Activation of the P2RX7/IL-18 pathway in immune cells attenuates lung fibrosis
Curation statements for this article:-
Curated by eLife
eLife assessment
This study presents a potentially valuable discovery which indicates that activation of the P2RX7 pathway by the small molecule HEI3090 can reduce lung fibrosis after its establishment by inflammatory damage. If confirmed, the study could clarify the role of specific immune networks in the establishment and progression of lung fibrosis. The presented data and analyses showing the efficacy of HEI3090 small molecule acting via the P2RX7 pathway in reducing lung fibrosis are solid. The studies also show that genetic deletion of P2RX7 itself can reduce the extent of fibrosis. P2RX7 can thus have distinct effects in various phases of the development of lung fibrosis. There is a need for additional definitive studies that specifically identify the discrete phases of when inflammasome activation via P2RX7 signaling can worsen fibrosis versus when the same signaling can be beneficial. It also needs to be established whether distinct immune cell populations mediate the detrimental and beneficial effects of P2RX7 activation in lung fibrosis.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Idiopathic pulmonary fibrosis (IPF) is an aggressive interstitial lung disease associated with progressive and irreversible deterioration of respiratory functions that lacks curative therapies. Despite IPF being associated with a dysregulated immune response, current antifibrotics aim only at limiting fibroproliferation. Transcriptomic analyses show that the P2RX7/IL18/IFNG axis is downregulated in IPF patients and that P2 R X7 has immunoregulatory functions. Using our positive modulator of P2 R X7, we show that activation of the P2 R X7/IL-18 axis in immune cells limits lung fibrosis progression in a mouse model by favoring an antifibrotic immune environment, with notably an enhanced IL-18-dependent IFN-γ production by lung T cells leading to a decreased production of IL-17 and TGFβ. Overall, we show the ability of the immune system to limit lung fibrosis progression by targeting the immunomodulator P2 R X7. Hence, treatment with a small activator of P2 R X7 may represent a promising strategy to help patients with lung fibrosis.
Article activity feed
-

