Shear and hydrostatic stress regulate fetal heart valve remodeling through YAP-mediated mechanotransduction
Curation statements for this article:-
Curated by eLife
eLife assessment
Determination of the biomechanical forces and downstream pathways that direct heart valve morphogenesis is an important area of research. In the current study, potential functions of localized Yap signaling in cardiac valve morphogenesis were examined. However, the evidence for Yap pathway activation and localization relative to areas of the valve subject to different mechanical stresses is not convincing.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Clinically serious congenital heart valve defects arise from improper growth and remodeling of endocardial cushions into leaflets. Genetic mutations have been extensively studied but explain less than 20% of cases. Mechanical forces generated by beating hearts drive valve development, but how these forces collectively determine valve growth and remodeling remains incompletely understood. Here, we decouple the influence of those forces on valve size and shape, and study the role of YAP pathway in determining the size and shape. The low oscillatory shear stress promotes YAP nuclear translocation in valvular endothelial cells (VEC), while the high unidirectional shear stress restricts YAP in cytoplasm. The hydrostatic compressive stress activated YAP in valvular interstitial cells (VIC), whereas the tensile stress deactivated YAP. YAP activation by small molecules promoted VIC proliferation and increased valve size. Whereas YAP inhibition enhanced the expression of cell-cell adhesions in VEC and affected valve shape. Finally, left atrial ligation was performed in chick embryonic hearts to manipulate the shear and hydrostatic stress in vivo. The restricted flow in the left ventricle induced a globular and hypoplastic left atrioventricular (AV) valves with an inhibited YAP expression. By contrast, the right AV valves with sustained YAP expression grew and elongated normally. This study establishes a simple yet elegant mechanobiological system by which transduction of local stresses regulates valve growth and remodeling. This system guides leaflets to grow into proper sizes and shapes with the ventricular development, without the need of a genetically prescribed timing mechanism.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
Determination of the biomechanical forces and downstream pathways that direct heart valve morphogenesis is an important area of research. In the current study, potential functions of localized Yap signaling in cardiac valve morphogenesis were examined. Extensive immunostainings were performed for Yap expression, but Yap activation status as indicated by nuclear versus cytoplasmic localization, Yap dephosphorylation, or expression of downstream target genes was not examined.
We thank the reviewer for appreciating the significance of this work, and we also thank the reviewer for the constructive suggestions. Following these suggestions, we have improved analysis of YAP activation status and used nuclear versus cytoplasmic localization to quantify YAP activation. To address the reviewer’s …
Author Response
Reviewer #1 (Public Review):
Determination of the biomechanical forces and downstream pathways that direct heart valve morphogenesis is an important area of research. In the current study, potential functions of localized Yap signaling in cardiac valve morphogenesis were examined. Extensive immunostainings were performed for Yap expression, but Yap activation status as indicated by nuclear versus cytoplasmic localization, Yap dephosphorylation, or expression of downstream target genes was not examined.
We thank the reviewer for appreciating the significance of this work, and we also thank the reviewer for the constructive suggestions. Following these suggestions, we have improved analysis of YAP activation status and used nuclear versus cytoplasmic localization to quantify YAP activation. To address the reviewer’s concerns, we have conducted extra qPCR analysis of YAP downstream target genes and YAP upstream genes in Hippo pathway. Please find the detailed revisions in our responses to the Recommendations for authors.
The goal of the work was to determine Yap activation status relative to different mechanical environments, but no biomechanical data on developing heart valves were provided in the study.
We appreciate the reviewer for raising this concern. We have previously published the biomechanical data of developing chick embryonic heart valves in the following study:
Buskohl PR, Gould RA, Butcher JT. Quantification of embryonic atrioventricular valve biomechanics during morphogenesis. Journal of Biomechanics. 2012;45(5):895-902.
