Iron chelation improves ineffective erythropoiesis and iron overload in myelodysplastic syndrome mice
Curation statements for this article:-
Curated by eLife
eLife assessment
This study presents a valuable finding that erythrocyte precursors could re-gain EPO responsiveness after DFP chelation therapy. In addition, the authors investigated iron trafficking in erythroblasts using the MDS mouse model. However, the evidence supporting the claims of the authors is still inadequate. The work will be of interest to medical biologists working on hematology.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Myelodysplastic syndrome (MDS) is a heterogeneous group of bone marrow stem cell disorders characterized by ineffective hematopoiesis and cytopenias, most commonly anemia. Red cell transfusion therapy for anemia in MDS results in iron overload, correlating with reduced overall survival. Whether the treatment of iron overload benefits MDS patients remains controversial. We evaluate underlying iron-related pathophysiology and the effect of iron chelation using deferiprone on erythropoiesis in NUP98-HOXD13 transgenic mice, a highly penetrant well-established MDS mouse model. Our results characterize an iron overload phenotype with aberrant erythropoiesis in these mice which was reversed by deferiprone-treatment. Serum erythropoietin levels decreased while erythroblast erythropoietin receptor expression increased in deferiprone-treated MDS mice. We demonstrate, for the first time, normalized expression of the iron chaperones Pcbp1 and Ncoa4 and increased ferritin stores in late-stage erythroblasts from deferiprone-treated MDS mice, evidence of aberrant iron trafficking in MDS erythroblasts. Importantly, erythroblast ferritin is increased in response to deferiprone, correlating with decreased erythroblast ROS. Finally, we confirmed increased expression of genes involved in iron uptake, sensing, and trafficking in stem and progenitor cells from MDS patients. Taken together, our findings provide evidence that erythroblast-specific iron metabolism is a novel potential therapeutic target to reverse ineffective erythropoiesis in MDS.
Article activity feed
-

-

Author Response
Reviewer #3 (Public Review):
Myelodysplastic syndrome (MDS) is a heterogenous, clonal hematopoietic stem cell disorder characterized by morphological dysplasia in one or more hematopoietic lineages, cytopenias (most frequently anemia), and ineffective hematopoiesis. In patients with MDS, transfusion therapy treatment causes clinical iron overload; however it has been unclear if treatment with iron chelation yields clinical benefits. In the present study, the authors use a transgenic mouse model of MDS, NUP98-HOXD13 (referred to here as "MDS mice") to investigate this area. Starting at 5 months of age (before MDS mice progress to acute leukemia), the authors administered DFP in the drinking water for 4 weeks, and compared parameters to untreated MDS mice and WT controls.
The authors first show that MDS mice exhibit …
Author Response
Reviewer #3 (Public Review):
Myelodysplastic syndrome (MDS) is a heterogenous, clonal hematopoietic stem cell disorder characterized by morphological dysplasia in one or more hematopoietic lineages, cytopenias (most frequently anemia), and ineffective hematopoiesis. In patients with MDS, transfusion therapy treatment causes clinical iron overload; however it has been unclear if treatment with iron chelation yields clinical benefits. In the present study, the authors use a transgenic mouse model of MDS, NUP98-HOXD13 (referred to here as "MDS mice") to investigate this area. Starting at 5 months of age (before MDS mice progress to acute leukemia), the authors administered DFP in the drinking water for 4 weeks, and compared parameters to untreated MDS mice and WT controls.
The authors first show that MDS mice exhibit systemic iron overload and macrocytic anemia that is improved by treatment with the iron chelator deferiprone (DFP). They then perform a detailed characterization the effects of DFP treatment on erythroid differentiation and various parameters related to iron transport and trafficking in MDS erythroblasts. Strengths of the work are the use of a well-characterized mouse model of MDS with appropriate animal group sizes and detailed analyses of systemic iron parameters and erythroid subpopulations. A remediable weakness is that in certain areas of the Results and Discussion, the authors overinterpret their findings by inferring causation when they have only shown a correlation. Additionally, when drawing conclusions based on changes in erythroblast mRNA expression levels between groups, the authors should consider that translation efficiency may be altered in MDS and that the NUP98 fusion protein itself, by acting as a chimeric transcription factor, may also impact gene expression profiles. Given that the application of chelators for treatment of MDS remains controversial, this work will be of interest to scientists focused on erythroid maturation and iron dysregulation in MDS, as well as clinicians caring for patients with this disorder.
Major Comments
- The authors define the stages of erythroblast differentiation using the CD44-FSC method, which assumes that CD44 expression levels during the stages of erythroid differentiation are not altered by MDS itself. Are morphologically abnormal erythroblasts, such as bi-nucleate forms, captured in this analysis, and if so, are they classified in the appropriate subset? The percentage of erythroblasts in the bone marrow of MDS mice in this current study is lower than that reported by Suragani et al (Nat Med 2014), who employed a different strategy to define erythroid precursors. While representative erythroblast gating is presented as Supplemental Figure 17, it would be important to present representative gating from all 3 animal groups: WT, MDS, and MDS+DFP mice.
We appreciate this comment and have added representative gating for all 3 groups to Supplemental Figure 17 (new Figure 3 – figure supplement 6 in the revised manuscript).
- Methods, "Statistical analysis." The authors state that all comparisons were done with 2-tailed student paired t test, which would not be appropriate for comparisons being made between independent animals groups (i.e. when groups are not "paired").
We appreciate this comment and have reanalyzed all revised mouse data using one-way ANOVA with multiple comparisons and Tukey post-test analyses when more than 2 groups were compared. This has been edited in the Methods section in the revised manuscript.
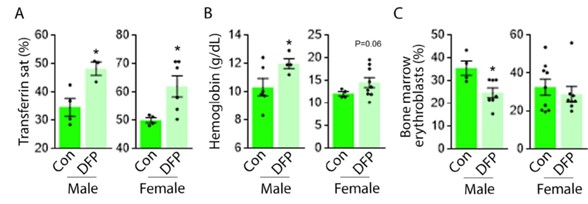
- The Results (p.7) indicates that both sexes showed similar responses to DFP; however, the figure legends do not indicate sex. Given that systemic iron metabolism in mice shows sex-related differences, sex should be specified.
We appreciate this comment and present here the gender-specific data for the reviewers’ evaluation (Author respone image 1). Similarly elevated transferrin saturation (a) (n = 3-4 male mice/group and n = 4-6 female mice/group) and hemoglobin (b) (n = 4-6 male mice/group and n = 4-9 female mice/group) are observed in male and female DFP-treated MDS mice. (c) Bone marrow erythroblasts are decreased to a greater degree in male relative to female DFP-treated MDS mice (n = 4-7 male mice/group and n = 8-9 female mice/group). We have added the data on gender-specific measures to new Figure 1 - figure supplement 3, Figure 2 – figure supplement 1, and Figure 3 – figure supplement 1 in the revised manuscript.
Author respone image 1.
-

