Quantitative modeling of the emergence of macroscopic grid-like representations
Curation statements for this article:-
Curated by eLife
eLife assessment
This computational work represents a valuable and long overdue assessment of the potential mechanisms associating patterns of activity of entorhinal grid cells, recorded mostly in rodents, with the population property of hexasymmetry detected in non-invasive human studies. The methodic comparison of alternative hypotheses is compelling, and the conclusions are important for the future design of experiments assessing the neural correlates of human navigation across physical, virtual, or conceptual spaces.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
When subjects navigate through spatial environments, grid cells exhibit firing fields that are arranged in a triangular grid pattern. Direct recordings of grid cells from the human brain are rare. Hence, functional magnetic resonance imaging (fMRI) studies proposed an indirect measure of entorhinal grid-cell activity, quantified as hexadirectional modulation of fMRI activity as a function of the subject’s movement direction. However, it remains unclear how the activity of a population of grid cells may exhibit hexadirectional modulation. Here, we use numerical simulations and analytical calculations to suggest that this hexadirectional modulation is best explained by head-direction tuning aligned to the grid axes, whereas it is not clearly supported by a bias of grid cells toward a particular phase offset. Firing-rate adaptation can result in hexadirectional modulation, but the available cellular data is insufficient to clearly support or refute this option. The magnitude of hexadirectional modulation furthermore depends considerably on the subject’s navigation pattern, indicating that future fMRI studies could be designed to test which hypothesis most likely accounts for the fMRI measure of grid cells. Our findings also underline the importance of quantifying the properties of human grid cells to further elucidate how hexadirectional modulations of fMRI activity may emerge.
Article activity feed
-

-

Author response:
Reviewer #1 (Public Review):
Reviewer #1, comment #1: The study is thorough and systematic, and in comparing three well-separated hypotheses about the mechanism leading from grid cells to hexasymmetry it takes a neutral stand above the fray which is to be particularly appreciated. Further, alternative models are considered for the most important additional factor, the type of trajectory taken by the agent whose neural activity is being recorded. Different sets of values, including both "ideal" and "realistic" ones, are considered for the parameters most relevant to each hypothesis. Each of the three hypotheses is found to be viable under some conditions, and less so in others. Having thus given a fair chance to each hypothesis, nevertheless, the study reaches the clear conclusion that the first one, based on …
Author response:
Reviewer #1 (Public Review):
Reviewer #1, comment #1: The study is thorough and systematic, and in comparing three well-separated hypotheses about the mechanism leading from grid cells to hexasymmetry it takes a neutral stand above the fray which is to be particularly appreciated. Further, alternative models are considered for the most important additional factor, the type of trajectory taken by the agent whose neural activity is being recorded. Different sets of values, including both "ideal" and "realistic" ones, are considered for the parameters most relevant to each hypothesis. Each of the three hypotheses is found to be viable under some conditions, and less so in others. Having thus given a fair chance to each hypothesis, nevertheless, the study reaches the clear conclusion that the first one, based on conjunctive grid-by-head-direction cells, is much more plausible overall; the hypothesis based on firing rate adaptation has intermediate but rather weak plausibility; and the one based on clustering of cells with similar spatial phases in practice would not really work. I find this conclusion convincing, and the procedure to reach it, a fair comparison, to be the major strength of the study.
Response: Thanks for your positive assessment of our manuscript.
Reviewer #1, comment #2: What I find less convincing is the implicit a priori discarding of a fourth hypothesis, that is, that the hexasymmetry is unrelated to the presence of grid cells. Full disclosure: we have tried unsuccessfully to detect hexasymmetry in the EEG signal from vowel space and did not find any (Kaya, Soltanipour and Treves, 2020), so I may be ranting off my disappointment, here. I feel, however, that this fourth hypothesis should be at least aired, for a number of reasons. One is that a hexasymmetry signal has been reported also from several other cortical areas, beyond entorhinal cortex (Constantinescu et al, 2016); true, also grid cells in rodents have been reported in other cortical areas as well (Long and Zhang, 2021; Long et al, bioRxiv, 2021), but the exact phenomenology remains to be confirmed.
