Three-dimensional imaging of vascular development in the mouse epididymis
Curation statements for this article:-
Curated by eLife
eLife assessment
There are many strengths in this paper that examines patterns of epididymal blood and lymphatic vasculature, supported by quantitative methods, and well-conducted 3D imaging studies (graphics and videos). Minor weaknesses include the lack of higher magnification images and the organization of image panels in some figures. Overall, this is a very important contribution to the epididymis research field.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Long considered an accessory tubule of the male reproductive system, the epididymis is proving to be a key determinant of male fertility. In addition to its secretory role in ensuring functional maturation and survival of spermatozoa, the epididymis has a complex immune function. Indeed, it must manage both peripheral tolerance to sperm antigens foreign to the immune system and the protection of spermatozoa as well as the organ itself against pathogens ascending the epididymal tubule. Although our knowledge of the immunobiology of this organ is beginning to accumulate at the molecular and cellular levels, the organization of blood and lymphatic networks of this tissue, important players in the immune response, remains largely unknown. In the present report, we have taken advantage of a VEGFR3:YFP transgenic mouse model. Using high-resolution three-dimensional (3D) imaging and organ clearing coupled with multiplex immunodetections of lymphatic (LYVE1, PDPN, PROX1) and/or blood (PLVAP/Meca32) markers, we provide a simultaneous deep 3D view of the lymphatic and blood epididymal vasculature in the mature adult mouse as well as during postnatal development.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
- Although the images and videos were of great quality, the results derived from them provided little new knowledge and few conceptual insights into male reproductive tract biology and basically confirmed what has been published using traditional methods. For example, the high intensity of the vascular network in the initial segment was previously reported by Abe in 1984 and Suzuki in 1982; the pattern of the major lymphatic vessel and drainage was beautifully depicted by Perez-Clavier, 1982.
We thank the reviewer for his/her appreciative comments regarding the quality of the images/videos we provide in this study. We do not fully agree with his/her assessment of the lack of novelty. Our work confirms earlier reports that are now dated (1980s), which in itself is worth mentioning for the …
Author Response
Reviewer #2 (Public Review):
- Although the images and videos were of great quality, the results derived from them provided little new knowledge and few conceptual insights into male reproductive tract biology and basically confirmed what has been published using traditional methods. For example, the high intensity of the vascular network in the initial segment was previously reported by Abe in 1984 and Suzuki in 1982; the pattern of the major lymphatic vessel and drainage was beautifully depicted by Perez-Clavier, 1982.
We thank the reviewer for his/her appreciative comments regarding the quality of the images/videos we provide in this study. We do not fully agree with his/her assessment of the lack of novelty. Our work confirms earlier reports that are now dated (1980s), which in itself is worth mentioning for the interested community, especially when the confirmation uses the most advanced technologies available today. We have never said that nothing was done in the past, and we have acknowledged all past contributors (including those mentioned by the reviewer) by pointing out the limitations of the technical tools that were available at the time. In addition, our current work provides a more comprehensive and global view by extending our approach to the entire mouse epididymis, whereas previous work was much more limited.
- The authors were very cautious when interpreting the results of marker immunostaining however these markers were not specific for a definite cell type. For example, as the authors stated, VEGFR3 marks both lymphatic vessels and fenestrated blood vessels. how could the authors claim the VEGFR3+ network was lymphatic? The authors claimed that they used three markers for the lymphatic vessel. But staining results of the networks were very different. How could the author make conclusions about the network of lymphatic vessels in the epididymis?
We broadly agree with the reviewer and have made it clear that one cannot be 100% sure that all the VEGFR3+ structures we present are lymphatic. However, in total, we used 4 documented lymphatic markers (not 3 as mentioned by the reviewer) which are (VEGFR3, LYVE1, PROX1 and PDPN). Three of them give very similar profiles, while only PDPN shows some differences. We are currently studying in more detail the expression of PDPN in the mouse epididymis because we speculate that this marker may target a population of pluripotent cells in this tissue. Therefore, with the 3 similar profiles and with the subtraction of PVLAP+ structures, we are pretty confident that what we show corresponds to the different lymphatic structures.
- To understand the vascular network development in the epididymis, would the authors please look at the fetal stage when the vascular network is established in the first place? Wolffian duct tissues are much smaller and thinner and would be amenable for 3D imaging probably even without clearing.
