The effect of weight loss following 18 months of lifestyle intervention on brain age assessed with resting-state functional connectivity
Curation statements for this article:-
Curated by eLife
eLife assessment
This potentially important study examines brain age based on resting state functional connectivity (RSFC) following an 18-month lifestyle intervention. The design of the intervention study is generally solid; the randomized controlled trial includes three intervention groups and assessments at two-time points of numerous health markers, however, the methodology for brain age prediction appears somewhat incomplete and would benefit from more rigorous approaches. The lack of control groups also prevents firm conclusions about the extent to which the observed RSFC changes are linked to the intervention. With these parts strengthened, the paper would be of broad interest to neuroscientists and biologists working on obesity, lifestyle interventions, and brain health.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Obesity negatively impacts multiple bodily systems, including the central nervous system. Retrospective studies that estimated chronological age from neuroimaging have found accelerated brain aging in obesity, but it is unclear how this estimation would be affected by weight loss following a lifestyle intervention.
Methods:
In a sub-study of 102 participants of the Dietary Intervention Randomized Controlled Trial Polyphenols Unprocessed Study (DIRECT-PLUS) trial, we tested the effect of weight loss following 18 months of lifestyle intervention on predicted brain age based on magnetic resonance imaging (MRI)-assessed resting-state functional connectivity (RSFC). We further examined how dynamics in multiple health factors, including anthropometric measurements, blood biomarkers, and fat deposition, can account for changes in brain age.
Results:
To establish our method, we first demonstrated that our model could successfully predict chronological age from RSFC in three cohorts (n=291;358;102). We then found that among the DIRECT-PLUS participants, 1% of body weight loss resulted in an 8.9 months’ attenuation of brain age. Attenuation of brain age was significantly associated with improved liver biomarkers, decreased liver fat, and visceral and deep subcutaneous adipose tissues after 18 months of intervention. Finally, we showed that lower consumption of processed food, sweets and beverages were associated with attenuated brain age.
Conclusions:
Successful weight loss following lifestyle intervention might have a beneficial effect on the trajectory of brain aging.
Funding:
The German Research Foundation (DFG), German Research Foundation - project number 209933838 - SFB 1052; B11, Israel Ministry of Health grant 87472511 (to I Shai); Israel Ministry of Science and Technology grant 3-13604 (to I Shai); and the California Walnuts Commission 09933838 SFB 105 (to I Shai).
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
It is now widely accepted that the age of the brain can differ from the person's chronological age and neuroimaging methods are ideally suited to analyze the brain age and associated biomarkers. Preclinical studies of rodent models with appropriate neuroimaging do attest that lifestyle-related prevention approaches may help to slow down brain aging and the potential of BrainAGE as a predictor of age-related health outcomes. However, there is a paucity of data on this in humans. It is in this context the present manuscript receives its due attention.
Comments:
- Lifestyle intervention benefits need to be analyzed using robust biomarkers which should be profiled non-invasively in a clinical setting. There is increasing evidence of the role of telomere length in brain aging. Gampawar et al …
Author Response
Reviewer #1 (Public Review):
It is now widely accepted that the age of the brain can differ from the person's chronological age and neuroimaging methods are ideally suited to analyze the brain age and associated biomarkers. Preclinical studies of rodent models with appropriate neuroimaging do attest that lifestyle-related prevention approaches may help to slow down brain aging and the potential of BrainAGE as a predictor of age-related health outcomes. However, there is a paucity of data on this in humans. It is in this context the present manuscript receives its due attention.
Comments:
- Lifestyle intervention benefits need to be analyzed using robust biomarkers which should be profiled non-invasively in a clinical setting. There is increasing evidence of the role of telomere length in brain aging. Gampawar et al (2020) have proposed a hypothesis on the effect of telomeres on brain structure and function over the life span and named it as the "Telomere Brain Axis". In this context, if the authors could measure telomere length before and after lifestyle intervention, this will give a strong biomarker utility and value addition for the lifestyle modification benefits.
- Authors should also consider measuring BDNF levels before and after lifestyle intervention.