Response: Thank you for the suggestion to add the hypothesis that the neural hexasymmetry observed in previous fMRI and intracranial EEG studies may be unrelated to grid cells. Following your suggestion, we have now mentioned at the end of the fourth paragraph of the Introduction that “the conjunctive grid by head-direction cell hypothesis does not necessarily depend on an alignment between the preferred head directions with the grid axes”. Furthermore, at the end of section “Potential mechanisms underlying hexadirectional population signals in the entorhinal cortex” (in the Discussion) we write: “However, none of the three hypotheses described here may be true and another mechanism may explain macroscopic grid-like representations. This includes the possibility that neural hexasymmetry is completely unrelated to grid-cell activity, previously summarized as the ‘independence hypothesis' (Kunz et al., 2019). For example, a population of head-direction cells whose preferred head directions occur at offsets of 60 degrees from each other could result in neural hexasymmetry in the absence of grid cells. The conjunctive grid by head-direction cell hypothesis thus also works without grid cells, which may explain why grid-like representations have been observed (using fMRI) in regions outside the entorhinal cortex, where rodent studies have not yet identified grid cells (Doeller et al., 2010; Constantinescu et al., 2016). In that case, however, another mechanism would be needed that could explain why the preferred head directions of different head-direction cells occur at multiples of 60 degrees. Attractor-network structures may be involved in such a mechanism, but this remains speculative at the current stage.” We now also mention the results from Long and Zhang (second paragraph of the Introduction): “Surprisingly, grid cells have also been observed in the primary somatosensory cortex in foraging rats (Long and Zhang, 2021).”
Regarding your EEG study, we have added a reference to it in the manuscript and state that it is an example for a study that did not find evidence for neural hexasymmetry (end of first paragraph of the Discussion): “We note though that some studies did not find evidence for neural hexasymmetry. For example, a surface EEG study with participants “navigating” through an abstract vowel space did not observe hexasymmetry in the EEG signal as a function of the participants’ movement direction through vowel space (Kaya et al., 2020). Another fMRI study did not find evidence for grid-like representations in the ventromedial prefrontal cortex while participants performed value-based decision making (Lee et al., 2021). This raises the question whether the detection of macroscopic grid-like representations is limited to some recording techniques (e.g., fMRI and iEEG but not surface EEG) and to what extent they are present in different tasks.”
Reviewer #1, comment #3: Second, as the authors note, the conjunctive mechanism is based on the tight coupling of a narrow head direction selectivity to one of the grid axes. They compare "ideal" with "Doeller" parameters, but to me the "Doeller" ones appear rather narrower than commonly observed and, crucially, they are applied to all cells in the simulations, whereas in reality only a proportion of cells in mEC are reported to be grid cells, only a proportion of them to be conjunctive, and only some of these to be narrowly conjunctive. Further, Gerlei et al (2020) find that conjunctive grid cells may have each of their fields modulated by different head directions, a truly surprising phenomenon that, if extensive, seems to me to cast doubts on the relation between mass activity hexasymmetry and single grid cells.
Response: We have revised the manuscript in several ways to address the different aspects of this comment.
Firstly, we agree with the reviewer that our “Doeller” parameter for the tuning width is narrower than commonly observed. We have therefore reevaluated the concentration parameter κ_c in the ‘realistic’ case from 10 rad-2 (corresponding to a tuning width of 18o) to 4 rad-2 (corresponding to a tuning width of 29o). We chose this value by referring to Supplementary Figure 3 of Doeller et al. (2010). In their figure, the tuning curves usually cover between one sixth and one third of a circle. Since stronger head-direction tuning contributes the most to the resulting hexasymmetry, we chose a value of κ_c=4 for the tuning parameter, which corresponds to a tuning width (= half width) of 29o (full width of roughly one sixth of a circle). Regarding the coupling of the preferred head directions to the grid axes, the specific value of the jitter σc = 3 degrees that quantifies the coupling of the head-direction preference to the grid axes was extracted from the 95% confidence interval given in the third row of the Table in Supplementary Figure 5b of Doeller et al. 2010. We now better explain the origin of these values in our new Methods section “Parameter estimation” and provide an overview of all parameter values in Table 1.
