The Slingshot phosphatase 2 is required for acrosome biogenesis during spermatogenesis in mice
Curation statements for this article:-
Curated by eLife
eLife assessment
This important study reports the physiological role of Ssh2 in spermatogenesis as a critical factor for acrosome biogenesis. Loss of SSh2 in round spermatids prevents the fusion of proacrosomal vesicles leading to fragmented acrosomes due to impaired actin bundling and dephosphorylation of COFILIN. This work would be more convincing if mutations in this gene could be identified in human infertile men. Moreover, the proposed mechanism needs cross-validation in future work.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The acrosome is a membranous organelle positioned in the anterior portion of the sperm head and is essential for male fertility. Acrosome biogenesis requires the dynamic cytoskeletal shuttling of vesicles toward nascent acrosome which is regulated by a series of accessory proteins. However, much remains unknown about the molecular basis underlying this process. Here, we generated Ssh2 knockout (KO) mice and HA-tagged Ssh2 knock-in (KI) mice to define the functions of Slingshot phosphatase 2 (SSH2) in spermatogenesis and demonstrated that as a regulator of actin remodeling, SSH2 is essential for acrosome biogenesis and male fertility. In Ssh2 KO males, spermatogenesis was arrested at the early spermatid stage with increased apoptotic index and the impaired acrosome biogenesis was characterized by defective transport/fusion of proacrosomal vesicles. Moreover, disorganized F-actin structures accompanied by excessive phosphorylation of COFILIN were observed in the testes of Ssh2 KO mice. Collectively, our data reveal a modulatory role for SSH2 in acrosome biogenesis through COFILIN-mediated actin remodeling and the indispensability of this phosphatase in male fertility in mice.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
The manuscript by Xu et. al. does a very thorough characterization and molecular dissection of the role of SSH2 in spermatogenesis. Loss of SSh2 in germ cells results in germ cell arrest In step2-3 spermatids and eventually leads to germ cell loss by apoptosis. Molecular characterization of the mutant mice shows that the loss of SSH2 prevents the fusion of proacrosomal vesicles leading to the formation of a fragmented acrosome. The fragmentation of the acrosome is due to the impaired actin bundling and dephosphorylation of COFILIN. In short, this is a comprehensive body of work.
We thank the referee for these insightful comments.
Reviewer #2 (Public Review):
The acrosome is a unique sperm-specific subcellular organelle required for the fertilization process, and it is also an organelle …
Author Response
Reviewer #1 (Public Review):
The manuscript by Xu et. al. does a very thorough characterization and molecular dissection of the role of SSH2 in spermatogenesis. Loss of SSh2 in germ cells results in germ cell arrest In step2-3 spermatids and eventually leads to germ cell loss by apoptosis. Molecular characterization of the mutant mice shows that the loss of SSH2 prevents the fusion of proacrosomal vesicles leading to the formation of a fragmented acrosome. The fragmentation of the acrosome is due to the impaired actin bundling and dephosphorylation of COFILIN. In short, this is a comprehensive body of work.
We thank the referee for these insightful comments.
Reviewer #2 (Public Review):
The acrosome is a unique sperm-specific subcellular organelle required for the fertilization process, and it is also an organelle undergoing extensive morphological and structural transformation during sperm development. The mechanism underlying the extensive acrosome morphogenesis and biogenesis remains incompletely understood. Xu et al in their manuscript entitled "The Slingshot phosphatase 2 is required for acrosome biogenesis during spermatogenesis in mice" reported that the Slingshot Phosphatase 2 is essential for acrosome biogenesis and male fertility through their characterization of spermatogenic and acrosomal defects in Ssh2 knockout mice they generated. Specifically, the authors provided molecular, genetic, and subcellular evidence supporting that Ssh2 mutation impaired the phosphorylation of an acting-binding protein, COFILIN during spermiogenesis and accordingly actin cytoskeleton remodeling, crucial for proacrosomal vesicle trafficking and acrosome biogenesis. The manuscript by Xu et. al. does a very thorough characterization and molecular dissection of the role of SSH2 in spermatogenesis. Loss of SSh2 in germ cells results in germ cell arrest In step2-3 spermatids and eventually leads to germ cell loss by apoptosis. Molecular characterization of the mutant mice shows that the loss of SSH2 prevents the fusion of proacrosomal vesicles leading to the formation of a fragmented acrosome. The fragmentation of the acrosome is due to the impaired actin bundling and dephosphorylation of COFILIN. In short, this is a comprehensive body of work.