eLife assessment
This study presents a valuable finding that erythrocyte precursors could re-gain EPO responsiveness after DFP chelation therapy. In addition, the authors investigated iron trafficking in erythroblasts using the MDS mouse model. However, the evidence supporting the claims of the authors is still inadequate. The work will be of interest to medical biologists working on hematology.
-

Reviewer #1 (Public Review):
Myelodysplastic syndrome (MDS) represents as a rather complex and serious hematologic malignancy that affects the production of normal blood cells in the bone marrow. Some types of MDS could stay mild for years and other types of MDS could be more serious and progressed into AML. Tremendous efforts have been made to investigate the pathogenesis and treatment of MDS. For instance, a pile of papers has found that iron chelation therapy could benefit the overall survival in low risk MDS patients. Yet, the risk and benefit of this therapy remain in much debate. The authors demonstrated that erythrocyte precursors could re-gain EPO responsiveness after DFP chelation therapy. In addition, the authors investigated iron trafficking in erythroblasts using the MDS mouse model. The paper is rather interesting as it …
Reviewer #1 (Public Review):
Myelodysplastic syndrome (MDS) represents as a rather complex and serious hematologic malignancy that affects the production of normal blood cells in the bone marrow. Some types of MDS could stay mild for years and other types of MDS could be more serious and progressed into AML. Tremendous efforts have been made to investigate the pathogenesis and treatment of MDS. For instance, a pile of papers has found that iron chelation therapy could benefit the overall survival in low risk MDS patients. Yet, the risk and benefit of this therapy remain in much debate. The authors demonstrated that erythrocyte precursors could re-gain EPO responsiveness after DFP chelation therapy. In addition, the authors investigated iron trafficking in erythroblasts using the MDS mouse model. The paper is rather interesting as it discussed the biological effects and underlying mechanisms of DFP for the treatment of low-risk MDS. More importantly, the paper adds practical values and theoretical evidences for chelation therapy towards low-risk MDS. The paper is overall well-written.
-

