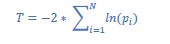Distinct roles of forward and backward alpha-band waves in spatial visual attention
Curation statements for this article:-
Curated by eLife
eLife assessment
Alamia and colleagues investigate the direction of traveling waves in the alpha frequency band during visual spatial attention. The authors' novel perspective adopted here is valuable to understanding the functional relevance of alpha oscillations for spatial attention. The observed pattern of results is consistent with distinct roles for travelling alpha waves in spatially opposite directions and makes a solid case for considering this new perspective on alpha rhythms in human cognitive function.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Previous research has associated alpha-band [8–12 Hz] oscillations with inhibitory functions: for instance, several studies showed that visual attention increases alpha-band power in the hemisphere ipsilateral to the attended location. However, other studies demonstrated that alpha oscillations positively correlate with visual perception, hinting at different processes underlying their dynamics. Here, using an approach based on traveling waves, we demonstrate that there are two functionally distinct alpha-band oscillations propagating in different directions. We analyzed EEG recordings from three datasets of human participants performing a covert visual attention task (one new dataset with N = 16, two previously published datasets with N = 16 and N = 31). Participants were instructed to detect a brief target by covertly attending to the screen’s left or right side. Our analysis reveals two distinct processes: allocating attention to one hemifield increases top-down alpha-band waves propagating from frontal to occipital regions ipsilateral to the attended location, both with and without visual stimulation. These top-down oscillatory waves correlate positively with alpha-band power in frontal and occipital regions. Yet, different alpha-band waves propagate from occipital to frontal regions and contralateral to the attended location. Crucially, these forward waves were present only during visual stimulation, suggesting a separate mechanism related to visual processing. Together, these results reveal two distinct processes reflected by different propagation directions, demonstrating the importance of considering oscillations as traveling waves when characterizing their functional role.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
The authors push a fresh perspective with a sufficiently sophisticated and novel methodology. I have some remaining reservations that concern the actual make-up of the data basis and consistency of results between the two (N=16) samples, the statistical analysis, as well as the “travelling” part.
I previously commented on the fact that findings from both datasets were difficult to discern and more effort should be made to highlight these. Also, a major conclusion “the directionality effect [effect of attention on forward waves] only occurs for visual stimulation” only rested on a qualitative comparison between studies. The authors have improved on this here, e.g., by toning down this conclusion. One thing that is still missing is a graphical representation of the data from Foster et al. …
Author Response
Reviewer #1 (Public Review):
The authors push a fresh perspective with a sufficiently sophisticated and novel methodology. I have some remaining reservations that concern the actual make-up of the data basis and consistency of results between the two (N=16) samples, the statistical analysis, as well as the “travelling” part.
I previously commented on the fact that findings from both datasets were difficult to discern and more effort should be made to highlight these. Also, a major conclusion “the directionality effect [effect of attention on forward waves] only occurs for visual stimulation” only rested on a qualitative comparison between studies. The authors have improved on this here, e.g., by toning down this conclusion. One thing that is still missing is a graphical representation of the data from Foster et al. (the second dataset analysed here) that would support the statistical results and allow the reader a visual comparison between the sets of findings.
We are glad that the reviewer recognizes the improvement in the presentation of the conclusions. According to the suggestions, we have modified figure 2, not only by including a third dataset (see point below), but also in a way that allows a direct comparison between the three datasets. Specifically, the results from the three datasets are now shown in three columns next to each other. The first row shows the FW and BW waves in contra and ipsilateral lines of electrodes for each dataset: our dataset and the one from Feldmann-Wustefeld and colleagues (the first and the second column in the figure, both with visual stimulation) shows a clear interaction between direction and laterality, as confirmed by the statistical analysis. The dataset from Foster and colleagues (the third column, no visual stimulation) shows a laterality effect only in the backward waves but not in the forward ones, in line with the hypothesis that FW waves are modulated only in the presence of visual stimulation. The second row shows a schematic representation of the task, and the third row illustrate the electrodes’ lines used in each dataset. We hope the reviewer will be satisfied with the current data presentation.
Also, for any naive reader, the concept of travelling waves may be hard to grasp in the way data are currently presented - only based on the results of the 2D-FFT. Can forward and backward-travelling waves be illustrated in a representative example to make this more intuitive?
We thank the reviewer for the suggestion. We included in figure 1 an additional panel E that represents a schematic example of forward and backward waves in the temporal domain (i.e., in the EEG data). We hope this example will provide a better understanding of the data and the traveling wave concept.