-

-

-

-

Author Response
The following is the authors’ response to the previous reviews.
Point to point response for the editors
We are deeply grateful for the time you have devoted to reviewing this manuscript, and we sincerely thank you. Your insightful feedback has been instrumental in enhancing the quality of our work.
In the revised version of the manuscript, we have carefully addressed each of the concerns you raised. Below, you will find a detailed summary of how your feedback has been incorporated to improve the overall content and clarity of the document.
- P2RX7 effects: In Figure 2, the vehicle treated P2RX7 knockout (panel M) shows an Ashcroft score of about 1.5 after BLM. Comparing this to the Ashcroft score of 3 after BLM in the wildtype (panel C) suggests that P2RX7 deletion is an effective way to reduce fibrosis by half!.
The …
Author Response
The following is the authors’ response to the previous reviews.
Point to point response for the editors
We are deeply grateful for the time you have devoted to reviewing this manuscript, and we sincerely thank you. Your insightful feedback has been instrumental in enhancing the quality of our work.
In the revised version of the manuscript, we have carefully addressed each of the concerns you raised. Below, you will find a detailed summary of how your feedback has been incorporated to improve the overall content and clarity of the document.
- P2RX7 effects: In Figure 2, the vehicle treated P2RX7 knockout (panel M) shows an Ashcroft score of about 1.5 after BLM. Comparing this to the Ashcroft score of 3 after BLM in the wildtype (panel C) suggests that P2RX7 deletion is an effective way to reduce fibrosis by half!.
The argument that HEI3090 also reduces fibrosis by activating P2RX7 is of course very difficult to convey and it seems contradictory that P2RX7 deletion and P2RX7 activation can be both anti-fibrotic. This is an unusual claim and confuses the reviewers as well as the future readers.
This has many important health implications because activating an inflammatory pathway via P2RX7 and IL-18 could be risky in terms of a fibrosis treatment as inflammatory activation can also worsen fibrosis. The authors' own P2RX7 KO data (untreated vehicle groups) indeed confirms that P2RX7 can be pro-fibrotic.
We thank the editors for their comment highlighting the lack of clarity in our message. Indeed, we verified whether the antifibrotic action of HEI3090 depends on the expression of P2RX7 by inducing lung fibrosis in P2RX7 KO mice. In doing so, we initially observed that P2RX7 plays a role in the development of BLM-induced lung fibrosis. This is illustrated by a decrease of 50% in the Ashcroft score, as shown in Figure 2M and Supplemental Figure 2C of the revised manuscript.
To increase the clarity of your message, we added in the text the following paragraph:
"We further verified whether the antifibrotic action of HEI3090 depends on the expression of P2RX7 by inducing lung fibrosis in p2rx7 knockout (KO) mice. In doing so, we initially observed that P2RX7 plays a role in the development of BLM-induced lung fibrosis. This is illustrated by a decrease of 50% in the Ashcroft score, with a mean value of 1.7 in P2RX7 knockout mice compared to 3 in wild-type mice (Figure 2M and Supplemental Figure 2C). It is important to note that p2rx7 -/- mice still exhibit signs of lung fibrosis, such as thickening of the alveolar wall and a reduction in free air space, in comparison to naïve mice that received PBS instead of BLM (see Supplemental Figure 2A). This result confirms a previous report indicating that BLM-induced lung fibrosis partially depends on the activation of the P2RX7/pannexin-1 axis, leading to the production of IL-1β in the lung. Additionally, in contrast to the observations in WT mice, HEI3090 failed to attenuate the remaining lung fibrosis in p2rx7 -/- mice, as measured by the Ashcroft score (Figure 2M), the percentage of lung tissue with fibrotic lesions, or the intensity of collagen fibers (Supplemental Figure 2D). These results show that P2RX7 alone participates in fibrosis and that HEI3090 exerts a specific antifibrotic effect through this receptor (see Supplemental Figure 2C)."
Since we used the HEI3090 compound in this study and to be closer to the results, we have replaced the title of 2 chapters in the results section as followed:
“HEI3090 inhibits the onset of pulmonary fibrosis in the bleomycin mouse model” instead of P2RX7 activation inhibits the onset of pulmonary fibrosis in the bleomycin mouse model and “HEI3090 shapes immune cell infiltration in the lungs" instead of P2RX7 activation shapes immune cell infiltration in the lungs
We concur that the observation of both anti-fibrotic effects following P2RX7 deletion and P2RX7 activation appears contradictory. This specific aspect has been thoroughly addressed and extensively discussed in the revised manuscript.
“A major unmet need in the field of IPF is new treatment to fight this uncurable disease. In this preclinical study, we demonstrate the ability of immune cells to limit lung fibrosis progression. Based on the hypothesis that a local activation of a T cell immune response and upregulation of IFN-γ production has antifibrotic proprieties, we used the HEI3090 positive modulator of the purinergic receptor P2RX7, previously developed in our laboratory (Douguet et al., 2021), to demonstrate that activation of the P2RX7/IL-18 pathway attenuates lung fibrosis in the bleomycin mouse model. We have demonstrated that lung fibrosis progression is inhibited by HEI3090 in the fibrotic phase but also in the acute phase of the BLM fibrosis mouse model, i.e. during the period of inflammation. This lung fibrosis mouse model commonly employed in preclinical investigations, has recently been recognized as the optimal model for studying IPF (Jenkins et al., 2017). In this model, the intrapulmonary administration of BLM induces DNA damage in alveolar epithelial type 1 cells, triggering cellular demise and the release of ATP. The extracellular release of ATP from injured cells activates the P2RX7/pannexin 1 axis, initiating the maturation of IL1β and subsequent induction of inflammation and fibrosis. In line with this, mice lacking P2RX7 exhibited reduced neutrophil counts in their bronchoalveolar fluids and decreased levels of IL1β in their lungs compared to WT mice (Riteau et al., 2010). Based on these findings, Riteau and colleagues postulated that the inhibition of P2RX7 activity may offer a potential strategy for the therapeutic control of fibrosis in lung injury. In the present study we provided strong evidence showing that selective activation of P2RX7 on immune cells, through the use of HEI3090, can dampen inflammation and fibrosis by releasing IL-18. The efficacy of HEI3090 to inhibit lung fibrosis was evaluated histologically on the whole lung’s surface by evaluating the severity of fibrosis using three independent approaches applied to the whole lung, the Ashcroft score, quantification of fibroblasts/myofibroblasts (CD140a) and polarized-light microscopy of Sirius Red staining to quantify collagen fibers. All these methods of fibrosis assessment revealed that HEI3090 exerts an inhibitory effect on lung fibrosis, underscoring the necessity for a thorough pre-clinical assessment of HEI3090's mode of action. Notably, HEI3090 functions as an activator, rather than an inhibitor, of P2RX7, further emphasizing the importance of elucidating its intricate mechanisms.”