Reviewer #2 (Public Review):
There are reports that patients experience hematologic improvement after treatment with iron chelators but the mechanism of this improvement and the specific patient category that benefits are not known. This article uses a mouse model of MDS to explore the mechanism by which chelator therapy may lead to improved erythropoiesis. Although many changes were seen in the MDS mouse model treated with deferiprone, a causal mechanism was not demonstrated.
The authors provide solid evidence for the following:
1. The NUP98-HOXD13 mouse model of MDS recapitulates spontaneous (non-transfusion related) iron overload seen in some subtypes of MDS
2. In this model, iron chelation with deferiprone (DFP) improves not only iron overload but also improves anemia, decreases splenomegaly, decreases erythropoietin concentrations …Reviewer #2 (Public Review):
There are reports that patients experience hematologic improvement after treatment with iron chelators but the mechanism of this improvement and the specific patient category that benefits are not known. This article uses a mouse model of MDS to explore the mechanism by which chelator therapy may lead to improved erythropoiesis. Although many changes were seen in the MDS mouse model treated with deferiprone, a causal mechanism was not demonstrated.
The authors provide solid evidence for the following:
1. The NUP98-HOXD13 mouse model of MDS recapitulates spontaneous (non-transfusion related) iron overload seen in some subtypes of MDS
2. In this model, iron chelation with deferiprone (DFP) improves not only iron overload but also improves anemia, decreases splenomegaly, decreases erythropoietin concentrations and makes erythropoiesis more effective
3. DFP treatment does not change hepcidin mRNA but increases it relative to the iron load. Consistently, DFP treatment also lowers the expression of erythroferrone mRNA in erythroblasts.
4. DFP lowers erythroblast reactive oxygen speciesThe authors identify a number of changes that result from iron chelation in their model but do not causally link them to the improvements in iron overload, anemia or ineffective erythropoiesis:
5. DFP alters the expression of GATA-1, Bcl-XI, EpoR, TfR1 but not TfR2, as well as intracellular iron chaperone Pcbp1, and the cargo receptor Ncoa4
6. Analyses of the same genes in human CD34+ selected bone marrow samples from unclassified MDS patients are shown but no conclusion or comparison is (or can be) made to the mouse data.
7. The data therefore do not provide a mechanistic explanation of the effect of DFP on anemia and ineffective erythropoiesisThe manuscript has significant strengths and several substantial weaknesses. The strengths include the establishment of a mouse MDS model that manifests anemia, ineffective erythropoiesis and non-transfusional iron overload, with increased erythroferrone and inadequate hepcidin response to iron overload, features that improve after treatment with deferiprone. The main structural weakness is that the many changes in erythroid pathways documented in the manuscript do not establish the mechanism by which deferiprone mediates these beneficial effects.
-

Reviewer #3 (Public Review):
Myelodysplastic syndrome (MDS) is a heterogenous, clonal hematopoietic stem cell disorder characterized by morphological dysplasia in one or more hematopoietic lineages, cytopenias (most frequently anemia), and ineffective hematopoiesis. In patients with MDS, transfusion therapy treatment causes clinical iron overload; however it has been unclear if treatment with iron chelation yields clinical benefits. In the present study, the authors use a transgenic mouse model of MDS, NUP98-HOXD13 (referred to here as "MDS mice") to investigate this area. Starting at 5 months of age (before MDS mice progress to acute leukemia), the authors administered DFP in the drinking water for 4 weeks, and compared parameters to untreated MDS mice and WT controls.
The authors first show that MDS mice exhibit systemic iron overload …
Reviewer #3 (Public Review):
Myelodysplastic syndrome (MDS) is a heterogenous, clonal hematopoietic stem cell disorder characterized by morphological dysplasia in one or more hematopoietic lineages, cytopenias (most frequently anemia), and ineffective hematopoiesis. In patients with MDS, transfusion therapy treatment causes clinical iron overload; however it has been unclear if treatment with iron chelation yields clinical benefits. In the present study, the authors use a transgenic mouse model of MDS, NUP98-HOXD13 (referred to here as "MDS mice") to investigate this area. Starting at 5 months of age (before MDS mice progress to acute leukemia), the authors administered DFP in the drinking water for 4 weeks, and compared parameters to untreated MDS mice and WT controls.
The authors first show that MDS mice exhibit systemic iron overload and macrocytic anemia that is improved by treatment with the iron chelator deferiprone (DFP). They then perform a detailed characterization the effects of DFP treatment on erythroid differentiation and various parameters related to iron transport and trafficking in MDS erythroblasts. Strengths of the work are the use of a well-characterized mouse model of MDS with appropriate animal group sizes and detailed analyses of systemic iron parameters and erythroid subpopulations. A remediable weakness is that in certain areas of the Results and Discussion, the authors overinterpret their findings by inferring causation when they have only shown a correlation. Additionally, when drawing conclusions based on changes in erythroblast mRNA expression levels between groups, the authors should consider that translation efficiency may be altered in MDS and that the NUP98 fusion protein itself, by acting as a chimeric transcription factor, may also impact gene expression profiles. Given that the application of chelators for treatment of MDS remains controversial, this work will be of interest to scientists focused on erythroid maturation and iron dysregulation in MDS, as well as clinicians caring for patients with this disorder.
Major Comments
1. The authors define the stages of erythroblast differentiation using the CD44-FSC method, which assumes that CD44 expression levels during the stages of erythroid differentiation are not altered by MDS itself. Are morphologically abnormal erythroblasts, such as bi-nucleate forms, captured in this analysis, and if so, are they classified in the appropriate subset? The percentage of erythroblasts in the bone marrow of MDS mice in this current study is lower than that reported by Suragani et al (Nat Med 2014), who employed a different strategy to define erythroid precursors. While representative erythroblast gating is presented as Supplemental Figure 17, it would be important to present representative gating from all 3 animal groups: WT, MDS, and MDS+DFP mice.
2. Methods, "Statistical analysis." The authors state that all comparisons were done with 2-tailed student paired t test, which would not be appropriate for comparisons being made between independent animals groups (i.e. when groups are not "paired").
3. The Results (p.7) indicates that both sexes showed similar responses to DFP; however, the figure legends do not indicate sex. Given that systemic iron metabolism in mice shows sex-related differences, sex should be specified.
-