Furthermore, in response to your comment, we have revised Figure 2E to show neural hexasymmetries for a larger range of values of the jitter (σc from 0 to 30 degrees), going way beyond the values that Doeller et al. suggested. We have also added a new supplementary figure (Figure 2 – figure supplement 1) where we further extend the range of tuning widths (parameter κ_c) to 60 degrees. This provides the reader with a comprehensive understanding of what parameter values are needed to reach a particular hexasymmetry.
Regarding your comments on the prevalence of conjunctive grid by head-direction cells, we have revised the manuscript to make it explicit that the actual percentage of conjunctive cells with the necessary properties may be low in the entorhinal cortex (first paragraph of section “A note on our choice of the values of model parameters” of the Discussion): “Empirical studies in rodents found a wide range of tuning widths among grid cells ranging from broad to narrow (Doeller et al., 2010; Sargolini et al., 2006). The percentage of conjunctive cells in the entorhinal cortex with a sufficiently narrow tuning may thus be low. Such distributions (with a proportionally small amount of narrowly tuned conjunctive cells) lead to low values in the absolute hexasymmetry. The neural hexasymmetry in this case would be driven by the subset of cells with sufficiently narrow tuning widths. If this causes the neural hexasymmetry to drop below noise levels, the statistical evaluation of this hypothesis would change.” In addition, in Figure 5, we have applied the coupling between preferred head directions and grid axes to only one third of all grid cells (parameter pc= ⅓ in Table 1), following the values reported by Boccara et al. 2010 and Sargolini et al. 2006. To strengthen the link between Figure 5 and Figure 2, we now state the hexasymmetry when using pc= ⅓ along with a ‘realistic’ tuning width and jitter for head-direction modulated grid cells in Figure 2H. Additionally, we performed new simulations where we observed a linear relationship (above the noise floor) between the proportion of conjunctive cells and the hexasymmetry. This shall help the reader understand the effect of a reduced percentage of conjunctive cells on the absolute hexasymmetry values. We have added these results as a new supplementary figure (Figure 2 – figure supplement 2).
Finally, regarding your comment on the findings by Gerlei et al. 2020, we now reference this study in our manuscript and discuss the possible implications (second paragraph of section “A note on our choice of the values of model parameters” of the Discussion): “Additionally, while we assumed that all conjunctive grid cells maintain the same preferred head direction between different firing fields, conjunctive grid cells have also been shown to exhibit different preferred head directions in different firing fields (Gerlei et al., 2020). This could lead to hexadirectional modulation if the different preferred head directions are offset by 60o from each other, but will not give rise to hexadirectional modulation if the preferred head directions are randomly distributed. To the best of our knowledge, the distribution of preferred head directions was not quantified by Gerlei et al. (2020), thus this remains an open question.”
Reviewer #1, comment #4: Finally, a variant of the fourth hypothesis is that the hexasymmetry might be produced by a clustering of head direction preferences across head direction cells similar to that hypothesized in the first hypothesis, but without such cells having to fire in grid patterns. If head direction selectivity is so clustered, who needs the grids? This would explain why hexasymmetry is ubiquitous, and could easily be explored computationally by, in fact, a simplification of the models considered in this study.
Response: We fully agree with you. We now explain this possibility in the Introduction where we introduce the conjunctive grid by head-direction cell hypothesis (fourth paragraph of the Introduction) and return to it in the Discussion (section “Potential mechanisms underlying hexadirectional population signals in the entorhinal cortex”). There, we now also explain that in such a case another mechanism would be needed to ensure that the preferred head directions of head-direction cells exhibit six-fold rotational symmetry.