We appreciate and thank Referee #2 for the positive feedback and insightful comments.
Strengths:
Nicely written manuscript, addresses an important mechanistic question of the roles of cytoskeleton remodeling in acrosome biogenesis and provided genetic, subcellular, and molecular evidence to build up their support for their hypothesis that Ssh2 regulates actin cytoskeleton remodeling, a process essential for proacrosomal vesicle trafficking and acrosome biogenesis, through dephosphorylation actin-binding protein during spermiogenesis.
We again thank to the Referee #2 for appreciating and encouraging us regarding our current research work.
Weaknesses:
For body weight, and testis weight of the mutants, the authors concluded that there is no significant difference between the mutant and wildtype (Fig 1E -1G), but they appear to use mice between 6-8 wk old, both the testis and body weight of males at 6-8 wks is still growing, with the number of mice analyzed being six, you could easily miss the significant difference of the testis size and or body weight with such a varied age and a small sample size.
We thank the referee for their prompting of this important discussion point, which we now cover in our revised manuscript. In our originally submitted manuscript, we only presented the data for body weight, testis weight, and T/B ratio for mice between the age of 6–8 weeks, however, we have added the additional data of mice with age more than 8 weeks in the revised manuscript in a new Figure 1E-1G with the sample size of 12 for each genotype. We have also updated the relevant content in the figure caption. The revised figure caption for Figure 1 panels E–G reads as follows: “(E-G) Body weights (26.3609 ± 0.4914 for WT; 25.1741 ± 0.5189 for Ssh2 KO), weights of the testes (0.0862 ± 0.0036 for WT; 0.0788 ± 0.0023 for Ssh2 KO), and the testis-to-body weight ratio (0.3281 ± 0.0153 for WT; 0.3154 ± 0.0135 for Ssh2 KO) of adult WT and Ssh2 KO males (n = 12). Data are presented as the mean ± SEM; p > 0.05 calculated by Student’s t-test. Bars indicate the range of the data.”
Other points:
Comments: 1) Could the uniform cytoplasmic distribution of diminutive actin filaments in the wild type and disrupted actin filament remodeling be examined at the EM level on the round spermatids?
We apologize for the confusion. Previously, we conducted a transmission electron microscopy (TEM) analysis on the testes samples to discover the distribution and ultrastructural organization of F-actin in WT and Ssh2 KO round spermatids. Unfortunately, even at high magnification (30,000x, right panel of Figure R1-Response Figure 1) by TEM of testicular section no diminutive actin filament was observed in the cytoplasm of round spermatids except for the acroplaxome-an actin-rich specialized structure anchors the acrosome-in WT spermatids as well as some thick bundle-like structures located at the acrosomal region of Ssh2 KO spermatids (Fig. R1). According to their unique characteristic of appearance, we interpreted these electron-dense bundles as the aberrantly aggregated actin filaments whose lengths are in accordance with the lengths of COFILIN-saturated F-actin fragments (Bamburg et al., 2021), suggesting the disrupted actin filament remodeling during acrosome biogenesis resulted from Ssh2 KO. However, due to the technological limitations of TEM and the complexity of intracellular environment of round spermatids, we only recognized few aggregated actin bundles with the loss of filamentous appearance in Ssh2 KO spermatids and no typical diminutive actin filament was detected which had been imaged under high-resolution cryo-TEM (Haviv et al., 2008) or live-cell total internal reflection fluorescence microscopy (Johnson et al., 2015) on the purified actin bundles and cultured cells. Given the lack of effective approaches to culture murine round spermatids in vitro, confocal microscopy of flourescence-labelled F-actin (e.g., IF staining by FITC-phalloidin) is a more accessible method for visualizing the disruption of actin remodeling than EM in murine spermatids as the actin-related findings that several other studies demonstrated (Djuzenova et al., 2015; Meenderink et al., 2019).
Comments: 2) Any other defects are seen besides acrosome in the mutant testis given the important roles of actin cytoskeleton network and high expression of Ssh2 in spermatocytes, were chromatoid bodies or mitochondria affected in any way? Any other defects in the mice overall including female fertility and other organs, given the previously reported roles in the nervous system. It could be helpful information for others interested in Ssh 2 protein and actin cytoskeleton's roles in general.