Finally, the way Bayes Factors from the Bayesian ANOVA are presented, especially with those close to the ‘meaningful boundaries’ ⅓ and 3, as defined in the ‘Statistical analysis’ section, requires some unification/revision. For example, here: “We found a positive correlation between contra- and ipsi- lateral backward waves, and occipital (all Pearson’s r~=0.4, all BFs 10 ~=3) and -to a smaller extent- frontal areas (all Pearson’s r~=0.3, all BFs 10 ~=2).”, where the second part should strictly be labelled as inconclusive evidence. In the same vein, there is occasional mention of “negative effects”, where it should say that evidence favours the absence of an effect.
We agree with the reviewer and apologize for the inaccuracies in reporting the statistical analysis. We corrected as suggested (see below), replacing ‘negative effects’ with ‘evidence favors the absence of an effect’.
From the updated manuscript :
"We found moderate evidence of a positive correlation between contra- and ipsi- lateral backward waves, and occipital (all Pearson’s r~=0.4, all BFs10~=3) but inconclusive evidence in the frontal areas (all Pearson’s r~=0.3, all BFs10~=2)."
From the revised ‘Results’ section, now it reads:
[…] whereas all other factors and their interactions revealed evidence in favor of the absence of an effect (BFs10<0.3).
[…] but not in the forward waves (BF10=0.231, error<0.01%, supporting evidence in favor of the absence of an effect).
Reviewer #2 (Public Review):
The present manuscript takes a new perspective and investigates the functional relevance of traveling alpha waves’ direction for visual spatial attention. While the modulation of alpha oscillatory power - and especially the lateralization of alpha power - has been associated with spatial attention in the literature, the present investigation offers a new perspective that helps understand and differentiate the functional roles of alpha oscillations in the ipsi- versus contralateral hemisphere for spatial attention.
The present study uses a straightforward approach and provides an analysis of two EEG datasets, which are convergingly in line with the authors’ claim that two patterns of travelling alpha waves need to be differentiated in visual spatial attention. First, backward waves in the ipsilateral hemisphere, and second, forward waves in the contralateral hemisphere, which are only observed during visual stimulation. Importantly, the authors test the relation of these patterns of traveling waves to the overall power of alpha oscillations and to the hemispheric lateralization of alpha power. Furthermore, to test the functional significance, the authors demonstrate that the pattern of forward and backward waves around stimulus onset differentiates between hits and misses in task performance.
Although the results are in line with the conclusions drawn, some questions remain. The authors investigate the relationship between traveling alpha waves and the hemispheric lateralization of alpha power, which is a well-established neural signature of spatial attention. Surprisingly, the lateralization of alpha power shown in Figure 3B appears relatively weak in the present dataset (by visual inspection), which raises the question of whether the investigation of a relation between lateralized alpha power and alpha traveling waves is warranted in the first place.
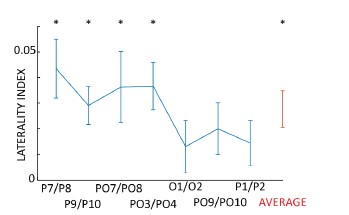
We agree with the reviewer that the effect seems reduced compared to other studies, despite the topography of alpha-band lateralization in our data is in line with the literature. In order to quantify the effect, we performed an analysis similar to (Thut et al., 2006), defining a laterality index as:
We computed such index for occipital electrodes and their average (in red in figure R1). The results reveal that for most electrodes, including their average, the laterality index is significantly larger than 0, confirming the presence of alpha-band lateralization. However, we also note that the amplitude of the effect (~0.04) is reduced compared to the study by Thut and colleagues, which was between 0.05 and 0.10.
Figure R1 – Laterality index for occipital electrodes, quantifying alpha-band lateralization during attention allocation. All electrodes go in the expected direction, revealing an increase of alpha-band power in the ipsilateral occipital hemisphere.
Furthermore, the authors employ between-subject correlations (with N = 16) to test the relationship between alpha traveling waves and (lateralized) alpha power. However, as inter- individual differences in patterns of travelling waves are not the main focus here, within- subject analyses of the same relations would be able to test the authors’ hypotheses much more directly.
As suggested, we included the recommended within-subject analysis in the revised manuscript by computing a trial-by-trial correlation between alpha power and traveling waves for each participant. First, we obtained a correlation coefficient and a p-value for each subject. Then, we tested whether the correlation coefficients had an overall positive or negative distribution (i.e., according to our previous results, we expected a positive correlation between backward waves and alpha power). Additionally, we combined the p-values to test for overall significance (using the Fisher method, see Methods section below). Our results corroborate the between-subject correlation, supporting the conclusion that alpha-band power correlates mostly with backward waves (especially contro-lateral to the attended location). The other correlations (i.e., forward waves and alpha power) were statistically inconclusive. We included in the revised manuscript these new results, as shown in the following.