We trust that the detailed explanation provided therein will adequately persuade both the reviewers and future readers.
- The statistical concerns are based on the phrasing of "the experiment was stopped when significantly statistical results were observed". This is different from the power analysis approach that the authors describe in their latest rebuttal. However, it raises the question why the power analysis was performed using "on a one-way ANOVA analysis comparing in each experiment the vehicle and the treated group". The analyses in the manuscript use the Mann-Whitney test for several comparisons which ahs the assumption that the samples do NOT have a normal distribution. An ANOVA and t-tests have the assumption that samples are normally distributed. If the power analysis and "statistical forecasting" assumed a normal distribution and used an ANOVA, then shouldn't all the analyses also use a statistical test appropriate for normally distributed samples such as ANOVA and t-tests?
Several of the data points in the figures seem to be normally distributed and therefore t-test for two group comparisons would be more appropriate. The most rigorous approach would be to check for normal distribution before choosing the correct statistical test and using the t-test/ANOVA in normally distributed data as well as Mann-Whitney for non-normally distributed data.
We described in the Material and Method section of the revised manuscript our approach to determine the size of experimental group.
“The determination of experimental group sizes involved conducting a pilot experiment with four mice in each group. Subsequently, a power analysis, based on the pilot experiment's findings (which revealed a 40% difference with a standard error of 0.9, α risk of 0.05, and power of 0.8), was performed to ascertain the appropriate group size for studying the effects of HEI3090 on BLM-induced lung fibrosis. The results of the pilot experiment and power analysis indicated that a group size of four mice was sufficient to characterize the observed effects. For each full-scale experiment, we initiated the study with 6 to 8 mice per group, ensuring a minimum of 5 mice in each group for robust statistical analysis. Additionally, we systematically employed the ROULT method to identify and subsequently exclude any outliers present in each experiment before conducting statistical analyses”.
We now described in the Material and Method section how we carried out the statistical analyses.
“Quantitative data were described and presented graphically as medians and interquartiles or means and standard deviations. The distribution normality was tested with the Shapiro's test and homoscedasticity with a Bartlett's test. For two categories, statistical comparisons were performed using the Student's t-test or the Mann–Whitney's test. For three and more categories, analysis of variance (ANOVA) or non-parametric data with Kruskal–Wallis was performed to test variables expressed as categories versus continuous variables. If this test was significant, we used the Tukey's test to compare these categories and the Bonferroni’s test to adjust the significant threshold. For the Gene Set Enrichment Analyses (GSEA), bilateral Kolmogorov–Smirnov test, and false discovery rate (FDR) were used. All statistical analyses were performed by biostatistician using Prism8 program from GraphPad software. Tests of significance was two-tailed and considered significant with an alpha level of P < 0.05. (graphically: * for P < 0.05, ** for P < 0.01, *** for P < 0.001).”
We also added in the legend of each figure, the statistical analysis used to determine each p-values.
- Adoptive transfer: The concerns of the reviewers include an unclear analysis of the effects of adoptive transfer itself and the approaches used to analyze the data independent of the HEI3090 effect. For example, in Figure 4, the adoptive transfer IL18-/- cells (vehicle group) leads to an Ashcroft score of about 1 and among the lowest of the BLM exposed mice. Does that mean that IL18 is pro-fibrotic and that its absence is beneficial? If yes, it would go against the core premise of the study that IL18 is beneficial. Statistical comparisons of the all the vehicle conditions in the adoptive transfer would help clarify whether adoptive transfer of NLRP3-/-, IL18-/- in wild-type and P2RX7-/- mice reduces or increases fibrosis. Such multiple comparisons are necessary to fully understand the adoptive transfer studies and would also require the appropriate statistical test with corrections for multiple comparisons such as Kruskal-Wallis for data without normal distribution and ANOVA with post hoc correction for normal distribution.
We added a new paragraph in the revised version of the manuscript to explain the adoptive transfer approach.
“We wanted to further investigate the mechanism of action of HEI3090 by identifying the cellular compartment and signaling pathway required for its activity. Since the expression of P2RX7 and the P2RX7-dependent release of IL-18 are mostly associated with immune cells (Ferrari et al., 2006), and since HEI3090 shapes the lung immune landscape (Figure 3), we investigated whether immune cells were required for the antifibrotic effect of HEI3090. To do so, we conducted adoptive transfer experiments wherein immune cells from a donor mouse were intravenously injected one day before BLM administration into an acceptor mouse. The intravenous injection route was chosen as it is a standard method for targeting the lungs, as previously documented (Wei and Zhao, 2014). This approach was previously used with success in our laboratory (Douguet et al., 2021). It is noteworthy that this adoptive transfer approach did not influence the response to HEI3090. This was observed consistently in both p2rx7 -/- mice and p2rx7 -/- mice that received splenocytes of the same genetic background. In both cases, HEI3090 failed to mitigate lung fibrosis, as depicted in Figure 2M and Supplemental Figures 2D and 6A and B.”
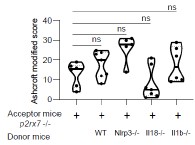
We added the Supplemental Figure 7 showing that the genetic background does not impact lung fibrosis at steady step levels where p-values were analyzed by one-way ANOVA, with Kruskal-Wallis test for multiple comparisons.
Author response image 1.
Supplemental Figure 7 : The genetic background does not impact lung fibrosis at steady step levels. p2rx7-/- mice were given 3.106 WT, nlrp3-/ , i118-/ or illb -l- splenocytes i_v_ one day prior to BLM delivery (i_n_ 2.5 LJ/kg)_ p2rx7-/- mice or p2rx7-/- mice adoptively transferred with splenocytes from indicated genetic background were treated daily i.p_ with mg/kg HE13090 or vehicle for 14 days. Fibrosis score assessed by the Ashcroft method. P-values were analyzed on all treated and non treated groups by one-way ANOVA, with Kruskal-Wallis test for multiple comparisons. The violin plot illustrates the distribution of Ashcroft scores across indicated experimental groups. The width of the violin at each point represents the density of data, and the central line indicates the median expression level. Each point represents one biological replicate. ns, not significant
-