Response to comments 1+2: we agree that associating both telomere length and BDNF level with brain age would be interesting and relevant. However, we did not measure these two variables. We would certainly consider adding these in future work. Regarding telomere length, we now include a short discussion of brain age in relation to other bodily ages, such as telomere length (Discussion section):
“Studying changes in functional brain aging is part of a broader field that examines changes in various biological ages, such as telomere length1, DNA methylation2, and arterial stiffness3. Evaluating changes in these bodily systems over time allows us to capture health and lifestyle-related factors that affect overall aging and may guide the development of targeted interventions to reduce age-related decline. For example, in the CENTRAL cohort, we recently reported that reducing body weight and intrahepatic fat following a lifestyle intervention was related to methylation age attenuation4. In the current work, we used RSFC for brain age estimation, which resulted in a MAE of ~8 years, which was larger than the intervention period. Nevertheless, we found that brain age attenuation was associated with changes in multiple health factors. The precision of an age prediction model based on RSFC is typically lower than a model based on structural brain imaging5. However, a higher model precision may result in a lower sensitivity to detect clinical effects6,7. Better tools for data harmonization among dataset6 and larger training sample size5 may improve the accuracy of such models in the future. We also suggest that examining the dynamics of multiple bodily ages and their interactions would enhance our understanding of the complex aging process8,9. “
And
“These findings complement the growing interest in bodily aging indicated, for example, by DNA methylation4 as health biomarkers and interventions that may affect them.”
Reviewer #2 (Public Review):
In this study, Levakov et al. investigated brain age based on resting-state functional connectivity (RSFC) in a group of obese participants following an 18-month lifestyle intervention. The study benefits from various sophisticated measurements of overall health, including body MRI and blood biomarkers. Although the data is leveraged from a solid randomized control set-up, the lack of control groups in the current study means that the results cannot be attributed to the lifestyle intervention with certainty. However, the study does show a relationship between general weight loss and RSFC-based brain age estimations over the course of the intervention. While this may represent an important contribution to the literature, the RSFC-based brain age prediction shows low model performance, making it difficult to interpret the validity of the derived estimates and the scale of change. The study would benefit from more rigorous analyses and a more critical discussion of findings. If incorporated, the study contributes to the growing field of literature indicating that weight-reduction in obese subjects may attenuate the detrimental effect of obesity on the brain.
The following points may be addressed to improve the study:
Brain age / model performance:
- Figure 2: In the test set, the correlation between true and predicted age is 0.244. The fitted slope looks like it would be approximately 0.11 (55-50)/(80-35); change in y divided by change in x. This means that for a chronological age change of 12 months, the brain age changes by 0.11*12 = 1.3 months. I.e., due to the relatively poor model performance, an 80-year-old participant in the plot (fig 2) has a predicted age of ~55. Hence, although the age prediction step can generate a summary score for all the RSFC data, it can be difficult to interpret the meaning of these brain age estimates and the 'expected change' since the scale is in years.
- In Figure 2 it could also help to add the x = y line to get a better overview of the prediction variance. The estimates are likely clustered around the mean/median age of the training dataset, and age is overestimated in younger subs and overestimated in older subs (usually referred to as "age bias"). It is important to inspect the data points here to understand what the estimates represent, i.e., is variation in RSFC potentially lost by wrapping the data in this summary measure, since the age prediction is not particularly accurate, and should age bias in the predictions be accounted for by adjusting the test data for the bias observed in the training data?
Response to comment 1+2: we agree with the reviewer that due to the relatively moderate correlation between the predicted and observed age, a large change in the observed age corresponds to a small change in the predicted age. We now state this limitation in Results section 2.1:
“Despite being significant and reproducible, we note that the correlations between the observed and predicted age were relatively moderate.”
And discuss this point in the Discussion section:
“In the current work, we used RSFC for brain age estimation, which resulted in a MAE of ~8 years, which was larger than the intervention period. Nevertheless, we found that brain age attenuation was associated with changes in multiple health factors. The precision of an age prediction model based on RSFC is typically lower than a model based on structural brain imaging5. However, a higher model precision may result in a lower sensitivity to detect clinical effects6,7. Better tools for data harmonization among dataset6 and larger training sample size5 may improve the accuracy of such models in the future.”