In that study, we used micropipette aspiration to measure the nonlinear biomechanics (strain energy) of chick embryonic heart valves at different developmental stages. Here in this study, we used the same method to measure the strain energy of YAP activated/inhibited cushion explants and compared it to the data from our previous study. Our findings were summarized in the Results: “YAP inhibition elevated valve stiffness”, and the detailed measurements, including images and data, are presented in Figure S4.
There are several major weaknesses that diminish enthusiasm for the study.
- The Hippo/Yap pathway activation leads to dephosphorylation of Yap, nuclear localization, and induced expression of downstream target genes. However, there are no data included in the study on Yap nuclear/cytoplasmic ratios, phosphorylation status, or activation of other Hippo pathway mediators. Analysis of Yap expression alone is insufficient to determine activation status since it is widely expressed in multiple cells throughout the valves. The specificity for activated Yap signaling is not apparent from the immunostainings.
We thank the reviewer for pointing out this weakness. We have now implemented nuclear versus cytoplasmic localization as recommended to quantify YAP activation. We have also conducted additional experiments to analyze via qPCR YAP downstream target genes and YAP upstream genes in Hippo pathway. Please see the detailed revisions in our responses to the Recommendations for authors.
- The specific regionalized biomechanical forces acting on different regions of the valves were not measured directly or clearly compared with Yap activation status. In some cases, it seems that Yap is not present in the nuclei of endothelial cells surrounding the valve leaflets that are subject to different flow forces (Fig 1B) and the main expression is in valve interstitial subpopulations. Thus the data presented do not support differential Yap activation in endothelial cells subject to different fluid forces. There is extensive discussion of different forces acting on the valve leaflets, but the relationship to Yap signaling is not entirely clear.
We thank the reviewer for these important questions. The region-specific biomechanics have been well mapped and studied, thanks to the help from Computational Fluid Dynamics supported by ultrasound velocity and pressure measurements. For example:
Yalcin, H.C., Shekhar, A., McQuinn, T.C. and Butcher, J.T. (2011), Hemodynamic patterning of the avian atrioventricular valve. Dev. Dyn., 240: 23-35.
Bharadwaj KN, Spitz C, Shekhar A, Yalcin HC, Butcher JT. Computational fluid dynamics of developing avian outflow tract heart valves. Ann Biomed Eng. 2012 Oct;40(10):2212-27. doi: 10.1007/s10439-012-0574-8.
Ayoub S, Ferrari G, Gorman RC, Gorman JH, Schoen FJ, Sacks MS. Heart Valve Biomechanics and Underlying Mechanobiology. Compr Physiol. 2016 Sep 15;6(4):1743-1780.
Salman HE, Alser M, Shekhar A, Gould RA, Benslimane FM, Butcher JT, et al. Effect of left atrial ligation-driven altered inflow hemodynamics on embryonic heart development: clues for prenatal progression of hypoplastic left heart syndrome. Biomechanics and Modeling in Mechanobiology. 2021;20(2):733-50.
Ho S, Chan WX, Yap CH. Fluid mechanics of the left atrial ligation chick embryonic model of hypoplastic left heart syndrome. Biomechanics and Modeling in Mechanobiology. 2021;20(4):1337-51.
Those studies have shown that USS develops on the inflow surface of valves while OSS develops on the outflow surface of valves, CS develops in the tip region of valves while TS develops in the regions of elongation and compaction. Here in this study, we mimic those forces in our in-vitro and ex-vivo models. This allows us to study the direct effect of specific force on the YAP activity in different cell lineages. The results showed that OSS promoted YAP activation in VECs while USS inhibited it, CS promoted YAP activation in VICs while TS inhibited it. This result well explained the spatiotemporal distribution of YAP activation in Figure 1. For example, nuclear YAP was mostly found in VECs on the fibrosa side, where OSS develops, and YAP was not expressed in the nuclei in VECs of the atrialis/ventricularis side, where USS develops. It is also worth noting that formation of OSS on the outflow side is slower, and thus the side specific YAP activation in VECs was not in effect at the early stage, from E11.5 to E14.5.