We generally agree with the reviewer that this could be an interesting addition. However, it represents a significant amount of additional work. Organ clearing will certainly be required because it is unlikely that Wolffian duct will be sufficiently transparent to allow lightsheet microscopy. In the literature, the study of Wolffian duct relies primarily on whole mounts, inclusions, and cryosections. Besides the fact that this represents a lot of extra work, we are not totally convinced that this would be of much use. A key reason is that the epididymis is an organ that differentiates completely after birth (Robaire and Hinton, 2015). It is reported that differentiation of mouse caput segment 1 occurs around 19DPN (Xu et al., 2016) and is intimately related to the development of the vasculature (Lebarr et al., 1986). Regarding the lymphatic network, Swingen et al, (2012) reports that lymphangiogenesis in the mouse testis and epididymis is initiated late in gestation after 15DPC. Videos showing the external lymphatic vessels of the testis and epididymis at 17.5DPC can be seen at https://doi.org/10.1371/journal.pone.0052620.s002. The authors indicate that lymphangiogenesis occurs via sprouting from the adjacent mesonephros. We hypothesize that the more internal lymphatics evolve between birth and 10DPN, which corresponds to the time when we observed LEPC Lyve1pos cells.
- Immunofluorescence staining of VEGF factors was not convincing. As a secreted factor, VEGF will be secreted out of the cells, would it be detected more in the interstitium? I am always skeptical about the results of immunostaining secreted growth factors. Would it be possible to perform in situ or RNAscope to confirm the spatial expression pattern of VEGFs?
Well, active VEGF factors result from alternative mRNA splicing events and posttranslational proteolytic cleavage. Therefore, in our opinion, the study of VEGF mRNA by in situ hybridization or RNAscope analysis will not be very informative about the actual presence of active forms of VEGF in the epididymis. If necessary, we can provide as supplementary material immunohistochemistry data showing the presence of VEFG-A in the epididymal principal cells. Our major objective with these