eLife assessment
This study presents a potentially valuable discovery which indicates that activation of the P2RX7 pathway by the small molecule HEI3090 can reduce lung fibrosis after its establishment by inflammatory damage. If confirmed, the study could clarify the role of specific immune networks in the establishment and progression of lung fibrosis. The presented data and analyses showing the efficacy of HEI3090 small molecule acting via the P2RX7 pathway in reducing lung fibrosis are solid. The studies also show that genetic deletion of P2RX7 itself can reduce the extent of fibrosis. P2RX7 can thus have distinct effects in various phases of the development of lung fibrosis. There is a need for additional definitive studies that specifically identify the discrete phases of when inflammasome activation via P2RX7 signaling can worsen …
eLife assessment
This study presents a potentially valuable discovery which indicates that activation of the P2RX7 pathway by the small molecule HEI3090 can reduce lung fibrosis after its establishment by inflammatory damage. If confirmed, the study could clarify the role of specific immune networks in the establishment and progression of lung fibrosis. The presented data and analyses showing the efficacy of HEI3090 small molecule acting via the P2RX7 pathway in reducing lung fibrosis are solid. The studies also show that genetic deletion of P2RX7 itself can reduce the extent of fibrosis. P2RX7 can thus have distinct effects in various phases of the development of lung fibrosis. There is a need for additional definitive studies that specifically identify the discrete phases of when inflammasome activation via P2RX7 signaling can worsen fibrosis versus when the same signaling can be beneficial. It also needs to be established whether distinct immune cell populations mediate the detrimental and beneficial effects of P2RX7 activation in lung fibrosis.
-

Reviewer #1 (Public Review):
In this revised preprint the authors investigate whether a presumably allosteric P2RX7 activating compound that they previously discovered reduces fibrosis in a bleomycin mouse model. They chose this particular model as publicly available mRNA data indicate that the P2RX7 pathway is downregulated in idiopathic pulmonary fibrosis patients compared to control individuals. In their revised manuscript, the authors use three proxies of lung damage, Ashcroft score, collagen fibers, and CD140a+ cells, to assess lung damage following the administration of bleomycin. These metrics are significantly reduced on HEI3090 treatment. Additional data implicate specific immune cell infiltrates and cytokines, namely inflammatory macrophages and damped release of IL-17A, as potential mechanistic links between their compound …
Reviewer #1 (Public Review):
In this revised preprint the authors investigate whether a presumably allosteric P2RX7 activating compound that they previously discovered reduces fibrosis in a bleomycin mouse model. They chose this particular model as publicly available mRNA data indicate that the P2RX7 pathway is downregulated in idiopathic pulmonary fibrosis patients compared to control individuals. In their revised manuscript, the authors use three proxies of lung damage, Ashcroft score, collagen fibers, and CD140a+ cells, to assess lung damage following the administration of bleomycin. These metrics are significantly reduced on HEI3090 treatment. Additional data implicate specific immune cell infiltrates and cytokines, namely inflammatory macrophages and damped release of IL-17A, as potential mechanistic links between their compound and reduced fibrosis. Finally, the researchers transplant splenocytes from WT, NLRP3-KO, and IL-18-KO mice into animals lacking the P2RX7 receptor to specifically ascertain how the transplanted splenocytes, which are WT for P2RX7 receptor, respond to HEI3090 (a P2RX7 agonist). Based on these results, the authors conclude that HEI3090 enhanced IL-18 production through the P2RX7-NLRP3 inflammasome axis to dampen fibrosis.
These findings could be interesting to the field, as there are conflicting results as to whether NLRP3 activation contributes to fibrosis and if so, at what stage(s) (e.g., acute damage phase versus progression). The revised manuscript is more convincing in that three orthogonal metrics for lung damage were quantified.
However, deletion of the P2RX7 receptor itself reduces the extent of fibrosis, suggesting that P2RX7 signaling can be pro-fibrotic. In the absence of P2RX7, the effects of HEI3900 are also abolished, suggesting that HEI3900 acts in part via P2RX7 signaling. This suggests a paradox that P2RX7 signaling can be both detrimental and beneficial in fibrosis and there is need for a better understanding of when P2RX7 signaling is beneficial and when it is detrimental in lung fibrosis. HEI3900-induced activation of P2RX7 seems to be beneficial but this primarily is shown for when fibrosis is already established. As the P2RX7 genetic deletion mouse model has less fibrosis, P2RX7 signaling and inflammasome activation may be deleterious during the formation of disease but it is also possible that HEI3900 has other beneficial effects that are not directly related to P2RX7.
Molecularly, additional evidence on specificity, such as thermal proteome profiling and direct biophysical binding experiments, would also enhance the authors' argument that the compound indeed binds P2RX7 directly and specifically. Since all small molecules have some degree of promiscuity, the absence of an additional P2RX7 modulator, or direct recombinant IL-18 administration, is needed to orthogonally validate the functional importance of this pathway. Another way the authors could probe pathway specificity would involve co-administering α-IL-18 with HEI3090 in several key experiments (similar to Figure 4L).
-

Reviewer #2 (Public Review):
In the study by Hreich et al, the potency of P2RX7-specific positive modulator HEI3090, developed by the authors, for the treatment of Idiopathic pulmonary fibrosis (IPF) was investigated. Recently, the authors have shown that HEI3090 can protect against lung cancer by stimulating dendritic cell P2RX7, resulting in IL-18 production that stimulates IFN-γ production by T and NK cells (DOI: 10.1038/s41467-021-20912-2). Interestingly, HEI3090 increases IL-18 levels only in the presence of high eATP. Since the treatment options for IPF are limited, new therapeutic strategies and targets are needed. The authors first show that P2RX7/IL-18/IFNG axis is downregulated in patients with IPF. Next, they used a bleomycin-induced lung fibrosis mouse model to show that the use of a positive modulator of P2RX7 leads to the …
Reviewer #2 (Public Review):
In the study by Hreich et al, the potency of P2RX7-specific positive modulator HEI3090, developed by the authors, for the treatment of Idiopathic pulmonary fibrosis (IPF) was investigated. Recently, the authors have shown that HEI3090 can protect against lung cancer by stimulating dendritic cell P2RX7, resulting in IL-18 production that stimulates IFN-γ production by T and NK cells (DOI: 10.1038/s41467-021-20912-2). Interestingly, HEI3090 increases IL-18 levels only in the presence of high eATP. Since the treatment options for IPF are limited, new therapeutic strategies and targets are needed. The authors first show that P2RX7/IL-18/IFNG axis is downregulated in patients with IPF. Next, they used a bleomycin-induced lung fibrosis mouse model to show that the use of a positive modulator of P2RX7 leads to the activation of the P2RX7/IL-18 axis in immune cells that limits lung fibrosis onset or progression. Mechanistically, treatment with HEI3090 enhanced IL-18-dependent IFN-γ production by lung T cells leading to a decreased production of IL-17 and TGFβ, major drivers of IPF. The major novelty is the use of the small molecule HEI3090 to stimulate the immune system to limit lung fibrosis progression by targeting the P2RX7, which could be potentially combined with current therapies available. Overall, the study was well performed and the manuscript is clear.
-