- The requirement for Yap signaling in heart valve remodeling as described in the title was not demonstrated through manipulation of Yap activity.
With respect, it is unclear what the reviewer is asking for given no experiments are suggested nor an elaboration of alternative interpretations of our results that emphasize against YAP requirement. It has been previously shown that YAP signaling is required for early EMT stages of valvulogenesis using conditional YAP deletion in mice:
Zhang H, von Gise A, Liu Q, Hu T, Tian X, He L, et al. Yap1 Is Required for Endothelial to Mesenchymal Transition of the Atrioventricular Cushion. Journal of Biological Chemistry. 2014;289(27):18681-92.
Signaling roles for early regulators at these later fetal stages are different, sometimes opposite early EndMT stages, thus contraindicating reliance on these early data to explain later events:
Bassen D, Wang M, Pham D, Sun S, Rao R, Singh R, et al. Hydrostatic mechanical stress regulates growth and maturation of the atrioventricular valve. Development. 2021;148(13).
However, embryos with YAP deletion failed to form endocardial cushions and could not survive long enough for the study of its roles in later cushion growth and remodeling into valve leaflets. In this work,
We first showed the localization of YAP activity and its direct link with local shear or pressure domains. Then we explicitly applied controlled gain and loss of function of YAP via specific molecules. We also applied critical mechanical gain or loss of function studies to demonstrate YAP mechanoactivation necessity and sufficiency to achieve growth and remodeling.
Reviewer #2 (Public Review)
This study by Wang et al. examines changes in YAP expression in embryonic avian cultured explants in response to high and low shear stress, as well as tensile and compressive stress. The authors show that YAP expression is increased in response to low, oscillatory shear stress, as well as high compressive stress conditions. Inhibition of YAP signaling prevents compressive stress-induced increases in circularity, decreased pHH3 expression, and increases VE-cadherin expression. On the other hand, YAP gain of function prevents tensile stress-induced decreases in pHH3 expression and VE-cadherin expansion. It also decreases the strain energy density of embryonic avian cushion explants. Finally, using an avian model of left atrial ligation, the authors demonstrate that unloaded regions within the primitive valve structures are associated with increased YAP expression, compared to regions of restricted flow where YAP expression is low. Overall, this study sheds light on the biomechanical regulation of YAP expression in developing valves.
We thank the reviewer for the accurate summary and their enthusiasm for this work.
Strengths of the manuscript include:
- Novel insights into the dynamic expression pattern of YAP in valve cell populations during post-EMT stages of embryonic valvulogenesis.
- Identify the positive regulation of YAP expression in response to low, oscillatory shear stress, as well as high compressive stress conditions.
- Identify a link between YAP signaling in regulating stress-induced cell proliferation and valve morphogenesis.
- The inclusion of the atrial left atrial ligation model is innovative, and the data showing distinguishable YAP expression levels between restricted, and non-restricted flow regions is insightful.
We thank the reviewer for appreciating the strengths of this work.
This is a descriptive study that focuses on changes in YAP expression following exposure to diverse stress conditions in embryonic avian cushion explants. Overall, the study currently lacks mechanistic insights, and conclusions based on data are highly over-interpreted, particularly given that the majority of experimental protocols rely on one method of readout.
We thank the reviewer for constructive suggestions.
Reviewer #3 (Public Review)
In this manuscript, Wang et al. assess the role of wall shear stress and hydrostatic pressure during valve morphogenesis at stages where the valve elongates and takes shape. The authors elegantly demonstrate that shear and pressure have different effects on cell proliferation by modulating YAP signaling. The authors use a combination of in vitro and in vivo approaches to show that YAP signaling is activated by hydrostatic pressure changes and inhibited by wall shear stress.
We thank the reviewer for their enthusiasm for the impact of our work.