data was to show that VEGF factors and their respective receptors were present in the epididymis. Nevertheless, in an attempt to convince the reviewer, we provide as accompanying data to this rebuttal letter new sets of figures (Figures VEGF-A-response editor & VEGFC /VEGF-D-response editor) that we believe can improve the perception of our data. If the editorial office feels it is necessary, these figures could be added to the supplementary figure set (as Figure 6figure supplement 1 and Figure 6-figure supplement 2). For VEGF-A the data exists already in the literature as we have indicated (Korpelainen, 1998). In fine, our goal was not to show which cell types of the epididymis epithelium produce VEGFs but rather than VEGF factors and their receptors where there in order to support angiogenesis or lymphangiogenic activity in the tissue. In addition, we hypothesize that because septa have been reported to constitute barriers between segments restricting passive diffusion of molecules (Turner et al., 2003; Stammler et al., 2015), the VEGF factors are expected to be produced locally.
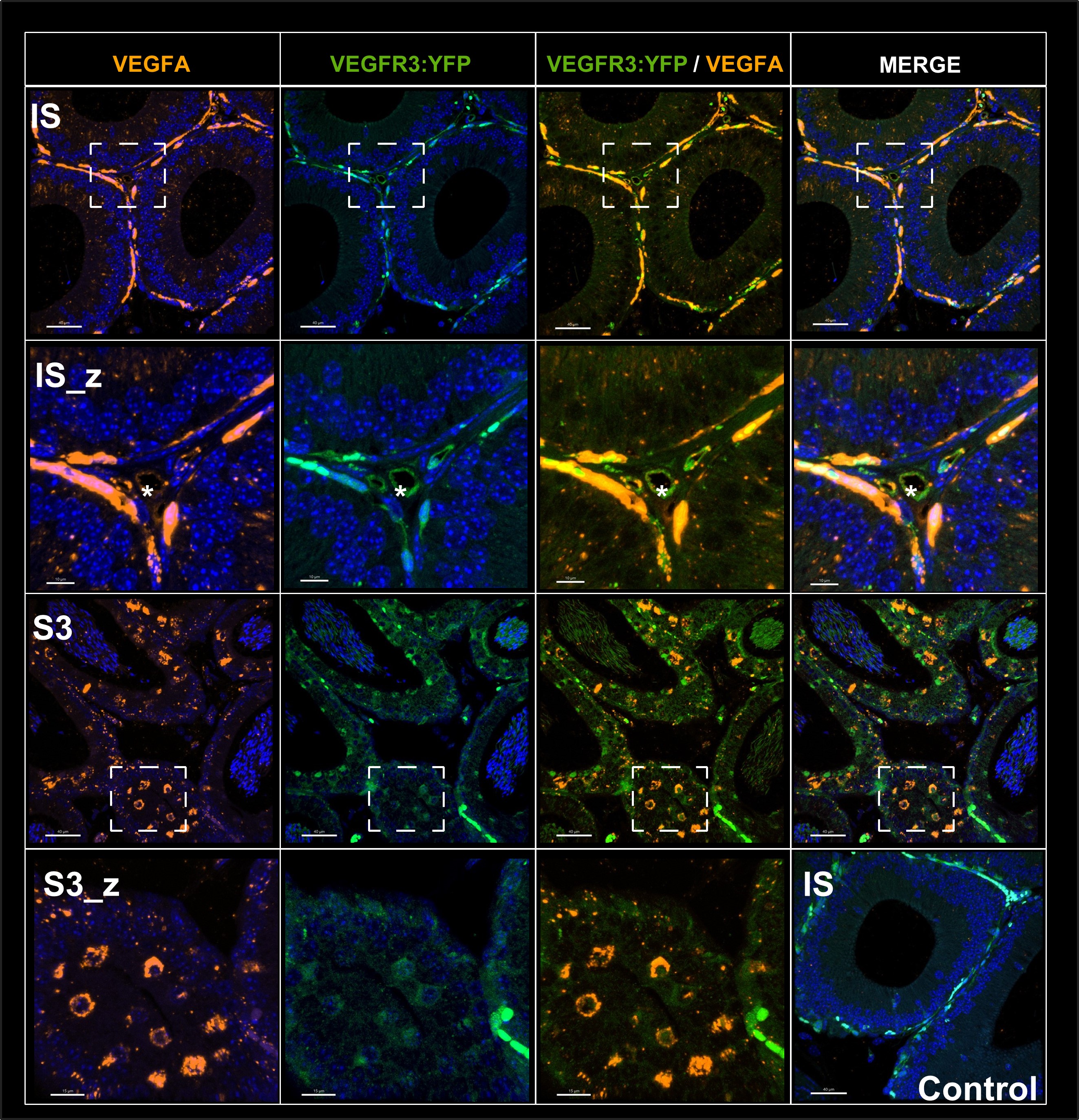
Figure VEGF-A - response editor : Immunofluorescence of the angiogenic ligand VEGF-A in the epididymis. Figure 6 shows that this ligand is mainly found in the caput and more precisely in S1.It is very strongly expressed in the peritubular microvascularization of the SI which expresses the VEGFR3:YFP transgene whereas it is less expressed by intertubular blood vessels (asterisk). This seems to indicate that it is the peritubular vessels that are in the majority responsible for the angiogenic activity measured in our study. Furthermore, it is expressed by the epithelium as secretory vesicles (IS, and S3 and enlargement) which is in agreement with in situ hybridization work performed by Korpelainene E.I et al J.Cell.biol 1998). The enlargement shown in S3_Z shows the sagital plane of the tubule where one can distinguish VEGFR:YFP positive cells that strongly express are also VEGF-A positive indicating that the same cells of the epithelium express both the receptor and the ligand. Here the transgene is detected directly without the use of an anti-GFP which allows to enhance the signal.