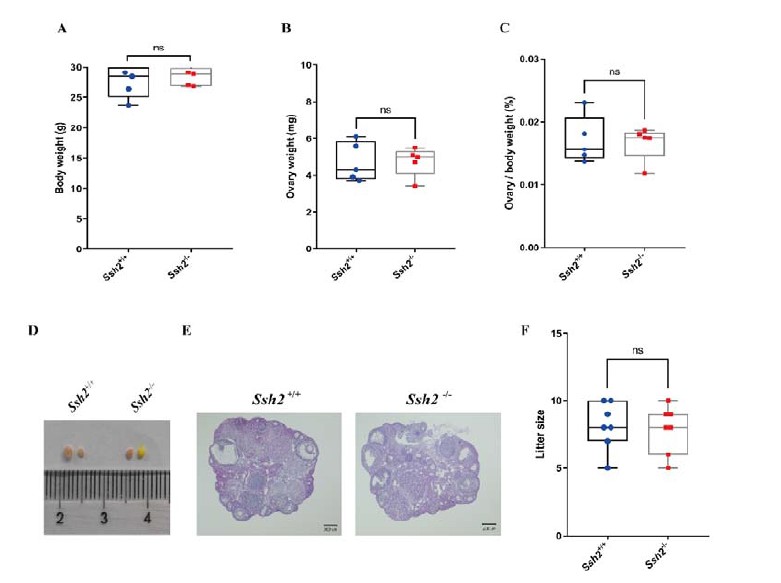
The referee has here raised an interesting point. Firstly, besides the acrosome-related defects in Ssh2 KO spermatids, we identified increased germ cell apoptosis and aberrant activation of apoptotic Bcl-2/Caspase-3 pathway in the testes of Ssh2 KO mice which were speculated to be triggered by the disordered COFILIN-mediated F-actin remodeling and have attracted our attention to further elucidate the underlying mechanisms in the future. Secondly, given the high expression of SSH2 in spermatocytes demonstrated by IF staining shown in figure 4B and 4C,we thus performed the surface chromosome spreading on spermatocytes to observe whether the morphology of chromatid bodies and the meiotic progression was affected by Ssh2 KO and no obvious defects were observed as shown in supplementary Figure S3 in originally submitted manuscript. Thirdly, no obvious morphological abnormality in chromatin or mitochondrial structure was detected in Ssh2 KO germ cells such as spermatocytes and round spermatids under TEM which prevents us to pursue it further. Fourthly, we have observed the potential effect(s) of Ssh2 KO on female fertility using Ssh2 KO female mice and did not find any obvious infertility defect in Ssh2 KO females compared to their WT littermates as demonstrated by the data of the body weight, ovary weight, ovary-to-body weight ratio, size of ovaries and fertility test as well as the images of ovarian HE staining (Fig. R1). Moreover, given that during our investigation period, Ssh2 KO males and females did not manifest any defective physical development, aberrant physiological status or mental disorder notwithstanding the roles of SSH2 in neurite extension had been reported (Endo, Ohashi, & Mizuno, 2007), we did not conduct the experiments to observe the effect(s) of SSH2 in other organs except for the female fertility.
Fig. R1 No reproductive defects were found in Ssh2 KO females. (A-C) Body weights, weights of the ovaries, and the ovary-to-body weight ratio of adult WT and Ssh2 KO females aged 8-10 weeks (n = 5); p > 0.05 calculated by Student’s t-test. Bars indicate the range of data. (D) The size of ovaries from Ssh2 KO were indistinguishable from ovaries of WT mice age 8 weeks, n = 4. (E) Histology of the ovaries from WT and Ssh2 KO mice. Sections were stained with hematoxylin and eosin. Scale bars: 200 μm. Images are representative of ovaries extracted from 8-week-old adult female mice per genotype. (F) Number of pups per litter from WT and Ssh2 KO male mice (8 weeks old) after crossing with WT adult male mice (n =3); p > 0.05 calculated by Student’s t-test. Bars indicate the range of the data.
Comments: 3) Providing detailed information on the number of animals used and cells analyzed in the legend is nice, but it might be even better for the readers to include sample size and the number of cells examined in the figure/graph if possible.