Moreover, , we now add the x=y line to Fig. 2, so the readers can better assess the prediction variance as suggested by the reviewer:
We prefer to avoid using different scales (year/month) in the x and y axes to avoid misleading the readers, but the list of observed and predicted ages are available as SI files with a precision of 2 decimals point (~3 days).
We note that despite the moderate precision accuracy, we replicated these results in three separate cohorts.
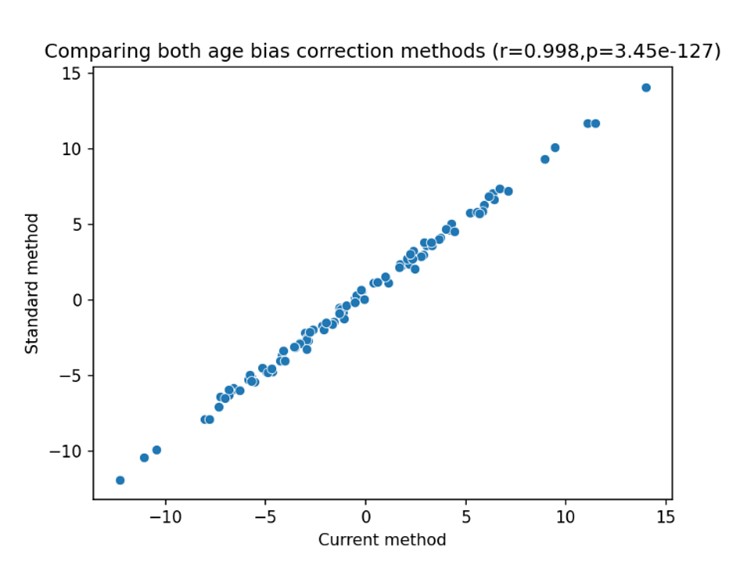
Regarding the effect of “age bias” (also known as “regression attenuation” or “regression dilution” 10), we are aware of this phenomenon and agree that it must be accounted for. In fact, the “age bias” is one of the reasons we chose to use the difference between the expected and observed ages as the primary outcome of the study, as this measure already takes this bias into account. To demonstrate this effect we now compute brain age attenuation in two ways: 1. As described and used in the current study (Methods 4.9); and 2. By regressing out the effect of age on the predicted brain age at both times separately, then subtracting the adjusted predicted age at T18 from the adjusted predicted age at T0. The second method is the standard method to account for age bias as described in a previous work 11. Below is a scatter plot of both measures across all participants:
The x-axis represents the first method, used in the current study, and the y-axis represents the second method, described in Smith et al., (2019). Across all subjects, we found a nearly perfect 1:1 correspondence between the two methods (r=.998, p<0.001; MAE=0.45), as the two are mathematically identical. The small gap between the two is because the brain age attenuation model also takes into account the difference in the exact time that passed between the two scans for each participant (mean=21.36m, std = 1.68m).
We now note this in Methods section 4.9:
“We note that the result of computing the difference between the bias-corrected brain age gap at both times was nearly identical to the brain age attenuation measure (r=.99, p<0.001; MAE=0.45). The difference between the two is because the brain age attenuation model takes into account the difference in the exact time that passed between the two scans for each participant (mean=21.36m, std = 1.68m).”
- In Figure 3, some of the changes observed between time points are very large. For example, one subject with a chronological age of 62 shows a ten-year increase in brain age over 18 months. This change is twice as large as the full range of age variation in the brain age estimates (average brain age increases from 50 to 55 across the full chronological age span). This makes it difficult to interpret RSFC change in units of brain age. E.g., is it reasonable that a person's brain ages by ten years, either up or down, in 18 months? The colour scale goes from -12 years to 14 years, so some of the observed changes are 14 / 1.5 = 9 times larger than the actual time from baseline to follow-up.
