediction for the relationship between folding wavelength λ and the mean thickness (𝑡) of the cortical layer: λ = 2π𝑡(µ/(3µ𝑠))1/3. (...)”
From this biomechanical framework, our answers to the questions of the Reviewer would be:
How is the folding of the cerebellum determined across mammals? By the expansion of a layer of reduced thickness on top of an elastic layer (the white matter)
Folial width, folial perimeter, and thickness of the molecular layer increase at a relatively slow rate across the species studied. Does this mean that these parameters have little influence on the cerebellar folding pattern? On the contrary, that indicates that the shape of individual folia is stable, providing the smallest level of granularity of a folding pattern. In the extreme case where all folia had exactly the same size, a small cerebellum would have enough space to accommodate only a few folia, whereas a large cerebellum would accommodate many more.
What mostly defines the folding patterns of the cerebellum given the results? Is it the interaction between section length and area? It’s the mostly 2D expansion of the cerebellar cortical layer and its thickness.
Can the authors explain why size does not seem to be a "limiting factor" for the folding of the cerebellum? Because even a cerebellum of very small volume would fold if its cortex were thin enough and expanded sufficiently. That’s why the cerebellum folds even while being smaller than the cerebrum: because its cortex is much thinner.
- One caveat or point to be raised is the fact that the authors use the median of the variables measured for the whole cerebellum (e.g., median width and median perimeter across all folia). Although the cerebellum is highly uniform in its gross internal morphology and circuitry's organization across most vertebrates, there is evidence showing that the cerebellum may be organized in different functional modules. In that way, different regions or folia of the cerebellum would have different olivo-cortico-nuclear circuitries, forming, each one, a single cerebellar zone. Although it is not completely clear how these modules/zones are organized within the cerebellum, I think the authors could acknowledge this at the end of their discussion, and raise potential ideas for future studies (e.g., analyse folding of the cerebellum within the brain structure - vermis vs lateral cerebellum, for example). I think this would be a good way to emphasize the importance of the results of this study and what are the main questions remaining to be answered. For example, the expansion of the lateral cerebellum in mammals is suggested to be linked with the evolution of vocal learning in different clades (see Smaers et al., 2018). An interesting question would be to understand how foliation within the lateral cerebellum varies across mammalian clades and whether this has something to do with the cellular composition or any other aspect of the microanatomy as well as the evolution of different cognitive skills in mammals.
We now address this point in a subsection of the discussion which details the implications of our methodological decisions and the limitations of our approach. It is true that the cerebellum is regionally variable. Our measurements of folial width, folial perimeter and molecular layer thickness are local, and we should be able to use them in the future to study regional variation. However, this comes with a number of difficulties. First, it would require sampling all the cerebellum (and the cerebrum) and not just one section. But even if that were possible that would increase the number of phenotypes, beyond the current scope of this study. Our central question about brain folding in the cerebellum compared to the cerebrum is addressed by providing data for a substantial number of mammalian species. As indicated by Reviewer #3, adding more variables makes phylogenetic comparative analyses very difficult because the models to fit become too large.
Reviewer #2 (Public Review):
- The methods section does not address all the numerical methods used to make sense of the different brain metrics.
We now provide more detailed descriptions of our measurements of foliation, phylogenetic models, analysis of partial correlations, phylogenetic principal components, and allometry. We have added illustrations (to Figs. 3 and 5), examples and references to the relevant literature.
- In the results section, it sometimes makes it difficult for the reader to understand the reason for a sub-analysis and the interpretation of the numerical findings.
The revised version of our manuscript includes motivations for the different types of analyses, and we have also added a paragraph providing a guide to the structure of our results.
- The originality of the article is not sufficiently brought forward:
a) the novel method to detect the depth of the molecular layer is not contextualized in order to understand the shortcomings of previously-established methods. This prevents the reader from understanding its added value and hinders its potential re-use in further studies.
The revised version of the manuscript provides additional context which highlights the novelty of our approach, in particular concerning the measurement of folding and the use of phylogenetic comparative models. The limitations of the previous approaches are stated more clearly, and illustrated in Figs. 3 and 5.
b) The numerous results reported are not sufficiently addressed in the discussion for the reader to get a full grasp of their implications, hindering the clarity of the overall conclusion of the article.