We appreciate the suggestions from the reviewer. We have integrated some information of sample size in the figures where appropriate. Firstly, we integrated sample size in the figure 1C, 1E, 1F, 1G and 1I. Secondly, we included sample size and the number of seminiferous tubule/epididymal duct we evaluated for TUNEL (+) cell counting in figure 2C and figure 2D. Thirdly, we included sample size and the number of spermatids for co-localization in figure 6B and figure 6D.
Comments: 4) Nice discussion and comparison with GOPC and GM130, how about comparison and discussion with other acrosome defective mutants like PICK1, and ATG to provide some insights into acrosome biogenesis and proacrosomal vesicle trafficking?
We greatly appreciate the referee for positive appraisal of our work with constructive suggestions, unfortunately, we are unable to address these defective mutants with certainty due to the lack of proper sample accessibility (only 3 of 16-month-old Ssh2 KO mice are accessible now). We compared the cytological staining of GM130 and GOPC in WT and Ssh2 KO spermatids using tubule squash sections as the description in the originally submitted manuscript which are prepared from fresh testes originated from 8-week-old mice and we now have several aged Ssh2 KO mice which prevent us to achieve the staining of PICK1 and ATG. PICK1 was previously reported to facilitate vesicle trafficking from the Golgi apparatus to the acrosome which co-localizes with GOPC in the proacrosomal granules (Xiao et al., 2009) and the phenotypes of Pick1 KO mice share a lot of similar characteristics with that of Ssh2 KO mice such as the fragmentation of the acrosome and increased germ cell apoptosis. Both autophagy-related ATG5 (Huang et al., 2021) and ATG7 (Wang et al., 2014) were reported to participate in the process of acrosome biogenesis and ATG7 is required for proacrosomal vesicle transportation/fusion by conjugating LC3 to the membrane of proacrosomal vesicles. Although the spermatids evaluated in these KO mice models could still be developed into spermatozoa with defective acrosome that is different from the situation in Ssh2 KO mice, it would be meaningful to discover the affects by Ssh2 KO on the localization of these regulators of acrosome biogenesis in spermatids and their potential interactions with SSH2. Indeed, in future work, we plan to pursue these issues and the content related to PICK1 has been added to the discussion in the revised manuscript as follows: “Moreover, it is intriguing to note that the phenotypes of Ssh2 KO mice share a lot of similarities with that of Pick1 KO model (Xiao et al., 2009) such as acrosome fragmentation and enhanced germ cell apoptosis, suggesting the possibility that SSH2 and PICK1 work together in a same trafficking machinery functioning in acrosome biogenesis which needs to be clarified further.”
Comments: 5) Given the literature on Cofilin's requirement for male fertility and the increased p-Cofilin in Ssh2 mutant testis by Western and IF, the authors have a strong case for their hypothesis. But given the general role of phosphatase, it might be prudent to discuss alternative possibilities.
We thank the reviewer for these valuable suggestions. Given that p-COFILIN is the only known substrate of SSH2 based on previous reports, we focused principally on this cascade to conduct our investigation. As a phosphatase, SSH2 is very likely to interact with many other proteins functioning in various cellular processes other than the actin-binding proteins which remain elusive. As directed, we now have added some content related to the regarding above concern in the discussion section of the revised manuscript as follows: “Given the diverse physiological roles reported for Slingshot family proteins, the possibility of the alternative mechanism underlying involvement of SSH2 in cellular events beyond the COFILIN-mediated actin remodeling should be noted. According to some publicly accessible databases as the indicators of potential protein–protein interactions such as BioGRID (Oughtred et al., 2019) and IntAct (Del Toro et al., 2022), SSH2 might interact with a set of actin-based molecular motors covering MYH9, MYO19 and MYO18A, which have been implicated in the maintenance of Golgi morphology and Golgi anterograde vesicular trafficking via the PI4P/GOLPH3/MYO18A/F-actin pathway (Rahajeng et al., 2019).”
-

eLife assessment
This important study reports the physiological role of Ssh2 in spermatogenesis as a critical factor for acrosome biogenesis. Loss of SSh2 in round spermatids prevents the fusion of proacrosomal vesicles leading to fragmented acrosomes due to impaired actin bundling and dephosphorylation of COFILIN. This work would be more convincing if mutations in this gene could be identified in human infertile men. Moreover, the proposed mechanism needs cross-validation in future work.