- The questions above should be investigated and addressed in the context of potential challenges with using brain age as a marker (see e.g., https://onlinelibrary.wiley.com/doi/full/10.1002/hbm.25837, https://onlinelibrary.wiley.com/doi/full/10.1002/hbm.26144).
We agree that our model precision was relatively low, especially compared to the period of the intervention, as also stated by reviewer #1. We now discuss this issue in light of the studies pointed out by the reviewer (Discussion section):
“In the current work, we used RSFC for brain age estimation, which resulted in a MAE of ~8 years, which was larger than the intervention period. Nevertheless, we found that brain age attenuation was associated with changes in multiple health factors. The precision of an age prediction model based on RSFC is typically lower than a model based on structural brain imaging5. However, a higher model precision may result in a lower sensitivity to detect clinical effects6,7. Better tools for data harmonization among datasets6 and larger training sample size5 may improve the accuracy of such models in the future.”
Again, we note that despite the moderate precision accuracy, we replicated these results in three separate cohorts and found that both the correlation and the MAE between the predicted and observed age were significant in all of them.
RSFC for age prediction:
- Several studies show better age prediction accuracy with structural MRI features compared to RSFC. If the focus of the study is to use an accurate estimate of brain ageing rather than specifically looking at changes in RSFC, adding structural MRI data could be helpful.
We focused on brain structural changes in a previous work, and the focus of the current work was assessing age-related functional connectivity alterations. We now added a few sentences in the Introduction section that would hopefully better motivate our choice:
“We previously found that weight loss, glycemic control, lowering of blood pressure, and increment in polyphenols-rich food were associated with an attenuation in brain atrophy 12. Obesity is also manifested in age-related changes in the brain’s functional organization as assessed with resting-state functional connectivity (RSFC). These changes are dynamic13 and can be observed in short time scales14 and thus of relevance when studying lifestyle intervention.”
- If changes in RSFC are the main focus, using brain age adds a complicated layer that is not necessarily helpful. It could be easier to simply assess RSFC change from baseline to follow up, and correlate potential changes with changes in e.g., BMI.
We are specifically interested in age-related changes as we described a-priori in the registration of the study: https://clinicaltrials.gov/ct2/show/NCT03020186
Moreover, age-related changes in RSFC are complex, multivariate and dependent upon the choice of theoretical network measures. We think that a data-driven brain age prediction approach might better capture these multifaceted changes and their relation to aging. We now state this in the Introduction section:
“Studies have linked obesity with decreased connectivity within the default mode network15,16 and increased connectivity with the lateral orbitofrontal cortex17, which are also seen in normal aging18,19. Longitudinal trials have reported changes in these connectivity patterns following weight reduction20,21, indicating that they can be altered. However, findings regarding functional changes are less consistent than those related to anatomical changes due to the multiple measures22 and scales23 used to quantify RSFC. Hence, focusing on a single measure, the functional brain age, may better capture these complex, multivariant changes and their relation to aging. “
The lack of control groups
- If no control group data is available, it is important to clarify this in the manuscript, and evaluate which conclusions can and cannot be drawn based on the data and study design.
We agree that this point should be made more clear, and we now state this in the limitation section of the Discussion:
“We also note that the lack of a no-intervention control group limits our ability to directly relate our findings to the intervention. Hence, we can only relate brain age attenuation to the observed changes in health biomarkers.”
Also, following reviewers’ #2 and #3 comments, we refer to the weight loss following 18 months of lifestyle intervention instead of to the intervention itself. This is now made clear in the title, abstract, and the main text.