There are a few elements that would require clarification:
- The impact of YAP on valve stiffness was unclear to me. How is YAP signaling affecting stiffness? is it through cell proliferation changes? I was unclear about the model put forward:
- Is it cell proliferation (cell proliferation fluidity tissue while non-proliferating tissue is stiffer?)
- Is it through differential gene expression?
This needs clarification.
We thank the reviewer for raising this important question. Cell proliferation can affect valve stiffness but is a minor factor compared with ECM deposition and cell contractility Our micropipette aspiration data showed that the higher cell proliferation rate induced by YAP activation did lead to stiffer valves when compared to the controls. This may be because at the early stages, cells are more elastic than the viscous ECM. However, the stiffness of YAP activated valves were only about half of that of YAP inhibited valves, showing that the transcriptional level factor plays a more important role. This also suggests that YAP inhibited valves exhibited a more mature phenotype. An analogous role of YAP has also been found in cardiomyocytes. Many theories propose that in cardiomyocytes when YAP is activated the proliferation programs are turned on, while when YAP is inhibited the proliferation programs are turned off and maturation programs are released. Similarly, here we hypothesize that YAP works like a mechanobiological switch, converting mechanical signaling into the decision between growth and maturation. We have revised the Discussion to include this hypothesis.
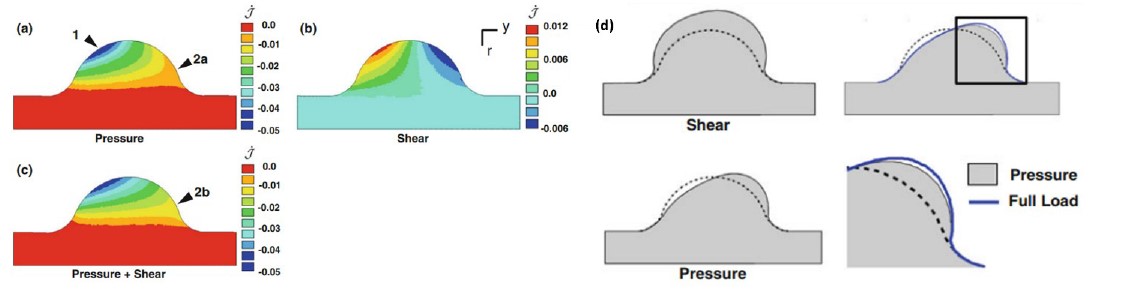
- The model proposes an early asymmetric growth of the cushion leading to different shear forces (oscillatory vs unidirectional shear stress). What triggers the initial asymmetry of the cushion shape? is YAP involved?
Although the initial geometry of the cushion model is symmetric, the force acting on it is asymmetric. The detailed numerical simulation of how the initial forces trigger the asymmetric morphogenesis can be found in our previous publication:
Buskohl PR, Jenkins JT, Butcher JT. Computational simulation of hemodynamic-driven growth and remodeling of embryonic atrioventricular valves. Biomechanics and Modeling in Mechanobiology. 2012;11(8):1205-17.
The color maps represent the dilatation rates when a) only pressure is applied, b) only shear stress is applied, and c) both pressure and shear stress are applied. It is such load that initiates an asymmetric morphological change, as shown in d). In addition, we believe YAP is involved during the initiation because it is directly nuclear activated by CS and OSS or cytoplasmically activated by TS and LSS.
- The differential expression of YAP and its correlation to cell proliferation is a little hard to see in the data presented. Drawings highlighting the main areas would help the reader to visualise the results better.
We thank the reviewer for this helpful suggestion, we have improved the visualization of Figure 3C and Figure 4C with insets of higher magnification.
- The origin of osmotic/hydrostatic pressure in vivo. While shear is clearly dependent upon blood flow, it is less clear that hydrostatic pressure is solely dependent upon blood flow. For example, it has been proposed that ECM accumulation such as hyaluronic acid could modify osmotic pressure (see for example Vignes et al.PMID: 35245444). Could the authors clarify the following questions:
- How blood flow affects osmotic pressure in vivo?