From the Results section:
“To further investigate the relation between alpha-band travelling waves and alpha power, we performed the same analysis focusing on the correlation within each participant. In particular, we correlated trial-by-trial forward and backward waves with alpha-band power for each subject, obtaining correlation coefficients ‘r’ and their respective p-values. As in the previous analysis, we correlated forward and backward waves with frontal and occipital electrodes in both contro- and ipsilateral hemispheres. We applied the Fisher method (Fisher, 1992, see Methods for details) to combine all subjects' p-values in every conditions. Overall, we found a significant effect of all combined p-values (p<0.0001), except in the lateralization condition (contra- minus ipsilateral hemisphere), similar to our previous analysis. Additionally, we tested for a consistent positive or negative distribution of the correlation coefficients. As shown in figure 3C, the results support a significant correlation between backward waves and alpha- power in the hemisphere contralateral to the attended location (BF10=10.7 and BF10=7.4 for occipital and frontal regions, respectively; all other BF10 were between 1 and 2, providing inconclusive evidence). Interestingly, this analysis also revealed a small but consistent effect in the correlation between lateralization effects, as we reported a consistently positive correlation in the contra- minus ipsilateral difference between forward waves and alpha power (BF10~5 for both frontal and occipital electrodes). However, it’s important to notice that the combined p-values obtained using the Fisher method did not reach the significance threshold in the lateralization condition, reducing the relevance of this specific result.“
From the Methods section:
“Additionally, we computed trial-by-trial correlations between waves and alpha power for all participants. First, we tested the correlation coefficient against zero in all conditions. Then, we obtained a combined p-value per condition using the log/lin regress Fisher method (Fisher, 1992), as shown in (Zoefel et al., 2019). Specifically, we computed the T value of a chi- square distribution with 2*N degrees of freedom from the pi values of the N participants as:
It needs to be appreciated that the authors analyze two datasets in the present study. However, the question remains whether the absence of the forward waves effect in paradigms without visual stimulation is a general one and would replicate in other datasets. Moreover, the manuscript would benefit from a discussion of the potential implications of traveling waves for functional connectivity between posterior and anterior regions.
We have now included a third dataset in the paper. In this dataset, from (Feldmann-Wüstefeld & Vogel, 2019), participants performed a visual working memory task by attending either the left or the right side of the screen where a stimulus was displayed. We analyzed the amount of waves during stimulus presentation, and we found the same results as in our own dataset: very strong evidence in favor of an interaction between LATERALITY (contra- and ipsilateral) and DIRECTION (FW and BW). We now included the results in figure 2 (see point above) and in the results section of the manuscript. Unfortunately, we couldn't find any other publicly available EEG dataset in which participants attend to either side of the screen without ongoing visual stimulation.
In addition, we re-analyzed our main findings (i.e. the interaction between LATERALITY and DIRECTION) in all three datasets using a classic ANOVA to report the effect size as 𝜂2 (see point above). Unlike the Bayesian ANOVA (which -in JASP- is based on linear mixed models), the classic one does not model the slope of the random effects. Yet, we observed that the LATERALITY x DIRECTION interaction in the Foster dataset proved very significant, with a large effect size (F(1,16)=9.81, p=0.003, 𝜂2=0.13). Supposedly, modeling the slope of the random effects in the Bayesian ANOVA lowered its statistical sensitivity. For the sake of completeness, we reported both results in the manuscript.
Concerning the potential implications of traveling waves on functional connectivity, we consider the interpretation based on the Predictive Coding scheme in the one before the last paragraph of the discussion (reported below for the reviewer’s convenience). In this framework, top-down connections have inhibitory functions, suppressing the predicted activity in lower regions. These interpretations align with our findings, relating the inhibitory role of backward travelling waves to visual attention. Similarly, in the same paragraph, we refer to the work of Spratling, which extensively investigates the relationship between selective attention and Predictive Coding.