Reviewer #2 (Public Review):
Reviewer #2, comment #1: Grid cells - originally discovered in single-cell recordings from the rodent entorhinal cortex, and subsequently identified in single-cell recordings from the human brain - are believed to contribute to a range of cognitive functions including spatial navigation, long-term memory function, and inferential reasoning. Following a landmark study by Doeller et al. (Nature, 2010), a plethora of human neuroimaging studies have hypothesised that grid cell population activity might also be reflected in the six-fold (or 'hexadirectional') modulation of the BOLD signal (following the six-fold rotational symmetry exhibited by individual grid cell firing patterns), or in the amplitude of oscillatory activity recorded using MEG or intracranial EEG. The mechanism by which these network-level dynamics might arise from the firing patterns of individual grid cells remains unclear, however.
In this study, Khalid and colleagues use a combination of computational modelling and mathematical analysis to evaluate three competing hypotheses that describe how the hexadirectional modulation of population firing rates (taken as a simple proxy for the BOLD, MEG, or iEEG signal) might arise from the firing patterns of individual grid cells. They demonstrate that all three mechanisms could account for these network-level dynamics if a specific set of conditions relating to the agent's movement trajectory and the underlying properties of grid cell firing patterns are satisfied.
The computational modelling and mathematic analyses presented here are rigorous, clearly motivated, and intuitively described. In addition, these results are important both for the interpretation of hexadirectional modulation in existing data sets and for the design of future experiments and analyses that aim to probe grid cell population activity. As such, this study is likely to have a significant impact on the field by providing a firmer theoretical basis for the interpretation of neuroimaging data. To my mind, the only weakness is the relatively limited focus on the known properties of grid cells in rodent entorhinal cortex, and the network level activity that these firing patterns might be expected to produce under each hypothesis. Strengthening the link with existing neurobiology would further enhance the importance of these results for those hoping to assay grid cell firing patterns in recordings of ensemble-level neural activity.
Response: Thank you very much for reviewing our manuscript and your positive assessment. Following your comments, we have revised the manuscript to more closely link our simulations to known properties of grid cells in the rodent entorhinal cortex.
Reviewer #3 (Public Review):
Reviewer #3, comment #1: This is an interesting and carefully carried out theoretical analysis of potential explanations for hexadirectional modulation of neural population activity that has been reported in the human entorhinal cortex and some other cortical regions. The previously reported hexadirectional modulation is of considerable interest as it has been proposed to be a proxy for the activation of grid cell networks. However, the extent to which this proposal is consistent with the known firing properties of grids hasn't received the attention it perhaps deserves. By comparing the predictions of three different models this study imposes constraints on possible mechanisms and generates predictions that can be tested through future experimentation.
Overall, while the conclusions of the study are convincing, I think the usefulness to the field would be increased if null hypotheses were more carefully considered and if the authors' new metric for hexadirectional modulation (H) could be directly contrasted with previously used metrics. For example, if the effect sizes for hexadirectional modulation in the previous fMRI and EEG data could be more directly compared with those of the models here, then this could help in establishing the extent to which the experimental hexadirectional modulation stands out from path hexasymmetry and how close it comes to the striking modulation observed with the conjunctive models. It could also be helpful to consider scenarios in which hexadirectional modulation is independent of grid firing, for example perhaps with appropriate coordination of head direction cell firing.
Response: Thanks for reviewing our manuscript and for the overall positive assessment. The new Methods section “Implementation of previously used metrics” starts with the following sentences: “We applied three previously used metrics to our framework: the Generalized Linear Model (GLM) method by Doeller et al. 2010; the GLM method with binning by Kunz et al. 2015; and the circular-linear correlation method by Maidenbaum et al. 2018.” We have created a new supplementary figure (Figure 5 – figure supplement 4) in which we compare the results from these other methods to the results of our new method. Overall, the results are highly similar, indicating that all these methods are equally suited to test for a hexadirectional modulation of neural activity.
In section “Implementation of previously used metrics” we then explain: “In brief, in the GLM method (e.g. used in Doeller et al., 2010), the hexasymmetry is found in two steps: the orientation of the hexadirectional modulation is first estimated on the first half of the data by using the regressors
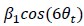 and
and 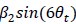 on the time-discrete fMRI activity (Equation 9), with θt being the movement direction of the subject in time step t. The amplitude of the signal is then estimated on the second half of the data using the single regressor
on the time-discrete fMRI activity (Equation 9), with θt being the movement direction of the subject in time step t. The amplitude of the signal is then estimated on the second half of the data using the single regressor 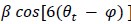 , where
, where 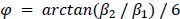 . The hexasymmetry is then evaluated as
. The hexasymmetry is then evaluated as 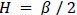 .