-

-

Author Response
The following is the authors’ response to the original reviews.
eLife assessment
This study presents a potentially valuable discovery which indicates that activation of the P2RX7 pathway can reduce the lung fibrosis after its establishment by inflammatory damage. If confirmed, the study could clarify the role of specific immune networks in the establishment and progression of lung fibrosis. However, the presented data and analyses are incomplete as they primarily rely on limited pharmacological treatments with modest effect sizes. I hope you will be convinced by the validity of our approaches with the following explanation/information and I remain at your disposal to discuss
Public Reviews:
Reviewer #1 (Public Review):
In this revised preprint the authors investigate whether a presumably allosteric P2RX7 …
Author Response
The following is the authors’ response to the original reviews.
eLife assessment
This study presents a potentially valuable discovery which indicates that activation of the P2RX7 pathway can reduce the lung fibrosis after its establishment by inflammatory damage. If confirmed, the study could clarify the role of specific immune networks in the establishment and progression of lung fibrosis. However, the presented data and analyses are incomplete as they primarily rely on limited pharmacological treatments with modest effect sizes. I hope you will be convinced by the validity of our approaches with the following explanation/information and I remain at your disposal to discuss
Public Reviews:
Reviewer #1 (Public Review):
In this revised preprint the authors investigate whether a presumably allosteric P2RX7 activating compound that they previously discovered reduces fibrosis in a bleomycin mouse model. They chose this particular model as publicly available mRNA data indicate that the P2RX7 pathway is downregulated in idiopathic pulmonary fibrosis patients compared to control individuals. In their revised manuscript, the authors use three proxies of lung damage, Ashcroft score, collagen fibers, and CD140a+ cells, to assess lung damage following the administration of bleomycin. These metrics are significantly reduced on HEI3090 treatment. Additional data implicate specific immune cell infiltrates and cytokines, namely inflammatory macrophages and damped release of IL-17A, as potential mechanistic links between their compound and reduced fibrosis. Finally, the researchers transplant splenocytes from WT, NLRP3-KO, and IL-18-KO mice into animals lacking the P2RX7 receptor to specifically ascertain how the transplanted splenocytes, which are WT for P2RX7 receptor, respond to HEI3090 (a P2RX7 agonist). Based on these results, the authors conclude that HEI3090 enhanced IL-18 production through the P2RX7-NLRP3 inflammasome axis to dampen fibrosis.
These findings could be interesting to the field, as there are conflicting results as to whether NLRP3 activation contributes to fibrosis and if so, at what stage(s) (e.g., acute damage phase versus progression). The revised manuscript is more convincing in that three orthogonal metrics for lung damage were quantified. However, major weaknesses of the study still include inconsistent and small effect sizes of HEI3090 treatment versus either batch effects from transplanted splenocytes or the effects of different genetic backgrounds. Moreover, the fundamental assumption that HEI3090 acts specifically and functionally through the P2RX7 pathway in this model cannot be directly tested, as the authors now provide results indicating that P2RX7 knockout mice do not establish lung fibrosis on bleomycin treatment.
I’m particularly concerned by the assumption made by reviewer 1 concerning the fact that P2RX7 knockout mice do not establish lung fibrosis on bleomycin treatment.
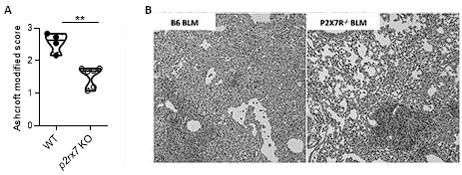
Indeed, what we showed in the point-to-point response is that BLM induces fibrosis in both WT and P2RX7 KO mice, but the intensity of the fibrosis is reduced in P2RX7KO mice, panel A. Therefore, as discussed in our first response, our results confirmed the previous publication of Riteau et al, that P2RX7 participates in BLM-induced lung fibrosis (see panel B).
Author response image 1.
Bleomycin induced lung fibrosis in WT versus p2rx7 KO mice. A: lung from BLM-treated mice were stained with HE and fibrosis was quantified using the Ashcroft protocol. Result showed that fibrosis induced by BLM in KO mice is reduced as compared to WT mice. B: Representative images of lung sections at day 14 after BLM treatment stained with H&E as published in Riteau et al. and illustrating that fibrosis induced by BLM in KO mice is reduced as compared to WT mice. WT mice vehicle (n=4) or p2rx7 KO (n=6) mice. Two-tailed Mann-Whitney test, p values: **p < 0.01.
Importantly, this lower intensity of lung fibrosis in P2RX7 KO mice, does not interfere with the capacity of our molecule to attenuate lung fibrosis, as demonstrated in the adoptive transfer of IL1B KO splenocytes in P2RX7 KO mice, in which HEI3090 decreases the Ashcroft score, the % of fibrosis and the collagen fibers (see below).
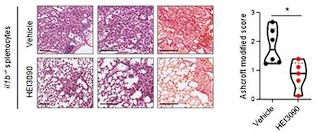
Author response image 2.
HEI3090 activity requires P2RX7’s expressing immune cells: Experimental design. p2rx7-/- mice were given 3.106 il1β-/- splenocytes i.v. one day prior to BLM delivery (i.n. 2.5 U/kg). Mice were treated daily i.p. with 1.5 mg/kg HEI3090 or vehicle for 14 days. (C) Representative images of lung sections at day 14 after treatment stained with H&E and Sirius Red with il1β-/- splenocytes, bar= 100 µm (left) and fibrosis score assessed by the Ashcroft method, the % of fibrosis and the content of collagen fibers (right). Each point represents one mouse (n=2 in WT and NLRP3 experiment, n =1 in IL18 and IL1B experiment), data represented as violin plot or mean±SEM, two-tailed Mann-Whitney test, *p < 0.05. WT: Wildtype, KO: P2RX7 knock-out
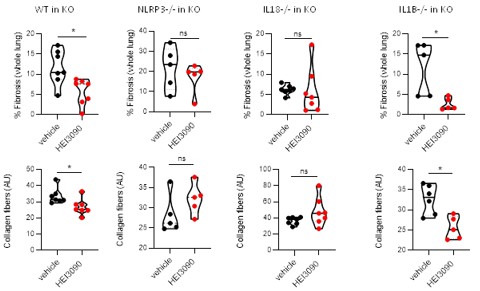
Importantly, in the same experimental setting, e.g adoptive transfer of splenocytes from different genetic backgrounds, HEI3090 decreases the fibrosis intensity only with WT and IL1B KO splenocytes and not with NLRP3 KO and IL18KO splenocytes.
Author response image 3.
HEI3090 activity requires P2RX7’s expressing immune cells: Experimental design. p2rx7-/- mice were given 3.106 WT, NLRP3-/-, IL18-/- or IL1β-/- splenocytes i.v. one day prior to BLM delivery (i.n. 2.5 U/kg). Mice were treated daily i.p. with 1.5 mg/kg HEI3090 or vehicle for 14 days. Fibrosis in whole lung was assessed by the % of fibrosis (upper panel) and the content of collagen fibers (lower panel). Each point represents one mouse (n=2 in WT and NLRP3 experiments, n =1 in IL18 and IL1B experiment). Data represented as violin plot or mean±SEM, two-tailed Mann-Whitney test, *p < 0.05. WT: Wildtype, KO: P2RX7 knock-out
In order to provide clear evidence that HEI3090 functions through P2RX7, a different lung fibrosis model that does not require P2RX7 would be necessary. For example, in such a system the authors could demonstrate a lack of HEI3090-mediated therapeutic effect on P2RX7 knockout.
Since BLM induces lung fibrosis in P2RX7 KO mice as we showed in this manuscript and as already published by Riteau in 2010, shown earlier in our response (first figure) and because HEI3090 is able to decrease the intensity of fibrosis in WT and IL1B-/- → P2RX7 KO mice but not in KO, NLRP3-/- → P2RX7 KO and IL18-/- → P2RX7 KO mice we believe that our data sustain the conclusion that
HEI3090 required the expression of P2RX7 in immune cells to mediate the antifibrotic activity,
IL1B is not a crucial effector mediating the antifibrotic effect of HEI3090.
Molecularly, additional evidence on specificity, such as thermal proteome profiling and direct biophysical binding experiments, would also enhance the authors' argument that the compound indeed binds P2RX7 directly and specifically. Since all small molecules have some degree of promiscuity, the absence of an additional P2RX7 modulator, or direct recombinant IL-18 administration (as suggested by another reviewer), is needed to orthogonally validate the functional importance of this pathway. Another way the authors could probe pathway specificity would involve co-administering α-IL-18 with HEI3090 in several key experiments (similar to Figure 4L).
At the moment we have no funds to do these experiments and given the high competition, we have decided to publish our story without these new data.
Reviewer #2 (Public Review):
In the study by Hreich et al, the potency of P2RX7-specific positive modulator HEI3090, developed by the authors, for the treatment of Idiopathic pulmonary fibrosis (IPF) was investigated. Recently, the authors have shown that HEI3090 can protect against lung cancer by stimulating dendritic cell P2RX7, resulting in IL-18 production that stimulates IFN-γ production by T and NK cells (DOI: 10.1038/s41467-021-20912-2). Interestingly, HEI3090 increases IL-18 levels only in the presence of high eATP. Since the treatment options for IPF are limited, new therapeutic strategies and targets are needed. The authors first show that P2RX7/IL-18/IFNG axis is downregulated in patients with IPF. Next, they used a bleomycin-induced lung fibrosis mouse model to show that the use of a positive modulator of P2RX7 leads to the activation of the P2RX7/IL-18 axis in immune cells that limits lung fibrosis onset or progression. Mechanistically, treatment with HEI3090 enhanced IL-18-dependent IFN-γ production by lung T cells leading to a decreased production of IL-17 and TGFβ, major drivers of IPF. The major novelty is the use of the small molecule HEI3090 to stimulate the immune system to limit lung fibrosis progression by targeting the P2RX7, which could be potentially combined with current therapies available. Overall, the study was well performed, and the manuscript is clear.
We thank the reviewer for this very positive comments.
However, there is need for more details on the description and interpretation of the adoptive transfer experiments, as well as the statistical analyses and number of replicate independent experiments.
I’m concerned by the reviewer’s comments, and I would like to bring additional information/explanation, which I hope will convince you on the validity of our approaches.
Author response image 4.
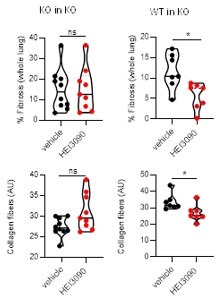
Adoptive transfer experiment. Adoptive transfer experiments are classically used to document which immune cells participate in immune cell responses (with more than 150 publications in pubmed with the key words adoptive transfer and onco immunology) and intravenous administration is a common route to trigger lungs (PMID: 23336716). To characterize the molecular effector (P2RX7, NLRP3, IL18 and IL1B) accounting for the antifibrotic effect of HEI3090 we purified splenocytes from donor mice and administrated them intra venously in P2RX7 KO mice. As shown in Author response image 4, HEI3090 has no antifibrotic activity when splenocyte isolated from mice invalidated for p2rx7 are iv into P2RX7 KO mice (KO in KO). By contrast, HEI3090 has antifibrotic activity when WT splenocytes expressing P2RX7 (isolated from WT mice) are transferred into P2RX7 KO mice (WT in KO).
This experiment brings strong evidence to demonstrate the efficacy of adoptive transfer approach to identify molecular effector required to mediate the antifibrotic effect of HEI3090.
Statistical analyses and number of replicate independent experiments
We thank the reviewer for his comment, and we apologize to not have been sufficiently clear in our previous response with this miss phrased statement “the experiment was stopped when significantly statistical results were observed” when we should have written “the experiment was stopped when each experimental group contained at least 5 mice”.
To define the size of experimental groups we did a pilot experiment, with 4 WT mice (e.g. 4 biological replicates) in each group (as shown aside), and a statistical forecasting based on the result of the pilot experiment (40% difference, standard error: 0.9, α risk: 0.05, power: 0.8). Since we focused on the effect of HEI3090 we based our statistical analysis on a one-way ANOVA analysis comparing in each experiment the vehicle and the treated group.
The pilot experiment and statistical forecasting indicated 4 mice per group to characterize the effect of HEI3090 on BLM-induced lung fibrosis. Each experiment was started with 6 to 8 mice per group. Being aware that 30% of mice can unexpectedly dye due to BLM treatment, we duplicated the experiment, when necessary, to include at least 5 mice in each group of each experiment meaning 5 biological replicates, knowing that 4 mice are sufficient to statistically analyze the results. In each experiment we have checked for the presence of outlier, using the ROULT method, and removed the outliers when necessary.
-