From the Results section:
"To confirm our previous results, we replicated the same traveling waves analysis on two publicly available EEG datasets in which participants performed similar attentional tasks (experiment 1 of Foster et al., 2017 and experiment 1 of Feldmann-Wüstefeld and Vogel, 2019). In the first experiment from the Feldmann-Wüstefeld and Vogel dataset, participants were instructed to perform a visual working memory task in which, while keeping a central fixation, they had to memorize a set of items while ignoring a group of distracting stimuli. We focused our analysis on those trials in which the visual items to remember were placed either to the right or the left side of the screen, while the distractors were either in the upper or lower part of the screen (we pulled together the trials with either 2 or 4 distractors, as this factor was irrelevant for the purposes of our analysis). The stimuli were shown for 200ms, and we computed the amount of forward and backward waves in the 500ms following stimulus onset. As shown in figure 2 (central column), the analysis confirmed our previous results, demonstrating a strong interaction between the factors DIRECTION and LATERALITY (BF10=667, error~2%; independently, the factors DIRECTION and LATERALITY had BF10=0.2 and BF10=0.4, respectively). These results confirmed that, in the presence of visual stimulation, spatial attention modulates both forward and backward waves. Next, we analyzed another publicly available dataset from Foster et al., 2017. [...]"
"Remarkably, as shown in figure 2 (right panel), our analysis demonstrated an effect of the lateralization (LATERALITY: BF10=3.571, error~1%), revealing more waves contralateral to the attended location, but inconclusive results regarding the interaction between DIRECTION and LATERALITY (BF10=2.056, error~1%). However, using a classical ANOVA (i.e., without modeling the slope of the random terms), the interaction between DIRECTION and LATERALITY proved significant (F(1,16)=9.81, p=0.003, 𝜂2=0.13)."
From the Methods section:
"We included two additional datasets in this study. In both studies, participants performed a visual attention task while keeping their fixation in the center of the screen. Regarding the Feldmann-Wüstefeld and Vogel, 2019 study, participants were asked to memorize the colors of two stimuli while ignoring a set of distractors stimuli. We analyzed uniquely those trials in which the visual stimuli were presented to the left or right side of the screen, while the distractors were placed above or below the fixation cross. After 500ms of the fixation cross, two colored 'target' stimuli were presented for 200ms. Participants were asked to memorize these stimuli, and a new 'probe’ stimulus was shown after an additional second. Participants reported whether the probe matched the target stimuli or not. We analyzed the traveling waves in the 500ms following the target stimulus onset. Participants performed a spatial attention task in the second dataset from Foster et al. 2017. First, the fixation cross cued participants to covertly attend one of eight possible spatial positions uniformly distributed around the center of the screen. After one second, a digit was displayed either in the cued location or in any other one. The remaining locations were filled with letters. Participants were instructed to report the only displayed digit. We analyzed the waves the second before the stimuli onset when participants attended to the locations cued to the left or right side of the screen (we discarded trials in which participants attended locations above or below the fixation cross). For additional details about both experimental procedures, we refer the reader to Foster et al., 2017 and Feldmann-Wüstefeld and Vogel, 2019.”
From the discussion:
"Our previous work proposed an alternative cause for the generation of cortical waves (Alamia and VanRullen, 2019). We demonstrated that a simple multi-level hierarchical model based on Predictive Coding (PC) principles and implementing biologically plausible constraints (temporal delays between brain areas and neural time constants) gives rise to oscillatory traveling waves propagating both forward and backward. This model is also consistent with the 2-dipoles hypothesis (Zhigalov and Jensen, 2022), considering the interaction between the parietal and occipital areas (i.e., a model of 2 hierarchical levels). However, dipoles in parietal regions are unlikely to explain the observed pattern of top-down waves, suggesting that more frontal areas may be involved in generating the feedback. This hypothesis is in line with the PC framework, in which top-down connections have an inhibitory function, suppressing the activity predicted by higher-level regions (Huang and Rao, 2011). Interestingly, Spratling proposed a simple reformulation of the terms in the PC equations that could describe it as a model of biased competition in visual attention, thus corroborating the interpretation of our finding within the PC framework (Spratling, 2008, 2012)."
-

eLife assessment
Alamia and colleagues investigate the direction of traveling waves in the alpha frequency band during visual spatial attention. The authors' novel perspective adopted here is valuable to understanding the functional relevance of alpha oscillations for spatial attention. The observed pattern of results is consistent with distinct roles for travelling alpha waves in spatially opposite directions and makes a solid case for considering this new perspective on alpha rhythms in human cognitive function.
-

Reviewer #1 (Public Review):
The authors push a fresh perspective with a sufficiently sophisticated and novel methodology. I have some remaining reservations that concern the actual make-up of the data basis and consistency of results between the two (N=16) samples, the statistical analysis, as well as the "travelling" part.