Following the Reviewer’s advice, we have thoroughly restructured our results and discussion section.
Reviewer #3 (Public Review):
- The first problem relates to their use of the Ornstein-Uhlenbeck (OU) model: they try fitting three evolutionary models, and conclude that the Ornstein-Uhlenbeck model provides the best fit. However, it has been known for a while that OU models are prone to bias and that the apparent superiority of OU models over Brownian Motion is often an artefact, a problem that increases with smaller sample sizes. (Cooper et al (2016) Biological Journal of the Linnean Society, 2016, 118, 64-77).
Cooper et al.’s (2016) article “A Cautionary Note on the Use of Ornstein Uhlenbeck Models in Macroevolutionary Studies” suggests that comparing evolutionary models using the model’s likelihood leads often to incorrectly selecting OU over BM even for data generated from a BM process. However, Grabowski et al (2023) in their article ‘A Cautionary Note on “A Cautionary Note on the Use of Ornstein Uhlenbeck Models in Macroevolutionary Studies”’ suggest that Cooper et al.’s (2016) claim may be misleading. The work of Clavel et al. (2019) and Clavel and Morlon (2017) shows that the penalised framework implemented in mvMORPH can successfully recover the parameters of a multivariate OU process. To address more directly the concern of the Reviewer, we used simulations to evaluate the chances that we would decide for an OU model when the correct model was BM – a similar procedure to the one used by Cooper et al.’s (2016). However, instead of using the likelihood of the fitted models directly as Cooper et al. (2016) – which does not control for the number of parameters in the model – we used the Akaike Information Criterion, corrected for small sample sizes: AICc. The standard Akaike Information Criterion takes the number of parameters of the model into account, but this is not sufficient when the sample size is small. AICc provides a score which takes both aspects into account: model complexity and sample size. This information has been added to the manuscript:
“We selected the best fitting model using the Akaike Information Criterion (AIC), corrected for 𝐴𝐼𝐶 = − 2 𝑙𝑜𝑔(𝑙𝑖𝑘𝑒𝑙𝑖ℎ𝑜𝑜𝑑) + 2 𝑝. This approximation is insufficient when the𝑝 sample size small sample sizes (AICc). AIC takes into account the number of parameters in the model: is small, in which case an additional correction is required, leading to the corrected AIC: 𝐴𝐼𝐶𝑐 = 𝐴𝐼𝐶 + (2𝑝2 + 2𝑝)/(𝑛 − 𝑝 − 1), where 𝑛 is the sample size.”
In 1000 simulations of 9 correlated multivariate traits for 56 species (i.e., 56*9 data points) using our phylogenetic tree, only 0.7% of the times we would decide for OU when the real model was BM.
- Second, for the partial correlations (e.g. fig 7) and Principal Components (fig 8) there is a concern about over-fitting: there are 9 variables and only 56 data points (violating the minimal rule of thumb that there should be >10 observations per parameter). Added to this, the inclusion of variables lacks a clear theoretical rationale. The high correlations between most variables will be in part because they are to some extent measuring the same things, e.g. the five different measures of cerebellar anatomy which include two measures of folial size. This makes it difficult to separate their effects. I get that the authors are trying to tease apart different aspects of size, but in practice, I think these results (e.g. the presence of negative coefficients in Fig 7) are really hard or impossible to interpret. The partial correlation network looks like a "correlational salad" rather than a theoretically motivated hypothesis test. It isn't clear to me that the PC analyses solve this problem, but it partly depends on the aims of these analyses, which are not made very clear.