eLife assessment
This study presents a potentially valuable discovery which indicates that activation of the P2RX7 pathway can reduce the lung fibrosis after its establishment by inflammatory damage. If confirmed, the study could clarify the role of specific immune networks in the establishment and progression of lung fibrosis. However, the presented data and analyses are incomplete as they primarily rely on limited pharmacological treatments with modest effect sizes.
-

Reviewer #1 (Public Review):
In this revised preprint the authors investigate whether a presumably allosteric P2RX7 activating compound that they previously discovered reduces fibrosis in a bleomycin mouse model. They chose this particular model as publicly available mRNA data indicate that the P2RX7 pathway is downregulated in idiopathic pulmonary fibrosis patients compared to control individuals. In their revised manuscript, the authors use three proxies of lung damage, Ashcroft score, collagen fibers, and CD140a+ cells, to assess lung damage following the administration of bleomycin. These metrics are significantly reduced on HEI3090 treatment. Additional data implicate specific immune cell infiltrates and cytokines, namely inflammatory macrophages and damped release of IL-17A, as potential mechanistic links between their compound …
Reviewer #1 (Public Review):
In this revised preprint the authors investigate whether a presumably allosteric P2RX7 activating compound that they previously discovered reduces fibrosis in a bleomycin mouse model. They chose this particular model as publicly available mRNA data indicate that the P2RX7 pathway is downregulated in idiopathic pulmonary fibrosis patients compared to control individuals. In their revised manuscript, the authors use three proxies of lung damage, Ashcroft score, collagen fibers, and CD140a+ cells, to assess lung damage following the administration of bleomycin. These metrics are significantly reduced on HEI3090 treatment. Additional data implicate specific immune cell infiltrates and cytokines, namely inflammatory macrophages and damped release of IL-17A, as potential mechanistic links between their compound and reduced fibrosis. Finally, the researchers transplant splenocytes from WT, NLRP3-KO, and IL-18-KO mice into animals lacking the P2RX7 receptor to specifically ascertain how the transplanted splenocytes, which are WT for P2RX7 receptor, respond to HEI3090 (a P2RX7 agonist). Based on these results, the authors conclude that HEI3090 enhanced IL-18 production through the P2RX7-NLRP3 inflammasome axis to dampen fibrosis.
These findings could be interesting to the field, as there are conflicting results as to whether NLRP3 activation contributes to fibrosis and if so, at what stage(s) (e.g., acute damage phase versus progression). The revised manuscript is more convincing in that three orthogonal metrics for lung damage were quantified. However, major weaknesses of the study still include inconsistent and small effect sizes of HEI3090 treatment versus either batch effects from transplanted splenocytes or the effects of different genetic backgrounds. Moreover, the fundamental assumption that HEI3090 acts specifically and functionally through the P2RX7 pathway in this model cannot be directly tested, as the authors now provide results indicating that P2RX7 knockout mice do not establish lung fibrosis on bleomycin treatment.
In order to provide clear evidence that HEI3090 functions through P2RX7, a different lung fibrosis model that does not require P2RX7 would be necessary. For example, in such a system the authors could demonstrate a lack of HEI3090-mediated therapeutic effect on P2RX7 knockout. Molecularly, additional evidence on specificity, such as thermal proteome profiling and direct biophysical binding experiments, would also enhance the authors' argument that the compound indeed binds P2RX7 directly and specifically. Since all small molecules have some degree of promiscuity, the absence of an additional P2RX7 modulator, or direct recombinant IL-18 administration (as suggested by another reviewer), is needed to orthogonally validate the functional importance of this pathway. Another way the authors could probe pathway specificity would involve co-administering α-IL-18 with HEI3090 in several key experiments (similar to Figure 4L).
-

Reviewer #2 (Public Review):
In the study by Hreich et al, the potency of P2RX7-specific positive modulator HEI3090, developed by the authors, for the treatment of Idiopathic pulmonary fibrosis (IPF) was investigated. Recently, the authors have shown that HEI3090 can protect against lung cancer by stimulating dendritic cell P2RX7, resulting in IL-18 production that stimulates IFN-γ production by T and NK cells (DOI: 10.1038/s41467-021-20912-2). Interestingly, HEI3090 increases IL-18 levels only in the presence of high eATP. Since the treatment options for IPF are limited, new therapeutic strategies and targets are needed. The authors first show that P2RX7/IL-18/IFNG axis is downregulated in patients with IPF. Next, they used a bleomycin-induced lung fibrosis mouse model to show that the use of a positive modulator of P2RX7 leads to the …
Reviewer #2 (Public Review):
In the study by Hreich et al, the potency of P2RX7-specific positive modulator HEI3090, developed by the authors, for the treatment of Idiopathic pulmonary fibrosis (IPF) was investigated. Recently, the authors have shown that HEI3090 can protect against lung cancer by stimulating dendritic cell P2RX7, resulting in IL-18 production that stimulates IFN-γ production by T and NK cells (DOI: 10.1038/s41467-021-20912-2). Interestingly, HEI3090 increases IL-18 levels only in the presence of high eATP. Since the treatment options for IPF are limited, new therapeutic strategies and targets are needed. The authors first show that P2RX7/IL-18/IFNG axis is downregulated in patients with IPF. Next, they used a bleomycin-induced lung fibrosis mouse model to show that the use of a positive modulator of P2RX7 leads to the activation of the P2RX7/IL-18 axis in immune cells that limits lung fibrosis onset or progression. Mechanistically, treatment with HEI3090 enhanced IL-18-dependent IFN-γ production by lung T cells leading to a decreased production of IL-17 and TGFβ, major drivers of IPF. The major novelty is the use of the small molecule HEI3090 to stimulate the immune system to limit lung fibrosis progression by targeting the P2RX7, which could be potentially combined with current therapies available. Overall, the study was well performed and the manuscript is clear. However, there is need for more details on the description and interpretation of the adoptive transfer experiments, as well as the statistical analyses and number of replicate independent experiments.
-