- Is ECM a factor that could affect osmotic pressure in this system?
We thank the reviewer for sharing this interesting study. The osmotic pressure plays a critical role in mechanotransduction and the development of many tissues including cardiovascular tissues and cartilage. As proposed in the reference, osmotic pressure is an interstitial force generated by cardiac contractility. Here in our study, the hydrostatic pressure is different, which is an external force applied by flowing blood. According to Bernoulli's law, when an incompressible fluid flows around a solid, the static pressure it applies on the solid is equal to its total pressure minus its dynamic pressure.
Despite the difference, the osmotic pressure can mimic the effect of hydrostatic pressure in-vitro. The in-vitro osmotic pressure model has been widely used in cartilage research, for example:
P. J. Basser, R. Schneiderman, R. A. Bank, E. Wachtel, and A. Maroudas, “Mechanical properties of the collagen network in human articular cartilage as measured by osmotic stress technique.,” Arch. Biochem. Biophys., vol. 351, no. 2, pp. 207–19, 1998.
D. a. Narmoneva, J. Y. Wang, and L. a. Setton, “Nonuniform swelling-induced residual strains in articular cartilage,” J. Biomech., vol. 32, no. 4, pp. 401–408, 1999.
C. L. Jablonski, S. Ferguson, A. Pozzi, and A. L. Clark, “Integrin α1β1 participates in chondrocyte transduction of osmotic stress,” Biochem. Biophys. Res. Commun., vol. 445, no. 1, pp. 184–190, 2014.
Z. I. Johnson, I. M. Shapiro, and M. V. Risbud, “Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: Evolving role of TonEBP,” Matrix Biol., vol. 40, pp. 10–16, 2014.
When maturing cushions shift from GAGs dominated ECM to collagen dominated ECM, the water and ion retention capacity of the tissue would be greatly changed, and thus reducing the osmotic pressure. This could in turn accelerate the maturation of cushions. By contrast, the ECM of growing cushions remain GAGs dominated, which would delay maturation and prolong the growth.
The revised second section of Results is as follows:
Shear and hydrostatic stress regulate YAP activity
In addition to the co-effector of the Hippo pathway, YAP is also a key mediator in mechanotransduction. Indeed, the spatiotemporal activation of YAP correlated with the changes in the mechanical environment. During valve remodeling, unidirectional shear stress (USS) develops on the inflow surface of valves, where YAP is rarely expressed in the nuclei of VECs (Figure 2A). On the other side, OSS develops on the outflow surface, where VECs with nuclear YAP localized. The YAP activation in VICs also correlated with hydrostatic pressure. The pressure generated compressive stress (CS) in the tips of valves, where VICs with nuclear YAP localized (Figure 2B). Whereas tensile stress (TS) was created in the elongated regions, where YAP was absent in VIC nuclei.
To study the effect of shear stress on the YAP activity in VECs, we applied USS and OSS directly onto a monolayer of freshly isolated VECs. The VEC was obtained from AV cushions of chick embryonic hearts at HH25. The cushions were placed on collagen gels with endocardium adherent to the collagen and incubated to enable the VECs to migrate onto the gel. We then removed the cushions and immediately applied the shear flow to the monolayer for 24 hours. The low stress OSS (2 dyn/cm2) promoted YAP nuclear translocation in VEC (Figure 2C, E), while high stress USS (20 dyn/cm2) restrained YAP in cytoplasm.