.The GLM method with binning (e.g. used in Kunz et al., 2015) uses the same procedure as the GLM method for estimating the grid orientation in the first half of the data, but the amplitude is estimated differently on the second half by a regressor that has a value 1 if θt is aligned with a peak of the hexadirectional modulation (aligned if
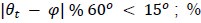 , modulo operator) and a value of -1 if θt is misaligned. The hexasymmetry is then calculated from the amplitude in the same way as in the GLM method.
, modulo operator) and a value of -1 if θt is misaligned. The hexasymmetry is then calculated from the amplitude in the same way as in the GLM method.The circular-linear correlation method (e.g. used in Maidenbaum et al., 2018) is similar to the GLM method in that it uses the regressors β1 cos(6θ_t) and β2 on the time-discrete mean activity, but instead of using β1 and β2 to estimate the orientation of the hexadirectional modulation, the beta values are directly used to estimate the hexasymmetry using the relation
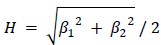 .”
.”For each of the three previously used metrics and our new method, we estimated the resulting hexasymmetry (new Figure 5 – figure supplement 4 in the manuscript). In the Methods section “Implementation of previously used metrics” we then continue with our explanations: “Regarding the statistical evaluation, each method evaluates the size of the neural hexasymmetry differently. Specifically, the new method developed in our manuscript compares the neural hexasymmetry to path hexasymmetry to test whether neural hexasymmetry is significantly above path hexasymmetry. For the two generalized linear model (GLM) methods, we compare the hexasymmetry to zero (using the Mann-Whitney U test) to establish significance. Hexasymmetry values can be negative in these approaches, allowing the statistical comparison against 0. Negative values occur when the estimated grid orientation from the first data half does not match the grid orientation from the second data half. Regarding the statistical evaluation of the circular-linear correlation method, we calculated a z-score by comparing each empirical observation of the hexasymmetry to hexasymmetries from a set of surrogate distributions (as in Maidenbaum et al., 2018). We then calculate a p-value by comparing the distribution of z-scores versus zero using a Mann-Whitney U test. We use the z-scores instead of the hexasymmetry for the circular-linear correlation method to match the procedure used in Maidenbaum et al. (2018). We obtained the surrogate distributions by circularly shifting the vector of movement directions relative to the time dependent vector of firing rates. For random walks, the vector is shifted by a random number drawn from a uniform distribution defined with the same length as the number of time points in the vector of movement directions. For the star-like walks and piecewise linear walks, the shift is a random integer multiplied by the number of time points in a linear segment. Circularly shifting the vector of movement directions scrambles the correlations between movement direction and neural activity while preserving their temporal structure.”
The results of these simulations, i.e. the comparison of our new method to previously used metrics, are summarized in Figure 5 – figure supplement 4 and show qualitatively identical findings when using the different methods. We have added this information also to the manuscript in the third paragraph of section “Quantification of hexasymmetry of neural activity and trajectories” of the Methods: “Empirical (fMRI/iEEG) studies (e.g. Doeller et al., 2010; Kunz et al., 2015; Maidenbaum et al., 2018) addressed this problem of trajectories spuriously contributing to hexasymmetry by fitting a Generalized Linear Model (GLM) to the time discrete fMRI/iEEG activity. In contrast, our new approach to hexasymmetry in Equation (12) quantifies the contribution of the path to the neural hexasymmetry explicitly, and has the advantage that it allows an analytical treatment (see next section). Comparing our new method with previous methods for evaluating hexasymmetry led to qualitatively identical statistical effects (Figure 5 – figure supplement 4).” We have also added a pointer to this new supplementary figure in the caption of Figure 5 in the manuscript: “For a comparison between our method and previously used methods for evaluating hexasymmetry, see Figure 5 – figure supplement 4.”