Reviewer #3 (Public Review):
The authors report on an interesting study that addresses the effects of a physical and dietary intervention on accelerated/decelerated brain ageing in obese individuals. More specifically, the authors examined potential associations between reductions in Body-Mass-Index (BMI) and a decrease in relative brain-predicted age after an 18-months period in N = 102 individuals. Brain age models were based on resting-state functional connectivity data. In addition to change in BMI, the authors also tested for associations between change in relative brain age and change in waist circumference, six liver markers, three glycemic markers, four lipid markers, and four MRI fat deposition measures. Moreover, change in self-reported consumption of food, stratified by categories such as 'processed food' and 'sweets and beverages', was tested for an association with change in relative brain age. Their analysis revealed no evidence for a general reduction in relative brain age in the tested sample. However, changes in BMI, as well as changes in several liver, glycemic, lipid, and fat-deposition markers showed significant covariation with changes in relative brain age. Three markers remained significant after additionally controlling for BMI, indicating an incremental contribution of these markers to change in relative brain age. Further associations were found for variables of subjective food consumption. The authors conclude that lifestyle interventions may have beneficial effects on brain aging.
Overall, the writing is concise and straightforward, and the langue and style are appropriate. A strength of the study is the longitudinal design that allows for addressing individual accelerations or decelerations in brain aging. Research on biological aging parameters has often been limited to cross-sectional analyses so inferences about intra-individual variation have frequently been drawn from inter-individual variation. The presented study allows, in fact, investigating within-person differences. Moreover, I very much appreciate that the authors seek to publish their code and materials online, although the respective GitHub project page did not appear to be set to 'public' at the time (error 404). Another strength of the study is that brain age models have been trained and validated in external samples. One further strength of this study is that it is based on a registered trial, which allows for the evaluation of the aims and motivation of the investigators and provides further insights into the primary and secondary outcomes measures (see the clinical trial identification code).
One weakness of the study is that no comparison between the active control group and the two experimental groups has been carried out, which would have enabled causal inferences on the potential effects of different types of interventions on changes in relative brain age. In this regard, it should also be noted that all groups underwent a lifestyle intervention. Hence, from an experimenter's perspective, it is problematic to conclude that lifestyle interventions may modulate brain age, given the lack of a control group without lifestyle intervention. This issue is fueled by the study title, which suggests a strong focus on the effects of lifestyle intervention. Technically, however, this study rather constitutes an investigation of the effects of successful weight loss/body fat reduction on brain age among participants who have taken part in a lifestyle intervention. In keeping with this, the provided information on the main effect of time on brain age is scarce, essentially limited to a sign test comparing the proportions of participants with an increase vs. decrease in relative brain age. Interestingly, this analysis did not suggest that the proportion of participants who benefit from the intervention (regarding brain age) significantly exceeds the number of participants who do not benefit. So strictly speaking, the data rather indicates that it's not the lifestyle intervention per sé that contributes to changes in brain age, but successful weight loss/body fat reduction. In sum, I feel that the authors' claims on the effects of the intervention cannot be underscored very well given the lack of a control group without lifestyle intervention.
We agree that this point, also raised by reviewer #2, should be made clear, and we now state this in the limitation section of the Discussion:
“We also note that the lack of a no-intervention control group limits our ability to directly relate our findings to the intervention. Hence, we can only relate brain age attenuation to the observed changes in health biomarkers.”
Also, following reviewers #2 and #3, we refer to the weight loss following 18 months of lifestyle intervention instead of to the intervention itself. This is now explicitly mentioned in the title, abstract, and within the text:
Title: “The effect of weight loss following 18 months of lifestyle intervention on brain age assessed with resting-state functional connectivity”
Abstract: “…, we tested the effect of weight loss following 18 months of lifestyle intervention on predicted brain age, based on MRI-assessed resting-state functional connectivity (RSFC).”
Another major weakness is that no rationale is provided for why the authors use functional connectivity data instead of structural scans for their age estimation models. This gets even more evident in view of the relatively low prediction accuracies achieved in both the validation and test sets. My notion of the literature is that the vast majority of studies in this field implicate brain age models that were trained on structural MRI data, and these models have achieved way higher prediction accuracies. Along with the missing rationale, I feel that the low model performances require some more elaboration in the discussion section. To be clear, low prediction accuracies may be seen as a study result and, as such, they should not be considered as a quality criterion of the study. Nevertheless, the choice of functional MRI data and the relevance of the achieved model performances for subsequent association analysis needs to be addressed more thoroughly.