To study the effect of hydrostatic stress on the YAP activation in VICs, we used media with different osmolarities to mimic the CS and TS. CS was induced by hypertonic condition while TS was created by hypotonic condition, and the Unloaded (U) condition refers to the osmotically balanced media. Notably, in-vivo hydrostatic pressure is generated by flowing blood, while in-vivo osmotic pressure is generated by cardiac contractility and plays a critical role in the mechanotransduction during valve development (30). Despite the different in-vivo origination, the osmotic pressure provides a reliable model to mimic the hydrostatic pressure in-vitro (31). We cultured HH34 AV cushion explants under different loading conditions for 24 hours and found that the trapezoidal cushions adopted a spherical shape (Figure 2D). TS loaded cushions significantly compacted, and the YAP activation in VICs of TS loaded cushions was significantly lower than that in CS loaded VICs (Figure 2F).
-

eLife assessment
Determination of the biomechanical forces and downstream pathways that direct heart valve morphogenesis is an important area of research. In the current study, potential functions of localized Yap signaling in cardiac valve morphogenesis were examined. However, the evidence for Yap pathway activation and localization relative to areas of the valve subject to different mechanical stresses is not convincing.
-

Reviewer #1 (Public Review):
Determination of the biomechanical forces and downstream pathways that direct heart valve morphogenesis is an important area of research. In the current study, potential functions of localized Yap signaling in cardiac valve morphogenesis were examined. Extensive immunostainings were performed for Yap expression, but Yap activation status as indicated by nuclear versus cytoplasmic localization, Yap dephosphorylation, or expression of downstream target genes was not examined. The goal of the work was to determine Yap activation status relative to different mechanical environments, but no biomechanical data on developing heart valves were provided in the study.
There are several major weaknesses that diminish enthusiasm for the study.
1. The Hippo/Yap pathway activation leads to dephosphorylation of Yap, …Reviewer #1 (Public Review):
Determination of the biomechanical forces and downstream pathways that direct heart valve morphogenesis is an important area of research. In the current study, potential functions of localized Yap signaling in cardiac valve morphogenesis were examined. Extensive immunostainings were performed for Yap expression, but Yap activation status as indicated by nuclear versus cytoplasmic localization, Yap dephosphorylation, or expression of downstream target genes was not examined. The goal of the work was to determine Yap activation status relative to different mechanical environments, but no biomechanical data on developing heart valves were provided in the study.
There are several major weaknesses that diminish enthusiasm for the study.
1. The Hippo/Yap pathway activation leads to dephosphorylation of Yap, nuclear localization, and induced expression of downstream target genes. However, there are no data included in the study on Yap nuclear/cytoplasmic ratios, phosphorylation status, or activation of other Hippo pathway mediators. Analysis of Yap expression alone is insufficient to determine activation status since it is widely expressed in multiple cells throughout the valves. The specificity for activated Yap signaling is not apparent from the immunostainings.2. The specific regionalized biomechanical forces acting on different regions of the valves were not measured directly or clearly compared with Yap activation status. In some cases, it seems that Yap is not present in the nuclei of endothelial cells surrounding the valve leaflets that are subject to different flow forces (Fig 1B) and the main expression is in valve interstitial subpopulations. Thus the data presented do not support differential Yap activation in endothelial cells subject to different fluid forces. There is extensive discussion of different forces acting on the valve leaflets, but the relationship to Yap signaling is not entirely clear.
3. The requirement for Yap signaling in heart valve remodeling as described in the title was not demonstrated through manipulation of Yap activity.