I previously commented on the fact that findings from both datasets were difficult to discern and more effort should be made to highlight these. Also, a major conclusion "the directionality effect [effect of attention on forward waves] only occurs for visual stimulation" only rested on a qualitative comparison between studies. The authors have improved on this here, e.g., by toning down this conclusion. One thing that is still missing is a graphical representation of the data from Foster et al. (the second dataset …
Reviewer #1 (Public Review):
The authors push a fresh perspective with a sufficiently sophisticated and novel methodology. I have some remaining reservations that concern the actual make-up of the data basis and consistency of results between the two (N=16) samples, the statistical analysis, as well as the "travelling" part.
I previously commented on the fact that findings from both datasets were difficult to discern and more effort should be made to highlight these. Also, a major conclusion "the directionality effect [effect of attention on forward waves] only occurs for visual stimulation" only rested on a qualitative comparison between studies. The authors have improved on this here, e.g., by toning down this conclusion. One thing that is still missing is a graphical representation of the data from Foster et al. (the second dataset analysed here) that would support the statistical results and allow the reader a visual comparison between the sets of findings.
Also, for any naive reader, the concept of travelling waves may be hard to grasp in the way data are currently presented - only based on the results of the 2D-FFT. Can forward and backward-travelling waves be illustrated in a representative example to make this more intuitive?
Finally, the way Bayes Factors from the Bayesian ANOVA are presented, especially with those close to the 'meaningful boundaries' ⅓ and 3, as defined in the 'Statistical analysis' section, requires some unification/revision. For example, here: "We found a positive correlation between contra- and ipsi- lateral backward waves, and occipital (all Pearson's r~=0.4, all BFs 10 ~=3) and -to a smaller extent- frontal areas (all Pearson's r~=0.3, all BFs 10 ~=2).", where the second part should strictly be labelled as inconclusive evidence. In the same vein, there is occasional mention of "negative effects", where it should say that evidence favours the absence of an effect.
-

Reviewer #2 (Public Review):
The present manuscript takes a new perspective and investigates the functional relevance of traveling alpha waves' direction for visual spatial attention. While the modulation of alpha oscillatory power - and especially the lateralization of alpha power - has been associated with spatial attention in the literature, the present investigation offers a new perspective that helps understand and differentiate the functional roles of alpha oscillations in the ipsi- versus contralateral hemisphere for spatial attention.
The present study uses a straightforward approach and provides an analysis of two EEG datasets, which are convergingly in line with the authors' claim that two patterns of travelling alpha waves need to be differentiated in visual spatial attention. First, backward waves in the ipsilateral …
Reviewer #2 (Public Review):
The present manuscript takes a new perspective and investigates the functional relevance of traveling alpha waves' direction for visual spatial attention. While the modulation of alpha oscillatory power - and especially the lateralization of alpha power - has been associated with spatial attention in the literature, the present investigation offers a new perspective that helps understand and differentiate the functional roles of alpha oscillations in the ipsi- versus contralateral hemisphere for spatial attention.
The present study uses a straightforward approach and provides an analysis of two EEG datasets, which are convergingly in line with the authors' claim that two patterns of travelling alpha waves need to be differentiated in visual spatial attention. First, backward waves in the ipsilateral hemisphere, and second, forward waves in the contralateral hemisphere, which are only observed during visual stimulation. Importantly, the authors test the relation of these patterns of traveling waves to the overall power of alpha oscillations and to the hemispheric lateralization of alpha power. Furthermore, to test the functional significance, the authors demonstrate that the pattern of forward and backward waves around stimulus onset differentiates between hits and misses in task performance.
Although the results are in line with the conclusions drawn, some questions remain. The authors investigate the relationship between traveling alpha waves and the hemispheric lateralization of alpha power, which is a well-established neural signature of spatial attention. Surprisingly, the lateralization of alpha power shown in Figure 3B appears relatively weak in the present dataset (by visual inspection), which raises the question of whether the investigation of a relation between lateralized alpha power and alpha traveling waves is warranted in the first place.
Furthermore, the authors employ between-subject correlations (with N = 16) to test the relationship between alpha traveling waves and (lateralized) alpha power. However, as inter-individual differences in patterns of travelling waves are not the main focus here, within-subject analyses of the same relations would be able to test the authors' hypotheses much more directly.
It needs to be appreciated that the authors analyze two datasets in the present study. However, the question remains whether the absence of the forward waves effect in paradigms without visual stimulation is a general one and would replicate in other datasets. Moreover, the manuscript would benefit from a discussion of the potential implications of traveling waves for functional connectivity between posterior and anterior regions.
-