We agree that age estimation from structural compared to functional imaging yields a higher prediction accuracy. In a previous publication using the same dataset12, we demonstrated that weight loss was associated with an attenuation in brain atrophy, as we describe in the introduction:
“We previously found that weight loss, glycemic control and lowering of blood pressure, as well as increment in polyphenols rich food, were associated with an attenuation in brain atrophy 12.”
Here we were specifically interested in age-related functional alterations that are associated with successful weight reduction. Compared to structural brain changes aging effect on functional connectivity is more complex and multifaced. Hence, we decided to utilize a data-driven or prediction-driven approach for assessing age-related changes in functional connectivity by predicting participants’ functional brain age. We now describe this rationale in the introduction section:
“Studies have linked obesity with decreased connectivity within the default mode network15,16 and increased connectivity with the lateral orbitofrontal cortex17, which are also seen in normal aging18,19. Longitudinal trials have reported changes in these connectivity patterns following weight reduction20,21, indicating that they can be altered. However, findings regarding functional changes are less consistent than those related to anatomical changes due to the multiple measures22 and scales23 used to quantify RSFC. Hence, focusing on a single measure, the functional brain age, may better capture these complex changes and their relation to aging.”
We address the point regarding the low model performance in response to reviewer #2, comment #2.
-

eLife assessment
This potentially important study examines brain age based on resting state functional connectivity (RSFC) following an 18-month lifestyle intervention. The design of the intervention study is generally solid; the randomized controlled trial includes three intervention groups and assessments at two-time points of numerous health markers, however, the methodology for brain age prediction appears somewhat incomplete and would benefit from more rigorous approaches. The lack of control groups also prevents firm conclusions about the extent to which the observed RSFC changes are linked to the intervention. With these parts strengthened, the paper would be of broad interest to neuroscientists and biologists working on obesity, lifestyle interventions, and brain health.
-

Reviewer #1 (Public Review):
It is now widely accepted that the age of the brain can differ from the person's chronological age and neuroimaging methods are ideally suited to analyze the brain age and associated biomarkers. Preclinical studies of rodent models with appropriate neuroimaging do attest that lifestyle-related prevention approaches may help to slow down brain aging and the potential of BrainAGE as a predictor of age-related health outcomes. However, there is a paucity of data on this in humans. It is in this context the present manuscript receives its due attention.
Comments:
Lifestyle intervention benefits need to be analyzed using robust biomarkers which should be profiled non-invasively in a clinical setting. There is increasing evidence of the role of telomere length in brain aging. Gampawar et al (2020) have proposed a …
Reviewer #1 (Public Review):
It is now widely accepted that the age of the brain can differ from the person's chronological age and neuroimaging methods are ideally suited to analyze the brain age and associated biomarkers. Preclinical studies of rodent models with appropriate neuroimaging do attest that lifestyle-related prevention approaches may help to slow down brain aging and the potential of BrainAGE as a predictor of age-related health outcomes. However, there is a paucity of data on this in humans. It is in this context the present manuscript receives its due attention.
Comments:
Lifestyle intervention benefits need to be analyzed using robust biomarkers which should be profiled non-invasively in a clinical setting. There is increasing evidence of the role of telomere length in brain aging. Gampawar et al (2020) have proposed a hypothesis on the effect of telomeres on brain structure and function over the life span and named it as the "Telomere Brain Axis". In this context, if the authors could measure telomere length before and after lifestyle intervention, this will give a strong biomarker utility and value addition for the lifestyle modification benefits.
Authors should also consider measuring BDNF levels before and after lifestyle intervention.