PCA is simply a rigid rotation of the data, distances among multivariate data points are all conserved. Neither our PCA nor our partial correlation analysis involve model fitting, the concept of overfitting does not apply. PCA and partial correlations are also not used here for hypothesis testing, but as exploratory methods which provide a transformation of the data aiming at capturing the main trends of multivariate change. The aim of our analysis of correlation structure is precisely to avoid the “correlational salad” that the Reviewer mentions. The Reviewer is correct: all our variables are correlated to a varying degree (note that there are 56 data points per variable = 56*9 data points, not just 56 data points). Partial correlations and PCA aim at providing a principled way in which correlated measurements can be explored. In the revised version of the manuscript we include a more detailed description of partial correlations and PCA (phylogenetic). Whenever variables measure the same thing, they will be combined into the same principal component (these are the combinations shown in Fig. 8 b and d). Additionally, two variables may be correlated because of their correlation with a third variable (or more). Partial correlations address this possibility by looking at the correlations between the residuals of each pair of variables after all other variables have been covaried out. We provide a simple example which should make this clear, providing in particular an intuition for the meaning of negative correlations:
“All our phenotypes were strongly correlated. We used partial correlations to better understand pairwise relationships. The partial correlation between 2 vectors of measurements a and b is the correlation between their residuals after the influence of all other measurements has been covaried out. Even if the correlation between a and b is strong and positive, their partial correlation could be 0 or even negative. Consider, for example, 3 vectors of measurements a, b, c, which result from the combination of uncorrelated random vectors x, y, z. Suppose that a = 0.5 x + 0.2 y + 0.1 z, b = 0.5 x - 0.2 y + 0.1 z, and c = x. The measurements a and b will be positively correlated because of the effect of x and z. However, if we compute the residuals of a and b after covarying the effect of c (i.e., x), their partial correlation will be negative because of the opposite effect of y on a and b. The statistical significance of each partial correlation being different than 0 was estimated using the edge exclusion test introduced by Whittaker (1990).”
The rationale for our analyses has been made more clear in the revised version of the manuscript, aided by the more detailed description of our methods. In particular, we describe better the reason for our 2 measurements of folial shape – width and perimeter – which measure independent dimensions of folding (this is illustrated in Fig. 3d).
- The claim of concerted evolution between cortical and cerebellar values (P 11-12) seems to be based on analyses that exclude body size and brain size. It, therefore, seems possible - or even likely - that all these analyses reveal overall size effects that similarly influence the cortex and cerebellum. When the authors state that they performed a second PC analysis with body and brain size removed "to better understand the patterns of neuroanatomical evolution" it isn't clear to me that is what this achieves. A test would be a model something like [cerebellar measure ~ cortical measure + rest of the brain measure], and this would deal with the problem of 'correlation salad' noted below.
The answer to this question is in the partial correlation diagram in Fig. 7c. This analysis does not exclude body weight nor brain weight. It shows that the strong correlation between cerebellar area and length is supported by a strong positive partial correlation, as is the link between cerebral area and length. There is a significant positive partial correlation between cerebellar section area and cerebral section length. That is, even after covarying everything else, there is still a correlation between cerebellar section area and cerebral section length (this partial correlation is equivalent to the suggestion of the Reviewer). Additionally, there is a positive partial correlation between body weight and cerebellar section area, but not significant partial correlation between body weight and cerebral section area or length. Our approach aims at obtaining a general view of all the relationships in the data. Testing an individual model would certainly decrease the number of correlations, however, it would provide only a partial view of the problem.
- It is not quite clear from fig 6a that the result does indeed support isometry between the data sets (predicted 2/3 slope), and no coefficient confidence intervals are provided.
We have now added the numerical values of the CIs to all our plots in addition to the graphical representations (grey regions) in the previous version of the manuscript. The isometry slope (0.67) is either within the CIs (both for the linear and orthogonal regressions) or at the margin, indicating that if the relationships are not isometric, they are very close to it.
Referencing/discussion/attribution of previous findings
- With respect to the discussion of the relationship between cerebellar architecture and function, and given the emphasis here on correlated evolution with cortex, Ramnani's excellent review paper goes into the issues in considerable detail, which may also help the authors develop their own discussion: Ramnani (2006) The primate cortico-cerebellar system: anatomy and function. Nature Reviews Neuroscience 7, 511-522 (2006)
We have added references to the work of Ramnani.
- The result that humans are outliers with a more folded cerebellum than expected is interesting and adds to recent findings highlighting evolutionary changes in the hominin human cerebellum, cerebellar genes, and epigenetics. Whilst Sereno et al (2020) are cited, it would be good to explain that they found that the human cerebellum has 80% of the surface area of the cortex.