-

Author Response
eLife assessment
This study presents a potentially valuable discovery which indicates that activation of the P2RX7 pathway can reduce the degree of lung fibrosis caused by other inflammatory pathways. If confirmed, the study could clarify the role of specific immune networks in the establishment and progression of lung fibrosis.
Thanks for this positive comment. Indeed, knowing that lung fibrosis is partly driven by inflammation, with a dysregulated Th1/Th2/Th17 ratio (PMID 20176803, PMID 19682929), we hypothesized that modulating the immune response would be able to attenuate lung fibrosis. To address this issue, we proposed to boost the activation of P2RX7, a purinergic receptor with immunomodulatory properties (PMID 8614837, PMID 11035104), in the well characterized bleomycin-induced lung fibrosis mouse model …
Author Response
eLife assessment
This study presents a potentially valuable discovery which indicates that activation of the P2RX7 pathway can reduce the degree of lung fibrosis caused by other inflammatory pathways. If confirmed, the study could clarify the role of specific immune networks in the establishment and progression of lung fibrosis.
Thanks for this positive comment. Indeed, knowing that lung fibrosis is partly driven by inflammation, with a dysregulated Th1/Th2/Th17 ratio (PMID 20176803, PMID 19682929), we hypothesized that modulating the immune response would be able to attenuate lung fibrosis. To address this issue, we proposed to boost the activation of P2RX7, a purinergic receptor with immunomodulatory properties (PMID 8614837, PMID 11035104), in the well characterized bleomycin-induced lung fibrosis mouse model (PMID 25959210). In this study, we used a pyroglutamic derivative compound (HEI3090) able to specifically enhance P2RX7-dependent biological activities (cationic channel and macropore opening) only in the presence of extracellular ATP, which was qualified as the first representative of an immunotherapy relying on the activation of P2RX7 expressed by dendritic cells (PMID 33510147), and we showed that lung fibrosis is attenuated in mice treated with HEI3090 as compared to vehicle treated mice.
However, the presented data and analyses are incomplete as they rely on limited pharmacological treatments and because there is an absence of key control studies, validation experiments and statistical analyses.
Quantification of lung fibrosis:
Quantification of lung fibrosis was made on the basis of a modified Ashcroft score which assigns 8 grades to quantify lung fibrosis reliably and reproducibly (PMID 18476815). To be even more accurate and not biased by patchy lesions observed in all existing lung fibrosis induced mouse models, the whole lungs (left and right lobes) were divided in section of 880 µm2 and each section was scored individually. A total of 80 to 110 sections were analyzed per mouse. We agree that our text requires clarification. In parallel, the collagen amount given by the polarization intensity of the Sirius red staining of the lung slices was quantified with a homemade ImageJ/Fiji macro program. Further, we recently analyzed by FACS the percentage of PDGFRα (a specific marker of fibroblasts and myofibroblasts) positive cells in lungs isolated from vehicle and HEI3090-treated mice. All these 3 different markers of lung damage show that HEI3090 attenuates bleomycin-induced lung fibrosis and therefore validate the use of the Ashcroft score to accurately study the extend of lung fibrosis. We are going add quantification of collagen fibers in all figures.
Limited pharmacological treatments:
We have designed and characterized HEI3090 in a previous study and have shown that it is a positive modulator of P2RX7 (PMID 33510147).
To test its effect on lung fibrosis, we tested two pharmacological regimens using HEI3090 and have shown that both regimens are effective in limiting the progression of fibrosis. While having shown the requirement of P2RX7 for the activity of HEI3090 (PMID 33510147), we used in this study p2rx7 KO mice which were adoptively transferred with splenocytes isolated from p2rx7 KO mice to demonstrate the involvement of P2RX7 to mediate the antifibrotic effect of HEI3090. This experiment also serves as control to validate the adoptive transfer experiment.
We agree that proving and validating furthermore that activation of the P2RX7/IL-18 pathway can limit the progression of fibrosis requires the use of other activators of P2RX7. However, to date, HEI3090 is the only pharmacological compound described to activate the receptor. Indeed, the other chemical compounds described in the literature are negative allosteric modulator of P2RX7 (PMID 27935479),
Absence of key control studies and validation experiments:
The importance of P2RX7 in the antifibrotic effect of HEI3090 was demonstrated thanks to P2RX7 KO mice (supplementary figures S6B). We are going to implement this figure with additional mice.
The importance of immune cells was demonstrated thanks to adoptive transfer of WT splenocytes (expressing P2RX7) into P2RX7 KO mice. We agree that lung fibrosis is attenuated in vehicle-treated P2RX7 KO mice, but lung fibrosis is still present and could be modulated by treatments as demonstrated by adoptive transfer of splenocytes isolated from IL-1B KO mice who still respond to HEI3090 as shown in Supplementary figure S6C.
As suggested by reviewers we examined the effect of genetic background using two-way Anova test and the result is “the interaction is considered not significant”.
The prevalence of transferred immune cells on endogenous cells is demonstrated in supplementary figure S5, where intravenous injection of splenocytes isolated from P2RX7 KO mice into WT mice abolishes the antifibrotic effect of HEI3090. This experiment further validates the requirement of immune cells and the efficacy of the adoptive transfer approach.
Statistical analyses:
In this study we compared side by side the effect of HEI3090 versus vehicle in different genetic backgrounds in order to characterize the implication of actors of the P2RX7/IL-18 pathway in the antifibrotic effect of HEI3090. We also examined the effect of genetic background using the two-way Anova test. Following European recommendations, and in agreement with the ARRIVE guidelines for mice studies, we performed provisional statistic to evaluate the number of mice required in the study and stopped the experiments when significantly statistical results were observed. We agree that results are heterogeneous, however this heterogeneity does not prevent data analyses as shown in supplementary figure S6D, where adoptive transfer of splenocytes isolated from IL-1B KO mice into P2RX7 KO mice dampens BLM-induced lung fibrosis (with an Ashcroft score of 1.8 versus 3 in WT mice) but still responds to HEI3090, thus indicating that IL-1B is not required to mediate the antifibrotic effect of HEI3090.
-

eLife assessment
This study presents a potentially valuable discovery which indicates that activation of the P2RX7 pathway can reduce the degree of lung fibrosis caused by other inflammatory pathways. If confirmed, the study could clarify the role of specific immune networks in the establishment and progression of lung fibrosis. However, the presented data and analyses are incomplete as they rely on limited pharmacological treatments and because there is an absence of key control studies, validation experiments and statistical analyses.
-

Reviewer #1 (Public Review):
In this study the authors investigate whether a presumably allosteric P2RX7 activating compound that they previously discovered reduces fibrosis in a bleomycin mouse model. They chose this particular model as publicly available mRNA data indicate that the P2XR7 pathway is downregulated in idiopathic pulmonary fibrosis patients compared to control individuals. The authors first demonstrate that two proxies of lung damage, Ashcroft score and collagen fibers, are significantly reduced in the bleomycin model on HEI3090 treatment. Additional data implicate specific immune cell infiltrates and cytokines, namely inflammatory macrophages and damped release of IL-17A, as potential mechanistic links between their compound and reduced fibrosis. Finally, the researchers transplant splenocytes from WT, NLRP3-KO, and …
Reviewer #1 (Public Review):
In this study the authors investigate whether a presumably allosteric P2RX7 activating compound that they previously discovered reduces fibrosis in a bleomycin mouse model. They chose this particular model as publicly available mRNA data indicate that the P2XR7 pathway is downregulated in idiopathic pulmonary fibrosis patients compared to control individuals. The authors first demonstrate that two proxies of lung damage, Ashcroft score and collagen fibers, are significantly reduced in the bleomycin model on HEI3090 treatment. Additional data implicate specific immune cell infiltrates and cytokines, namely inflammatory macrophages and damped release of IL-17A, as potential mechanistic links between their compound and reduced fibrosis. Finally, the researchers transplant splenocytes from WT, NLRP3-KO, and IL-18-KO mice into animals lacking the P2XR7 receptor to specifically ascertain how the transplanted splenocytes, which are WT for P2XR7 receptor, respond to HEI3090 (a P2XR7 agonist). Based on these results, the authors conclude that HEI3090 enhanced IL-18 production through the P2XR7-NLRP3 inflammasome axis to dampen fibrosis.
These findings could be interesting to the field, as there are conflicting results as to whether NLRP3 activation contributes to fibrosis and if so, at what stage(s) (e.g., acute damage phase versus progression). However, major weaknesses of the study include inconsistent and small effect sizes in key outcomes used to measure fibrosis, small animal cohorts that do not empower results, and lack of key experimental controls. For example, damage indicators for the vehicle-treated mice transplanted with splenocytes of various genetic background are markedly different, and there are no statistical tests of these effects because the data are presented as separate graphs. Moreover, the fundamental assumption that HEI3090 acts specifically through the P2XR7 pathway in this model is questionable, as P2XR7 knockout mice are not included in the initial key experiments. These issues must be addressed as stimulating an inflammasome response might lead to pathogenic inflammation, which could counterproductively exacerbate fibrosis in the clinic and harm people.
Experimental concerns:
1. Ashcroft method quantification throughout is outdated and prone to bias. The methods describing quantification are lacking, and only include a citation: there should be mention of researcher blinding, etc. In general, please re-quantify using an automated classifier, and consider staining for additional markers of lung damage that are appropriate in the field.
2. For Figure 2, P2XR7 knockout mice, and an additional P2XR7 activator, should be included (e.g, A74003, AZ10606120, others), to support the hypothesis that HEI3090 acts through this pathway to alleviate fibrosis. Moreover, these data are especially important as the author's conclusions are directly opposed to a previous study demonstrating that the P2XR7 receptor is required for inflammation/fibrosis in this model system (PMID: 20522787). Two-way ANOVA or similar statistical tests on all groups should be examined to see whether genetic knockout of this DAMP receptor alone is protective or exacerbates fibrosis (e.g., comparing the vehicle-alone groups), and whether compounds exert a specific effect through this receptor.
3. Fig. 3A: Please show the individual IFN/IL-17A plots in the supplement, as a ratiometric result might mask variance. Moreover, please conduct a statistical test for the outlier in the HEI3090 condition (to potentially remove it), as this sole data point might skew the entire mean, causing the observed statistical difference between means despite a very modest change. If the results are still significant, please comment on effect size.
4. Fig. 3: How is IL-17A measured and what is the abbreviation GMFI?
5. Fig. 3E: It's unclear how the left and right figures align-it looks like the gates are 45.8 % and 25 %, respectively, but the means on the right are between 2-3%. Also, is this effect size (2 versus 3 %) significant biologically?
6. For Figure 4B-G, the Ashcroft scores for the vehicle mice treated with HEI3090 are at entirely different starting points following adoptive transfer of cells with different genetic background. In Fig. 1, WT mice have starting scores of around 3 following the induction of fibrosis, with a modest decrease of about 0.8 following HEI3090 treatment. Here, there is a much greater effect of the genetic background itself rather than the treatment, with the IL-18 knockout mice having a much lower baseline "vehicle" score (~1) compared to Fig. 1F (both of which are 14 day treatments). In fact, adoptive transfer of WT splenocytes start at a baseline of 1.8 here, which is much lower than Fig. 1F, and NLRP3-KO splenocytes score nearly the same as Fig. 1F following BLM treatment, with a modest reduction following treatment with HEI3090. Please analyze all of these groups together with appropriate multiple hypothesis testing to examine the effect of the genetic background, and please comment on why IL-18-knockout splenocytes might be protective at vehicle baseline while NLRP3-knockout splenocytes might exacerbate the phenotype at vehicle baseline.
7. The variance on Supplemental Figure 5C is quite large. These data have a decrease in mean Ashcroft score between untreated and HEI3090 treatment of around 0.8, which is similar to the WT mice in Figure 1. This is very concerning, as the underlying assumption is that KO of the protein required for HEI3090's on-target effect would completely ablate response, and this would be required for the subsequent adoptive transfer experiments in Figure 4. Please conduct power analysis, comment, and provide additional evidence (other than Ashcroft score).
8. Figure 4: Should quantify collagen fibers or have an additional quantitative metric for lung damage, as in Fig. 2C/J.
9. Figure 4: Should group the quantification of C/E/G and perform a 2-way Anova to assess effects of genetic background versus treatment.
10. Fig. 4H, Supplemental Fig. 6D: Is it reasonable to expect differences in IL-1beta and IL-18 in sera compared to in lung tissue itself?
-