-

eLife assessment
This computational work represents a valuable and long overdue assessment of the potential mechanisms associating patterns of activity of entorhinal grid cells, recorded mostly in rodents, with the population property of hexasymmetry detected in non-invasive human studies. The methodic comparison of alternative hypotheses is compelling, and the conclusions are important for the future design of experiments assessing the neural correlates of human navigation across physical, virtual, or conceptual spaces.
-

Reviewer #1 (Public Review):
The study is thorough and systematic, and in comparing three well-separated hypotheses about the mechanism leading from grid cells to hexasymmetry it takes a neutral stand above the fray which is to be particularly appreciated. Further, alternative models are considered for the most important additional factor, the type of trajectory taken by the agent whose neural activity is being recorded. Different sets of values, including both "ideal" and "realistic" ones, are considered for the parameters most relevant to each hypothesis. Each of the three hypotheses is found to be viable under some conditions, and less so in others. Having thus given a fair chance to each hypothesis, nevertheless, the study reaches the clear conclusion that the first one, based on conjunctive grid-by-head-direction cells, is much …
Reviewer #1 (Public Review):
The study is thorough and systematic, and in comparing three well-separated hypotheses about the mechanism leading from grid cells to hexasymmetry it takes a neutral stand above the fray which is to be particularly appreciated. Further, alternative models are considered for the most important additional factor, the type of trajectory taken by the agent whose neural activity is being recorded. Different sets of values, including both "ideal" and "realistic" ones, are considered for the parameters most relevant to each hypothesis. Each of the three hypotheses is found to be viable under some conditions, and less so in others. Having thus given a fair chance to each hypothesis, nevertheless, the study reaches the clear conclusion that the first one, based on conjunctive grid-by-head-direction cells, is much more plausible overall; the hypothesis based on firing rate adaptation has intermediate but rather weak plausibility; and the one based on clustering of cells with similar spatial phases in practice would not really work. I find this conclusion convincing, and the procedure to reach it, a fair comparison, to be the major strength of the study.
What I find less convincing is the implicit a priori discarding of a fourth hypothesis, that is, that the hexasymmetry is unrelated to the presence of grid cells. Full disclosure: we have tried unsuccessfully to detect hexasymmetry in the EEG signal from vowel space and did not find any (Kaya, Soltanipour and Treves, 2020), so I may be ranting off my disappointment, here. I feel, however, that this fourth hypothesis should be at least aired, for a number of reasons. One is that a hexasymmetry signal has been reported also from several other cortical areas, beyond entorhinal cortex (Constantinescu et al, 2016); true, also grid cells in rodents have been reported in other cortical areas as well (Long and Zhang, 2021; Long et al, bioRxiv, 2021), but the exact phenomenology remains to be confirmed. Second, as the authors note, the conjunctive mechanism is based on the tight coupling of a narrow head direction selectivity to one of the grid axes. They compare "ideal" with "Doeller" parameters, but to me the "Doeller" ones appear rather narrower than commonly observed and, crucially, they are applied to all cells in the simulations, whereas in reality only a proportion of cells in mEC are reported to be grid cells, only a proportion of them to be conjunctive, and only some of these to be narrowly conjunctive. Further, Gerlei et al (2020) find that conjunctive grid cells may have each of their fields modulated by different head directions, a truly surprising phenomenon that, if extensive, seems to me to cast doubts on the relation between mass activity hexasymmetry and single grid cells.
Finally, a variant of the fourth hypothesis is that the hexasymmetry might be produced by a clustering of head direction preferences across head direction cells similar to that hypothesized in the first hypothesis, but without such cells having to fire in grid patterns. If head direction selectivity is so clustered, who needs the grids? This would explain why hexasymmetry is ubiquitous, and could easily be explored computationally by, in fact, a simplification of the models considered in this study.