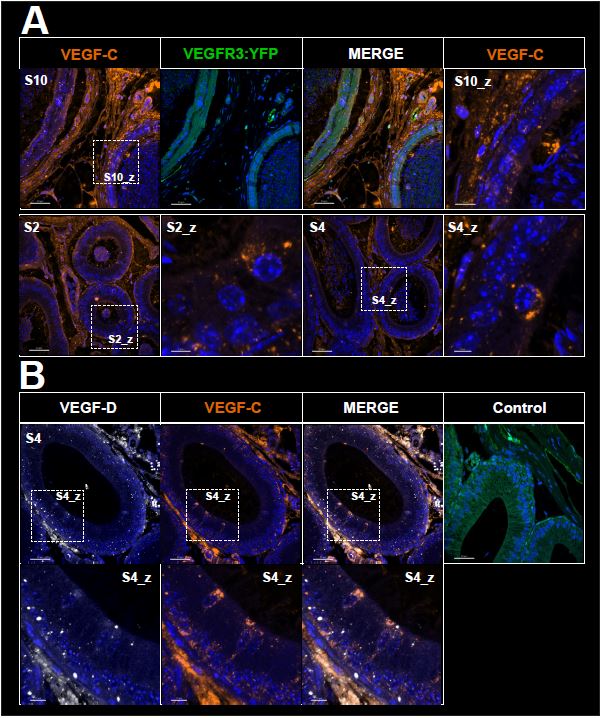
Figure VEGF-C / VEGF-D - response editor : Immunofluorescence of VEGF-C and VEGF-D lymphangiogenic ligands in the epididymis. This figure shows that these ligands are mainly found in the interstitial tissue throughout the organ with a higher proportion in the caudal part. This expression may be largely driven by fibroblasts, which are widely represented in the interstitium, or by endothelial cells, since these two ligands are expressed by these cell types. However, as shown in the figures and in the enlargement of panel A, VEGF-C is also produced by epithelial cells within what may appear as secretory vesicles. In contrast, for VEGF-D, we observe only few weakly positive epithelial cells (panel B). These ligands are also detected in the lumen of epididymal tubules (visible for VEGF-C Panel A S2). This presence may be explained by lumicrine transfer from the testis, in addition to secretion from epithelial cells. Here the transgene is detected directly without the use of an anti-GFP which allows to enhance the signal.
- The study is descriptive and does not provide functional and mechanistic insights. Maybe, the combination of 3D imaging with lineage tracing of endothelium cells or ligation study (removal/ligation of the certain vessel) would help better understand how the vascular network is established and their functional significance.
The technical approaches suggested by the reviewer could certainly improve our understanding of the rather complex epididymal vascular network. Taken together, they represent the body of a comprehensive follow-up study that is worth undertaking.
- Immune response is among many physiological processes in which vascular networks play significant roles. Discussion would be needed in other physiological processes, such as tissue metabolism and stem/progenitor cell niche microenvironment.
We agree with the reviewer that the mammalian vasculature is involved in other physiological processes beyond immune/inflammatory responses. We have deliberately chosen to focus our discussion on the inflammatory and immune context of the epididymis, as we believe this is the most relevant aspect. It is also in full agreement with the research that our team has been conducting for 15 years to try to understand the complex orchestration of tolerance versus immune surveillance in this territory. This is a finely tuned process that, if properly understood, can help to understand and appropriately treat clinical situations of infertility and/or urological problems. As our discussion section is already quite long, we feel that it was not justified to extend it further on other aspects. However, in response to the reviewer's suggestion, we now mention at the end of the first paragraph of the discussion that the epididymal vascular network is likely to serve different processes in this tissue (page 9, lines 299 to 303).
- How could the author determine the Cd-A labeled vessel in Fig 1 was an artery, not a vein? This leads to another critical question. Would it be possible to stain with artery and vein markers to help illustrate the blood flow directions of the vessel?
The reviewer is right on the fact that we arbitrarily called the Cd-A vessel in Figure 1 an artery. Cd-A is not an acronym we use anymore. What we have done is to use the acronym SEA (superior epididymal artery) to indicate what we firmly believe to be an artery, as also suggested by previous literature (e.g., Suzuki, 1982; Abe et al, 1982) in which this same structure has been consistently referred to as an artery. For other blood vessels, we now have used the acronym "Cd-BV" because we do not know whether we are dealing with a vein or an artery as rightfully pointed out by the reviewer. This is clearly stated in the legend of Figure 1.
-

eLife assessment
There are many strengths in this paper that examines patterns of epididymal blood and lymphatic vasculature, supported by quantitative methods, and well-conducted 3D imaging studies (graphics and videos). Minor weaknesses include the lack of higher magnification images and the organization of image panels in some figures. Overall, this is a very important contribution to the epididymis research field.
-

Reviewer #1 (Public Review):
Damon-Soubeyrand and colleagues use 3DISCO tissue clearing and light-sheet microscopy to provide a detailed atlas of the blood and lymphatic circulating networks of the mouse epididymis. While this manuscript does not address the function of these networks during the development or homeostasis of the epididymis, it is an outstanding example of a descriptive study that paves the way towards functional investigations of the role of epididymal vasculature in the post-testicular maturation of spermatozoa.