We have added this information to the introduction:
“In humans, the cerebellum has ~80% of the surface area of the cerebral cortex (Sereno et al. 2020), and contains ~80% of all brain neurons, although it represents only ~10% of the brain mass (Azevedo et al. 2009)”
- It would surely also be relevant to highlight some of the molecular work here, such as Harrison & Montgomery (2017). Genetics of Cerebellar and Neocortical Expansion in Anthropoid Primates: A Comparative Approach. Brain Behav Evol. 2017;89(4):274-285. doi: 10.1159/000477432. Epub 2017 (especially since this paper looks at both cerebellar and cortical genes); also Guevara et al (2021) Comparative analysis reveals distinctive epigenetic features of the human cerebellum. PLoS Genet 17(5): e1009506. https://doi.org/10.1371/journal. pgen.1009506. Also relevant here is the complex folding anatomy of the dentate nucleus, which is the largest structure linking cerebellum to cortex: see Sultan et al (2010) The human dentate nucleus: a complex shape untangled. Neuroscience. 2010 Jun 2;167(4):965-8. doi: 10.1016/j.neuroscience.2010.03.007.
The information is certainly important, and could have provided a wider perspective on cerebellar evolution, but we would prefer to keep a focus on cerebellar anatomy and address genetics only indirectly through phylogeny.
- The authors state that results confirm previous findings of a strong relationship between cerebellum and cortex (P 3 and p 16): the earliest reference given is Herculano-Houzel (2010), but this pattern was discovered ten years earlier (Barton & Harvey 2000 Nature 405, 1055-1058. https://doi.org/10.1038/35016580; Fig 1 in Barton 2002 Nature 415, 134-135 (2002). https://doi.org/10.1038/415134a) and elaborated by Whiting & Barton (2003) whose study explored in more detail the relationship between anatomical connections and correlated evolution within the cortico-cerebellar system (this paper is cited later, but only with reference to suggestions about the importance of functions of the cerebellum in the context of conservative structure, which is not its main point). In fact, Herculano-Houzel's analysis, whilst being the first to examine the question in terms of numbers of neurons, was inconclusive on that issue as it did not control for overall size or rest of the brain (A subsequent analysis using her data did, and confirmed the partially correlated evolution - Barton 2012, Philos Trans R Soc Lond B Biol Sci. 367:2097-107. doi: 10.1098/rstb.2012.0112.)
We apologise for this oversight, these references are now included.
-

eLife assessment
This important study gives novel insight into the folding diversity of the cerebellum compared to the cerebrum among 56 mammalian species. Based on impressive data, the analyses performed for anatomical descriptions and phylogenetic comparisons are solid, although some issues need to be addressed regarding the choice of statistical models, and the sample size versus the number of explanatory variables. This study will be of interest to neuroscientists, evolutionary and developmental biologists, and physicists interested in biomechanics, as these observations provide a basis for models of brain folding mechanisms.
-

Reviewer #1 (Public Review):
This paper provides valuable (and impressive) data on the geometry of cerebellar foliation among 56 species of mammals and gives novel insights into the evolution of cerebellar foliation and its relationship with the anatomy of the cerebrum. Thus far, the majority of the research on brain folding focuses on the cerebral cortex with little research on the cerebellum. The results from Heuer et al confirm that the evolution of the cerebellum and cerebrum follows a concerted fashion across mammals. Moreover, they suggest that both the cerebrum and cerebellum folding are explained by a similar mechanistic process.
1. Although I found the introduction well written, I think it lacks some information or needs to develop more on some ideas (e.g., differences between the cerebellum and cerebral cortex, and folding …
Reviewer #1 (Public Review):
This paper provides valuable (and impressive) data on the geometry of cerebellar foliation among 56 species of mammals and gives novel insights into the evolution of cerebellar foliation and its relationship with the anatomy of the cerebrum. Thus far, the majority of the research on brain folding focuses on the cerebral cortex with little research on the cerebellum. The results from Heuer et al confirm that the evolution of the cerebellum and cerebrum follows a concerted fashion across mammals. Moreover, they suggest that both the cerebrum and cerebellum folding are explained by a similar mechanistic process.