-

Reviewer #2 (Public Review):
Grid cells - originally discovered in single-cell recordings from the rodent entorhinal cortex, and subsequently identified in single-cell recordings from the human brain - are believed to contribute to a range of cognitive functions including spatial navigation, long-term memory function, and inferential reasoning. Following a landmark study by Doeller et al. (Nature, 2010), a plethora of human neuroimaging studies have hypothesised that grid cell population activity might also be reflected in the six-fold (or 'hexadirectional') modulation of the BOLD signal (following the six-fold rotational symmetry exhibited by individual grid cell firing patterns), or in the amplitude of oscillatory activity recorded using MEG or intracranial EEG. The mechanism by which these network-level dynamics might arise from the …
Reviewer #2 (Public Review):
Grid cells - originally discovered in single-cell recordings from the rodent entorhinal cortex, and subsequently identified in single-cell recordings from the human brain - are believed to contribute to a range of cognitive functions including spatial navigation, long-term memory function, and inferential reasoning. Following a landmark study by Doeller et al. (Nature, 2010), a plethora of human neuroimaging studies have hypothesised that grid cell population activity might also be reflected in the six-fold (or 'hexadirectional') modulation of the BOLD signal (following the six-fold rotational symmetry exhibited by individual grid cell firing patterns), or in the amplitude of oscillatory activity recorded using MEG or intracranial EEG. The mechanism by which these network-level dynamics might arise from the firing patterns of individual grid cells remains unclear, however.
In this study, Khalid and colleagues use a combination of computational modelling and mathematical analysis to evaluate three competing hypotheses that describe how the hexadirectional modulation of population firing rates (taken as a simple proxy for the BOLD, MEG, or iEEG signal) might arise from the firing patterns of individual grid cells. They demonstrate that all three mechanisms could account for these network-level dynamics if a specific set of conditions relating to the agent's movement trajectory and the underlying properties of grid cell firing patterns are satisfied.
The computational modelling and mathematic analyses presented here are rigorous, clearly motivated, and intuitively described. In addition, these results are important both for the interpretation of hexadirectional modulation in existing data sets and for the design of future experiments and analyses that aim to probe grid cell population activity. As such, this study is likely to have a significant impact on the field by providing a firmer theoretical basis for the interpretation of neuroimaging data. To my mind, the only weakness is the relatively limited focus on the known properties of grid cells in rodent entorhinal cortex, and the network level activity that these firing patterns might be expected to produce under each hypothesis. Strengthening the link with existing neurobiology would further enhance the importance of these results for those hoping to assay grid cell firing patterns in recordings of ensemble-level neural activity.
-

Reviewer #3 (Public Review):
This is an interesting and carefully carried out theoretical analysis of potential explanations for hexadirectional modulation of neural population activity that has been reported in the human entorhinal cortex and some other cortical regions. The previously reported hexadirectional modulation is of considerable interest as it has been proposed to be a proxy for the activation of grid cell networks. However, the extent to which this proposal is consistent with the known firing properties of grids hasn't received the attention it perhaps deserves. By comparing the predictions of three different models this study imposes constraints on possible mechanisms and generates predictions that can be tested through future experimentation.
Overall, while the conclusions of the study are convincing, I think the …
Reviewer #3 (Public Review):
This is an interesting and carefully carried out theoretical analysis of potential explanations for hexadirectional modulation of neural population activity that has been reported in the human entorhinal cortex and some other cortical regions. The previously reported hexadirectional modulation is of considerable interest as it has been proposed to be a proxy for the activation of grid cell networks. However, the extent to which this proposal is consistent with the known firing properties of grids hasn't received the attention it perhaps deserves. By comparing the predictions of three different models this study imposes constraints on possible mechanisms and generates predictions that can be tested through future experimentation.
Overall, while the conclusions of the study are convincing, I think the usefulness to the field would be increased if null hypotheses were more carefully considered and if the authors' new metric for hexadirectional modulation (H) could be directly contrasted with previously used metrics. For example, if the effect sizes for hexadirectional modulation in the previous fMRI and EEG data could be more directly compared with those of the models here, then this could help in establishing the extent to which the experimental hexadirectional modulation stands out from path hexasymmetry and how close it comes to the striking modulation observed with the conjunctive models. It could also be helpful to consider scenarios in which hexadirectional modulation is independent of grid firing, for example perhaps with appropriate coordination of head direction cell firing.
-