-

Reviewer #1 (Public Review):
The manuscript by Xu et. al. does a very thorough characterization and molecular dissection of the role of SSH2 in spermatogenesis. Loss of SSh2 in germ cells results in germ cell arrest In step2-3 spermatids and eventually leads to germ cell loss by apoptosis. Molecular characterization of the mutant mice shows that the loss of SSH2 prevents the fusion of proacrosomal vesicles leading to the formation of a fragmented acrosome. The fragmentation of the acrosome is due to the impaired actin bundling and dephosphorylation of COFILIN. In short, this is a comprehensive body of work.
-

Reviewer #2 (Public Review):
The acrosome is a unique sperm-specific subcellular organelle required for the fertilization process, and it is also an organelle undergoing extensive morphological and structural transformation during sperm development. The mechanism underlying the extensive acrosome morphogenesis and biogenesis remains incompletely understood. Xu et al in their manuscript entitled "The Slingshot phosphatase 2 is required for acrosome biogenesis during spermatogenesis in mice" reported that the Slingshot Phosphatase 2 is essential for acrosome biogenesis and male fertility through their characterization of spermatogenic and acrosomal defects in Ssh2 knockout mice they generated. Specifically, the authors provided molecular, genetic, and subcellular evidence supporting that Ssh2 mutation impaired the phosphorylation of an …
Reviewer #2 (Public Review):
The acrosome is a unique sperm-specific subcellular organelle required for the fertilization process, and it is also an organelle undergoing extensive morphological and structural transformation during sperm development. The mechanism underlying the extensive acrosome morphogenesis and biogenesis remains incompletely understood. Xu et al in their manuscript entitled "The Slingshot phosphatase 2 is required for acrosome biogenesis during spermatogenesis in mice" reported that the Slingshot Phosphatase 2 is essential for acrosome biogenesis and male fertility through their characterization of spermatogenic and acrosomal defects in Ssh2 knockout mice they generated. Specifically, the authors provided molecular, genetic, and subcellular evidence supporting that Ssh2 mutation impaired the phosphorylation of an acting-binding protein, COFILIN during spermiogenesis and accordingly actin cytoskeleton remodeling, crucial for proacrosomal vesicle trafficking and acrosome biogenesis.
Strengths:
Nicely written manuscript, addresses an important mechanistic question of the roles of cytoskeleton remodeling in acrosome biogenesis and provided genetic, subcellular, and molecular evidence to build up their support for their hypothesis that Ssh2 regulates actin cytoskeleton remodeling, a process essential for proacrosomal vesicle trafficking and acrosome biogenesis, through dephosphorylation actin-binding protein during spermiogenesis.Weaknesses:
For body weight, and testis weight of the mutants, the authors concluded that there is no significant difference between the mutant and wildtype (Fig 1E -1G), but they appear to use mice between 6-8 wk old, both the testis and body weight of males at 6-8 wks is still growing, with the number of mice analyzed being six, you could easily miss the significant difference of the testis size and or body weight with such a varied age and a small sample size.Could the uniform cytoplasmic distribution of diminutive actin filaments in the wild type and disrupted actin filament remodeling be examined at the EM level on the round spermatids?
Any other defects are seen besides acrosome in the mutant testis given the important roles of actin cytoskeleton network and high expression of Ssh2 in spermatocytes, were chromatoid bodies or mitochondria affected in any way? Any other defects in the mice overall including female fertility and other organs, given the previously reported roles in the nervous system. It could be helpful information for others interested in Ssh 2 protein and actin cytoskeleton's roles in general.
Providing detailed information on the number of animals used and cells analyzed in the legend is nice, but it might be even better for the readers to include sample size and the number of cells examined in the figure/graph if possible.
Nice discussion and comparison with GOPC and GM130, how about comparison and discussion with other acrosome defective mutants like PICK1, and ATG to provide some insights into acrosome biogenesis and proacrosomal vesicle trafficking?
Given the literature on Cofilin's requirement for male fertility and the increased p-Cofilin in Ssh2 mutant testis by Western and IF, the authors have a strong case for their hypothesis. But given the general role of phosphatase, it might be prudent to discuss alternative possibilities. -