1. Although I found the introduction well written, I think it lacks some information or needs to develop more on some ideas (e.g., differences between the cerebellum and cerebral cortex, and folding patterns of both structures). For example, after stating that "Many aspects of the organization of the cerebellum and cerebrum are, however, very different" (1st paragraph), I think the authors need to develop more on what these differences are. Perhaps just rearranging some of the text/paragraphs will help make it better for a broad audience (e.g., authors could move the next paragraph up, i.e., "While the cx is unique to mammals (...)").
2. Given that the authors compare the folding patterns between the cerebrum and cerebellum, another point that could be mentioned in the introduction is the fact that the cerebellum is convoluted in every mammalian species (and non-mammalian spp as well) while the cerebrum tends to be convoluted in species with larger brains. Why is that so? Do we know about it (check Van Essen et al., 2018)? I think this is an important point to raise in the introduction and to bring it back into the discussion with the results.
3. In the results, first paragraph, what do the authors mean by the volume of the medial cerebellum? This needs clarification.
4. In the results: When the authors mention 'frequency of cerebellar folding', do they mean the degree of folding in the cerebellum? At least in non-mammalian species, many studies have tried to compare the 'degree or frequency of folding' in the cerebellum by different proxies/measurements (see Iwaniuk et al., 2006; Yopak et al., 2007; Lisney et al., 2007; Yopak et al., 2016; Cunha et al., 2022). Perhaps change the phrase in the second paragraph of the result to: "There are no comparative analyses of the frequency of cerebellar folding in mammals, to our knowledge".
5. Sultan and Braitenberg (1993) measured cerebella that were sagittally sectioned (instead of coronal), right? Do you think this difference in the plane of the section could be one of the reasons explaining different results on folial width between studies? Why does the foliation index calculated by Sultan and Braitenberg (1993) not provide information about folding frequency?
6. Another point that needs to be clarified is the log transformation of the data. Did the authors use log-transformed data for all types of analyses done in the study? Write this information in the material and methods.
7. The discussion needs to be expanded. The focus of the paper is on the folding pattern of the cerebellum (among different mammalian species) and its relationship with the anatomy of the cerebrum. Therefore, the discussion on this topic needs to be better developed, in my opinion (especially given the interesting results of this paper). For example, with the findings of this study, what can we say about how the folding of the cerebellum is determined across mammals? The authors found that the folial width, folial perimeter, and thickness of the molecular layer increase at a relatively slow rate across the species studied. Does this mean that these parameters have little influence on the cerebellar folding pattern? What mostly defines the folding patterns of the cerebellum given the results? Is it the interaction between section length and area? Can the authors explain why size does not seem to be a "limiting factor" for the folding of the cerebellum (for example, even relatively small cerebella are folded)? Is that because the 'white matter' core of the cerebellum is relatively small (thus more stress on it)?
8. One caveat or point to be raised is the fact that the authors use the median of the variables measured for the whole cerebellum (e.g., median width and median perimeter across all folia). Although the cerebellum is highly uniform in its gross internal morphology and circuitry's organization across most vertebrates, there is evidence showing that the cerebellum may be organized in different functional modules. In that way, different regions or folia of the cerebellum would have different olivo-cortico-nuclear circuitries, forming, each one, a single cerebellar zone. Although it is not completely clear how these modules/zones are organized within the cerebellum, I think the authors could acknowledge this at the end of their discussion, and raise potential ideas for future studies (e.g., analyse folding of the cerebellum within the brain structure - vermis vs lateral cerebellum, for example). I think this would be a good way to emphasize the importance of the results of this study and what are the main questions remaining to be answered. For example, the expansion of the lateral cerebellum in mammals is suggested to be linked with the evolution of vocal learning in different clades (see Smaers et al., 2018). An interesting question would be to understand how foliation within the lateral cerebellum varies across mammalian clades and whether this has something to do with the cellular composition or any other aspect of the microanatomy as well as the evolution of different cognitive skills in mammals.