Reviewer #2 (Public Review):
In the study by Hreich et al, the potency of P2RX7 positive modulator HEI3090, developed by the authors, for the treatment of Idiopathic pulmonary fibrosis (IPF) was investigated. Recently, the authors have shown that HEI3090 can protect against lung cancer by stimulating dendritic cell P2RX7, resulting in IL-18 production that stimulates IFN-γ production by T and NK cells (DOI: 10.1038/s41467-021-20912-2). Interestingly, HEI3090 increases IL-18 levels only in the presence of high eATP. Since the treatment options for IPF are limited, new therapeutic strategies and targets are needed. The authors first show that P2RX7/IL-18/IFNG axis is downregulated in patients with IPF. Next, they used a bleomycin-induced lung fibrosis mouse model to show that the use of a positive modulator of P2RX7 leads to the …
Reviewer #2 (Public Review):
In the study by Hreich et al, the potency of P2RX7 positive modulator HEI3090, developed by the authors, for the treatment of Idiopathic pulmonary fibrosis (IPF) was investigated. Recently, the authors have shown that HEI3090 can protect against lung cancer by stimulating dendritic cell P2RX7, resulting in IL-18 production that stimulates IFN-γ production by T and NK cells (DOI: 10.1038/s41467-021-20912-2). Interestingly, HEI3090 increases IL-18 levels only in the presence of high eATP. Since the treatment options for IPF are limited, new therapeutic strategies and targets are needed. The authors first show that P2RX7/IL-18/IFNG axis is downregulated in patients with IPF. Next, they used a bleomycin-induced lung fibrosis mouse model to show that the use of a positive modulator of P2RX7 leads to the activation of the P2RX7/IL-18 axis in immune cells that limits lung fibrosis onset or progression. Mechanistically, treatment with HEI3090 enhanced IL-18-dependent IFN-γ production by lung T cells leading to a decreased production of IL-17 and TGFβ, major drivers of IPF. The major novelty is the use of the small molecule HEI3090 to stimulate the immune system to limit lung fibrosis progression by targeting the P2RX7, which could be potentially combined with current therapies available. However, there is the lack of information on the reproducibility of data, especially for the data presented in Figures 3 and 4, and related supplementary figures, as well as the lack of support data for experiments that emphasize the role of P2RX7 expressed on immune cells (e.g. frequency of transferred cells compared to endogenous cells).
-

Reviewer #3 (Public Review):
Idiopathic pulmonary fibrosis (IPF) is an aggressive interstitial lung disease with progressive and irreversible deterioration of respiratory functions that lacks curative therapies. The authors investigated a new therapeutic approach to treat idiopathic pulmonary fibrosis by targeting P2RX7/IL-18/IFNG axis.
The current data are mainly based on P2RX7 activator HEI3090 and genetic experiments are lacking to support the primary claim that activation P2RX7/IL-18/IFNG axis is beneficial for IPF.
- Parenteral systemic administration of IFN-γ failed in clinical trials (INSPIRE; NCT00075998). However, this study used i.p. administration of P2RX7 activator HEI3090 to activate P2RX7/IL-18/IFNG axis.
- Activation of P2RX7 NLRP3 inflammasome triggers cell death and the current experiments do not explore IL-18 as a …
Reviewer #3 (Public Review):
Idiopathic pulmonary fibrosis (IPF) is an aggressive interstitial lung disease with progressive and irreversible deterioration of respiratory functions that lacks curative therapies. The authors investigated a new therapeutic approach to treat idiopathic pulmonary fibrosis by targeting P2RX7/IL-18/IFNG axis.
The current data are mainly based on P2RX7 activator HEI3090 and genetic experiments are lacking to support the primary claim that activation P2RX7/IL-18/IFNG axis is beneficial for IPF.
- Parenteral systemic administration of IFN-γ failed in clinical trials (INSPIRE; NCT00075998). However, this study used i.p. administration of P2RX7 activator HEI3090 to activate P2RX7/IL-18/IFNG axis.
- Activation of P2RX7 NLRP3 inflammasome triggers cell death and the current experiments do not explore IL-18 as a potential therapy that would avoid harmful cell death as a consequence of P2RX7/NLRP3 inflammasome activation.
- Reciprocal bone marrow chimera model is needed to demonstrate the requirement of a hematopoietic compartment for HEI3090's antifibrotic effect.
- There is no evidence to show whether P2RX7 interferes with bleomycin during the generation of the IPF model. Independent IPF models would validate the therapeutic effect of P2RX7.
-

-

-

-

-