-

Reviewer #2 (Public Review):
In this study, Levakov et al. investigated brain age based on resting-state functional connectivity (RSFC) in a group of obese participants following an 18-month lifestyle intervention. The study benefits from various sophisticated measurements of overall health, including body MRI and blood biomarkers. Although the data is leveraged from a solid randomized control set-up, the lack of control groups in the current study means that the results cannot be attributed to the lifestyle intervention with certainty. However, the study does show a relationship between general weight loss and RSFC-based brain age estimations over the course of the intervention. While this may represent an important contribution to the literature, the RSFC-based brain age prediction shows low model performance, making it difficult to …
Reviewer #2 (Public Review):
In this study, Levakov et al. investigated brain age based on resting-state functional connectivity (RSFC) in a group of obese participants following an 18-month lifestyle intervention. The study benefits from various sophisticated measurements of overall health, including body MRI and blood biomarkers. Although the data is leveraged from a solid randomized control set-up, the lack of control groups in the current study means that the results cannot be attributed to the lifestyle intervention with certainty. However, the study does show a relationship between general weight loss and RSFC-based brain age estimations over the course of the intervention. While this may represent an important contribution to the literature, the RSFC-based brain age prediction shows low model performance, making it difficult to interpret the validity of the derived estimates and the scale of change. The study would benefit from more rigorous analyses and a more critical discussion of findings. If incorporated, the study contributes to the growing field of literature indicating that weight-reduction in obese subjects may attenuate the detrimental effect of obesity on the brain.
The following points may be addressed to improve the study:
Brain age / model performance:
1. Figure 2: In the test set, the correlation between true and predicted age is 0.244. The fitted slope looks like it would be approximately 0.11 (55-50)/(80-35); change in y divided by change in x. This means that for a chronological age change of 12 months, the brain age changes by 0.11*12 = 1.3 months. I.e., due to the relatively poor model performance, an 80-year-old participant in the plot (fig 2) has a predicted age of ~55. Hence, although the age prediction step can generate a summary score for all the RSFC data, it can be difficult to interpret the meaning of these brain age estimates and the 'expected change' since the scale is in years.
2. In Figure 2 it could also help to add the x = y line to get a better overview of the prediction variance. The estimates are likely clustered around the mean/median age of the training dataset, and age is overestimated in younger subs and overestimated in older subs (usually referred to as "age bias"). It is important to inspect the data points here to understand what the estimates represent, i.e., is variation in RSFC potentially lost by wrapping the data in this summary measure, since the age prediction is not particularly accurate, and should age bias in the predictions be accounted for by adjusting the test data for the bias observed in the training data?
3. In Figure 3, some of the changes observed between time points are very large. For example, one subject with a chronological age of 62 shows a ten-year increase in brain age over 18 months. This change is twice as large as the full range of age variation in the brain age estimates (average brain age increases from 50 to 55 across the full chronological age span). This makes it difficult to interpret RSFC change in units of brain age. E.g., is it reasonable that a person's brain ages by ten years, either up or down, in 18 months? The colour scale goes from -12 years to 14 years, so some of the observed changes are 14 / 1.5 = 9 times larger than the actual time from baseline to follow-up.
- The questions above should be investigated and addressed in the context of potential challenges with using brain age as a marker (see e.g., https://onlinelibrary.wiley.com/doi/full/10.1002/hbm.25837, https://onlinelibrary.wiley.com/doi/full/10.1002/hbm.26144).
RSFC for age prediction:
1. Several studies show better age prediction accuracy with structural MRI features compared to RSFC. If the focus of the study is to use an accurate estimate of brain ageing rather than specifically looking at changes in RSFC, adding structural MRI data could be helpful.
2. If changes in RSFC is the main focus, using brain age adds a complicated layer that is not necessarily helpful. It could be easier to simply assess RSFC change from baseline to follow up, and correlate potential changes with changes in e.g., BMI.
The lack of control groups
1. If no control group data is available, it is important to clarify this in the manuscript, and evaluate which conclusions can and cannot be drawn based on the data and study design.