-

Reviewer #2 (Public Review):
This study explores the variability of cerebellar anatomy in the mammal. By capturing a set of anatomical measures in the cerebellum and including previously reported cerebral and cerebellar metrics in a set of 58 different mammalian species, this study depicts both consistency and heterogeneity in the co-occurrence of different brain features, with a focus on cerebellar structures such as folial wavelength or median depth of the molecular layer. This is very informative as the cerebellum is currently under-explored and the phylogenetic aspect of this work gives insights into evolutionary processes linked to the morphology of the cerebellum.
Strengths:
- The methods used to capture the different brain features are relevant, and include the reuse of previously reported metrics, which makes sense and valorises …
Reviewer #2 (Public Review):
This study explores the variability of cerebellar anatomy in the mammal. By capturing a set of anatomical measures in the cerebellum and including previously reported cerebral and cerebellar metrics in a set of 58 different mammalian species, this study depicts both consistency and heterogeneity in the co-occurrence of different brain features, with a focus on cerebellar structures such as folial wavelength or median depth of the molecular layer. This is very informative as the cerebellum is currently under-explored and the phylogenetic aspect of this work gives insights into evolutionary processes linked to the morphology of the cerebellum.
Strengths:
- The methods used to capture the different brain features are relevant, and include the reuse of previously reported metrics, which makes sense and valorises the previous work of other teams.
- One interesting novel method to detect the depth of the molecular layer is implemented.
- A generous amount of results are reported (including correlations, phylogenetic principal component analyses, ancestor character state estimation, and allometries), with visually effective figures to support them.
- A remarkable effort has been made to make data and code available, which will be of great use to the community.Weaknesses:
- The methods section does not address all the numerical methods used to make sense of the different brain metrics. In the results section, it sometimes makes it difficult for the reader to understand the reason for a sub-analysis and the interpretation of the numerical findings.
- The originality of the article is not sufficiently brought forward:
a) the novel method to detect the depth of the molecular layer is not contextualized in order to understand the shortcomings of previously-established methods. This prevents the reader from understanding its added value and hinders its potential re-use in further studies.
b) The numerous results reported are not sufficiently addressed in the discussion for the reader to get a full grasp of their implications, hindering the clarity of the overall conclusion of the article. -

Reviewer #3 (Public Review):
This paper enhances our understanding of the evolution of cerebellar size and structure and is a potentially valuable addition to the recent literature on this. The examination of both the correlated evolution and divergent patterns of folding in the cerebellum and cortex may help us to understand what processes are involved and how these relate to the structural organisation at macro- and micro-levels. The study combines careful anatomical measurements based on a curated, publicly available mammalian brain collection, consideration of theoretical explanation of folding patterns, and for the most part a good comparative sample size. However, questions about the sample size arise in the authors' more complex statistical models (see below).
The main issues I have are with the statistical analyses. The authors …
Reviewer #3 (Public Review):
This paper enhances our understanding of the evolution of cerebellar size and structure and is a potentially valuable addition to the recent literature on this. The examination of both the correlated evolution and divergent patterns of folding in the cerebellum and cortex may help us to understand what processes are involved and how these relate to the structural organisation at macro- and micro-levels. The study combines careful anatomical measurements based on a curated, publicly available mammalian brain collection, consideration of theoretical explanation of folding patterns, and for the most part a good comparative sample size. However, questions about the sample size arise in the authors' more complex statistical models (see below).
The main issues I have are with the statistical analyses. The authors use a standard phylogenetic approach - Phylogenetic Generalised Least Squares - which is adequate for these questions. I think the authors need to be a bit more cautious in interpreting their results in two respects.