Strengths: The authors used a wide range of markers to carefully assess the differential patterns of epididymal blood and lymphatic vasculature, and elegantly describe each image in great detail. Where possible, the authors used appropriate quantitative methods to support their descriptive data, which are …
Reviewer #1 (Public Review):
Damon-Soubeyrand and colleagues use 3DISCO tissue clearing and light-sheet microscopy to provide a detailed atlas of the blood and lymphatic circulating networks of the mouse epididymis. While this manuscript does not address the function of these networks during the development or homeostasis of the epididymis, it is an outstanding example of a descriptive study that paves the way towards functional investigations of the role of epididymal vasculature in the post-testicular maturation of spermatozoa.
Strengths: The authors used a wide range of markers to carefully assess the differential patterns of epididymal blood and lymphatic vasculature, and elegantly describe each image in great detail. Where possible, the authors used appropriate quantitative methods to support their descriptive data, which are useful metrics for readers seeking to characterize vascular and lymphatic networks in disease models.
Weaknesses: In its current form, it is unclear which of the elements presented in the manuscript are novel discoveries about the blood and lymphatic networks of the epididymis, as the text lacks concise and precise statements about the major findings of the study. In addition, the authors frame this study of the vasculature as a way to understand the immune context of spermatozoa in the epididymis but do not integrate their data on blood and lymphatic networks with the immune system.
-

Reviewer #2 (Public Review):
This manuscript illustrates a vascular network in the postnatal developing and adult epididymis using high-resolution three-dimensional (3D) imaging and organ clearing coupled with multiplex immunodetections of lymphatic and blood markers.
Strengths:
The cutting-edge imaging technique to visualize the three-dimensional vascular network.
The images and videos were of great quality.
The authors were very cautious and careful when interpreting the results of marker immunostaining.Weaknesses:
1. Although the images and videos were of great quality, the results derived from them provided little new knowledge and few conceptual insights into male reproductive tract biology and basically confirmed what has been published using traditional methods. For example, the high intensity of the vascular network in the …Reviewer #2 (Public Review):
This manuscript illustrates a vascular network in the postnatal developing and adult epididymis using high-resolution three-dimensional (3D) imaging and organ clearing coupled with multiplex immunodetections of lymphatic and blood markers.
Strengths:
The cutting-edge imaging technique to visualize the three-dimensional vascular network.
The images and videos were of great quality.
The authors were very cautious and careful when interpreting the results of marker immunostaining.Weaknesses:
1. Although the images and videos were of great quality, the results derived from them provided little new knowledge and few conceptual insights into male reproductive tract biology and basically confirmed what has been published using traditional methods. For example, the high intensity of the vascular network in the initial segment was previously reported by Abe in 1984 and Suzuki in 1982; the pattern of the major lymphatic vessel and drainage was beautifully depicted by Perez-Clavier, 1982.2. The authors were very cautious when interpreting the results of marker immunostaining however these markers were not specific for a definite cell type. For example, as the authors stated, VEGFR3 marks both lymphatic vessels and fenestrated blood vessels. how could the authors claim the VEGFR3+ network was lymphatic? The authors claimed that they used three markers for the lymphatic vessel. But staining results of the networks were very different. How could the author make conclusions about the network of lymphatic vessels in the epididymis?
3. To understand the vascular network development in the epididymis, would the authors please look at the fetal stage when the vascular network is established in the first place? Wolffian duct tissues are much smaller and thinner and would be amenable for 3D imaging probably even without clearing.
4. Immunofluorescence staining of VEGF factors was not convincing. As a secreted factor, VEGF will be secreted out of the cells, would it be detected more in the interstitium? I am always skeptical about the results of immunostaining secreted growth factors. Would it be possible to perform in situ or RNAscope to confirm the spatial expression pattern of VEGFs?
5. The study is descriptive and does not provide functional and mechanistic insights. Maybe, the combination of 3D imaging with lineage tracing of endothelium cells or ligation study (removal/ligation of the certain vessel) would help better understand how the vascular network is established and their functional significance.
6. Immune response is among many physiological processes in which vascular networks play significant roles. Discussion would be needed in other physiological processes, such as tissue metabolism and stem/progenitor cell niche microenvironment.
7. How could the author determine the Cd-A labeled vessel in Fig 1 was an artery, not a vein? This leads to another critical question. Would it be possible to stain with artery and vein markers to help illustrate the blood flow directions of the vessel?
-