-

Reviewer #3 (Public Review):
The authors report on an interesting study that addresses the effects of a physical and dietary intervention on accelerated/decelerated brain ageing in obese individuals. More specifically, the authors examined potential associations between reductions in Body-Mass-Index (BMI) and a decrease in relative brain-predicted age after an 18-months period in N = 102 individuals. Brain age models were based on resting-state functional connectivity data. In addition to change in BMI, the authors also tested for associations between change in relative brain age and change in waist circumference, six liver markers, three glycemic markers, four lipid markers, and four MRI fat deposition measures. Moreover, change in self-reported consumption of food, stratified by categories such as 'processed food' and 'sweets and …
Reviewer #3 (Public Review):
The authors report on an interesting study that addresses the effects of a physical and dietary intervention on accelerated/decelerated brain ageing in obese individuals. More specifically, the authors examined potential associations between reductions in Body-Mass-Index (BMI) and a decrease in relative brain-predicted age after an 18-months period in N = 102 individuals. Brain age models were based on resting-state functional connectivity data. In addition to change in BMI, the authors also tested for associations between change in relative brain age and change in waist circumference, six liver markers, three glycemic markers, four lipid markers, and four MRI fat deposition measures. Moreover, change in self-reported consumption of food, stratified by categories such as 'processed food' and 'sweets and beverages', was tested for an association with change in relative brain age. Their analysis revealed no evidence for a general reduction in relative brain age in the tested sample. However, changes in BMI, as well as changes in several liver, glycemic, lipid, and fat-deposition markers showed significant covariation with changes in relative brain age. Three markers remained significant after additionally controlling for BMI, indicating an incremental contribution of these markers to change in relative brain age. Further associations were found for variables of subjective food consumption. The authors conclude that lifestyle interventions may have beneficial effects on brain aging.
Overall, the writing is concise and straightforward, and the langue and style are appropriate. A strength of the study is the longitudinal design that allows for addressing individual accelerations or decelerations in brain aging. Research on biological aging parameters has often been limited to cross-sectional analyses so inferences about intra-individual variation have frequently been drawn from inter-individual variation. The presented study allows, in fact, investigating within-person differences. Moreover, I very much appreciate that the authors seek to publish their code and materials online, although the respective GitHub project page did not appear to be set to 'public' at the time (error 404). Another strength of the study is that brain age models have been trained and validated in external samples. One further strength of this study is that it is based on a registered trial, which allows for the evaluation of the aims and motivation of the investigators and provides further insights into the primary and secondary outcomes measures (see the clinical trial identification code).
One weakness of the study is that no comparison between the active control group and the two experimental groups has been carried out, which would have enabled causal inferences on the potential effects of different types of interventions on changes in relative brain age. In this regard, it should also be noted that all groups underwent a lifestyle intervention. Hence, from an experimenter's perspective, it is problematic to conclude that lifestyle interventions may modulate brain age, given the lack of a control group without lifestyle intervention. This issue is fueled by the study title, which suggests a strong focus on the effects of lifestyle intervention. Technically, however, this study rather constitutes an investigation of the effects of successful weight loss/body fat reduction on brain age among participants who have taken part in a lifestyle intervention. In keeping with this, the provided information on the main effect of time on brain age is scarce, essentially limited to a sign test comparing the proportions of participants with an increase vs. decrease in relative brain age. Interestingly, this analysis did not suggest that the proportion of participants who benefit from the intervention (regarding brain age) significantly exceeds the number of participants who do not benefit. So strictly speaking, the data rather indicates that it's not the lifestyle intervention per sé that contributes to changes in brain age, but successful weight loss/body fat reduction. In sum, I feel that the authors' claims on the effects of the intervention cannot be underscored very well given the lack of a control group without lifestyle intervention.
Another major weakness is that no rationale is provided for why the authors use functional connectivity data instead of structural scans for their age estimation models. This gets even more evident in view of the relatively low prediction accuracies achieved in both the validation and test sets. My notion of the literature is that the vast majority of studies in this field implicate brain age models that were trained on structural MRI data, and these models have achieved way higher prediction accuracies. Along with the missing rationale, I feel that the low model performances require some more elaboration in the discussion section. To be clear, low prediction accuracies may be seen as a study result and, as such, they should not be considered as a quality criterion of the study. Nevertheless, the choice of functional MRI data and the relevance of the achieved model performances for subsequent association analysis needs to be addressed more thoroughly.
-