1. The first problem relates to their use of the Ornstein-Uhlenbeck (OU) model: they try fitting three evolutionary models, and conclude that the Ornstein-Uhlenbeck model provides the best fit. However, it has been known for a while that OU models are prone to bias and that the apparent superiority of OU models over Brownian Motion is often an artefact, a problem that increases with smaller sample sizes. (Cooper et al (2016) Biological Journal of the Linnean Society, 2016, 118, 64-77,
2. Second, for the partial correlations (e.g. fig 7) and Principal Components (fig 8) there is a concern about over-fitting: there are 9 variables and only 56 data points (violating the minimal rule of thumb that there should be >10 0bservations per parameter). Added to this, the inclusion of variables lacks a clear theoretical rationale. The high correlations between most variables will be in part because they are to some extent measuring the same things, e.g. the five different measures of cerebellar anatomy which include two measures of folial size. This makes it difficult to separate their effects. I get that the authors are trying to tease apart different aspects of size, but in practice, I think these results (e.g. the presence of negative coefficients in Fig 7) are really hard or impossible to interpret. The partial correlation network looks like a "correlational salad" rather than a theoretically motivated hypothesis test. It isn't clear to me that the PC analyses solve this problem, but it partly depends on the aims of these analyses, which are not made very clear.
The claim of concerted evolution between cortical and cerebellar values (P 11-12) seems to be based on analyses that exclude body size and brain size. It, therefore, seems possible - or even likely - that all these analyses reveal overall size effects that similarly influence the cortex and cerebellum. When the authors state that they performed a second PC analysis with body and brain size removed "to better understand the patterns of neuroanatomical evolution" it isn't clear to me that is what this achieves. A test would be a model something like [cerebellar measure ~ cortical measure + rest of the brain measure], and this would deal with the problem of 'correlation salad' noted below.
It is not quite clear from fig 6a that the result does indeed support isometry between the data sets (predicted 2/3 slope), and no coefficient confidence intervals are provided.
Referencing/discussion/attribution of previous findings
- With respect to the discussion of the relationship between cerebellar architecture and function, and given the emphasis here on correlated evolution with cortex, Ramnani's excellent review paper goes into the issues in considerable detail, which may also help the authors develop their own discussion: Ramnani (2006) The primate cortico-cerebellar system: anatomy and function. Nature Reviews Neuroscience 7, 511-522 (2006
- The result that humans are outliers with a more folded cerebellum than expected is interesting and adds to recent findings highlighting evolutionary changes in the hominin human cerebellum, cerebellar genes, and epigenetics. Whilst Sereno et al (2020) are cited, it would be good to explain that they found that the human cerebellum has 80% of the surface area of the cortex. It would surely also be relevant to highlight some of the molecular work here, such as Harrison & Montgomery (2017). Genetics of Cerebellar and Neocortical Expansion in Anthropoid Primates: A Comparative Approach. Brain Behav Evol. 2017;89(4):274-285. doi: 10.1159/000477432. Epub 2017 (especially since this paper looks at both cerebellar and cortical genes); also Guevara et al (2021) Comparative analysis reveals distinctive epigenetic features of the human cerebellum. PLoS Genet 17(5): e1009506. https://doi.org/10.1371/journal. pgen.1009506. Also relevant here is the complex folding anatomy of the dentate nucleus, which is the largest structure linking cerebellum to cortex: see Sultan et al (2010) The human dentate nucleus: a complex shape untangled. Neuroscience. 2010 Jun 2;167(4):965-8. doi: 10.1016/j.neuroscience.2010.03.007.
- The authors state that results confirm previous findings of a strong relationship between cerebellum and cortex (P 3 and p 16): the earliest reference given is Herculano-Houzel (2010), but this pattern was discovered ten years earlier (Barton & Harvey 2000 Nature 405, 1055-1058. https://doi.org/10.1038/35016580; Fig 1 in Barton 2002 Nature 415, 134-135 (2002). https://doi.org/10.1038/415134a) and elaborated by Whiting & Barton (2003) whose study explored in more detail the relationship between anatomical connections and correlated evolution within the cortico-cerebellar system (this paper is cited later, but only with reference to suggestions about the importance of functions of the cerebellum in the context of conservative structure, which is not its main point). In fact, Herculano-Houzel's analysis, whilst being the first to examine the question in terms of numbers of neurons, was inconclusive on that issue as it did not control for overall size or rest of the brain (A subsequent analysis using her data did, and confirmed the partially correlated evolution - Barton 2012, Philos Trans R Soc Lond B Biol Sci. 367:2097-107. doi: 10.1098/rstb.2012.0112.) -