-

Reviewer #2 (Public Review):
This study by Wang et al. examines changes in YAP expression in embryonic avian cultured explants in response to high and low shear stress, as well as tensile and compressive stress. The authors show that YAP expression is increased in response to low, oscillatory shear stress, as well as high compressive stress conditions. Inhibition of YAP signaling prevents compressive stress-induced increases in circularity, decreased pHH3 expression, and increases VE-cadherin expression. On the other hand, YAP gain of function prevents tensile stress-induced decreases in pHH3 expression and VE-cadherin expansion. It also decreases the strain energy density of embryonic avian valve explants. Finally, using an avian model of left atrial ligation, the authors demonstrate that unloaded regions within the primitive valve …
Reviewer #2 (Public Review):
This study by Wang et al. examines changes in YAP expression in embryonic avian cultured explants in response to high and low shear stress, as well as tensile and compressive stress. The authors show that YAP expression is increased in response to low, oscillatory shear stress, as well as high compressive stress conditions. Inhibition of YAP signaling prevents compressive stress-induced increases in circularity, decreased pHH3 expression, and increases VE-cadherin expression. On the other hand, YAP gain of function prevents tensile stress-induced decreases in pHH3 expression and VE-cadherin expansion. It also decreases the strain energy density of embryonic avian valve explants. Finally, using an avian model of left atrial ligation, the authors demonstrate that unloaded regions within the primitive valve structures are associated with increased YAP expression, compared to regions of restricted flow where YAP expression is low. Overall, this study sheds light on the biomechanical regulation of YAP expression in developing valves.
Strengths of the manuscript include:
- Novel insights into the dynamic expression pattern of YAP in valve cell populations during post-EMT stages of embryonic valvulogenesis.
- Identify the positive regulation of YAP expression in response to low, oscillatory shear stress, as well as high compressive stress conditions.
- Identify a link between YAP signaling in regulating stress-induced cell proliferation and valve morphogenesis.
- The inclusion of the atrial left atrial ligation model is innovative, and the data showing distinguishable YAP expression levels between restricted, and non-restricted flow regions is insightful.This is a descriptive study that focuses on changes in YAP expression following exposure to diverse stress conditions in embryonic avian valve explants. Overall, the study currently lacks mechanistic insights, and conclusions based on data are highly over-interpreted, particularly given that the majority of experimental protocols rely on one method of readout.
-

Reviewer #3 (Public Review):
In this manuscript, Wang et al. assess the role of wall shear stress and hydrostatic pressure during valve morphogenesis at stages where the valve elongates and takes shape. The authors elegantly demonstrate that shear and pressure have different effects on cell proliferation by modulating YAP signaling. The authors use a combination of in vitro and in vivo approaches to show that YAP signaling is activated by hydrostatic pressure changes and inhibited by wall shear stress.
There are a few elements that would require clarification:
The impact of YAP on valve stiffness was unclear to me. How is YAP signaling affecting stiffness? is it through cell proliferation changes? I was unclear about the model put forward:
- Is it cell proliferation (cell proliferation fluidity tissue while non-proliferating tissue is …
Reviewer #3 (Public Review):
In this manuscript, Wang et al. assess the role of wall shear stress and hydrostatic pressure during valve morphogenesis at stages where the valve elongates and takes shape. The authors elegantly demonstrate that shear and pressure have different effects on cell proliferation by modulating YAP signaling. The authors use a combination of in vitro and in vivo approaches to show that YAP signaling is activated by hydrostatic pressure changes and inhibited by wall shear stress.
There are a few elements that would require clarification:
The impact of YAP on valve stiffness was unclear to me. How is YAP signaling affecting stiffness? is it through cell proliferation changes? I was unclear about the model put forward:
- Is it cell proliferation (cell proliferation fluidity tissue while non-proliferating tissue is stiffer?)
- Is it through differential gene expression?
This needs clarification.The model proposes an early asymmetric growth of the cushion leading to different shear forces (oscillatory vs unidirectional shear stress). What triggers the initial asymmetry of the cushion shape? is YAP involved?
The differential expression of YAP and its correlation to cell proliferation is a little hard to see in the data presented. Drawings highlighting the main areas would help the reader to visualise the results better.
The origin of osmotic/hydrostatic pressure in vivo. While shear is clearly dependent upon blood flow, it is less clear that hydrostatic pressure is solely dependent upon blood flow. For example, it has been proposed that ECM accumulation such as hyaluronic acid could modify osmotic pressure (see for example Vignes et al.PMID: 35245444). Could the authors clarify the following questions:
- How blood flow affects osmotic pressure in vivo?
- Is ECM a factor that could affect osmotic pressure in this system?
-