Connexin 43 hemichannels regulate mitochondrial ATP generation, mobilization, and mitochondrial homeostasis against oxidative stress
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The manuscript is well organized and clearly written. The discussion provides the required information to easily understand the relevance of each finding. the authors demonstrated using an osteocyte cell model that connexin43 is localized to mitochondria and that this is enhanced in response to oxidative stress. Several lines of evidence were presented showing that mitochondrial connexin43 forms functional hemichannels and that connexin43 is required for optimal mitochondrial respiration and ATP generation. These aspects were major strengths of the study.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Oxidative stress is a major risk factor that causes osteocyte cell death and bone loss. Prior studies primarily focus on the function of cell surface expressed Cx43 channels. Here, we reported a new role of mitochondrial Cx43 (mtCx43) and hemichannels (HCs) in modulating mitochondria homeostasis and function in bone osteocytes under oxidative stress. In murine long bone osteocyte-Y4 cells, the translocation of Cx43 to mitochondria was increased under H 2 O 2 -induced oxidative stress. H 2 O 2 increased the mtCx43 level accompanied by elevated mtCx43 HC activity, determined by dye uptake assay. Cx43 knockdown (KD) by the CRISPR-Cas9 lentivirus system resulted in impairment of mitochondrial function, primarily manifested as decreased ATP production. Cx43 KD had reduced intracellular reactive oxidative species levels and mitochondrial membrane potential. Additionally, live-cell imaging results demonstrated that the proton flux was dependent on mtCx43 HCs because its activity was specifically inhibited by an antibody targeting Cx43 C-terminus. The co-localization and interaction of mtCx43 and ATP synthase subunit F (ATP5J2) were confirmed by Förster resonance energy transfer and a protein pull-down assay. Together, our study suggests that mtCx43 HCs regulate mitochondrial ATP generation by mediating K + , H + , and ATP transfer across the mitochondrial inner membrane and the interaction with mitochondrial ATP synthase, contributing to the maintenance of mitochondrial redox levels in response to oxidative stress.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
There is emerging evidence that connexin43 hemichannels localized to mitochondria can influence their function. Here the authors demonstrated using an osteocyte cell model that connexin43 is localized to mitochondria and that this is enhanced in response to oxidative stress. Several lines of evidence were presented showing that mitochondrial connexin43 forms functional hemichannels and that connexin43 is required for optimal mitochondrial respiration and ATP generation. These aspects were major strengths of the study.
The authors also show that connexin43 is recruited to mitochondria in response to oxidant stress, as a cell protective mechanism. This was primarily done using hydrogen peroxide to generate oxidant stress; primary osteocytes from Csf-1+/- mice, which are prone to Nox4 …
Author Response
Reviewer #2 (Public Review):
There is emerging evidence that connexin43 hemichannels localized to mitochondria can influence their function. Here the authors demonstrated using an osteocyte cell model that connexin43 is localized to mitochondria and that this is enhanced in response to oxidative stress. Several lines of evidence were presented showing that mitochondrial connexin43 forms functional hemichannels and that connexin43 is required for optimal mitochondrial respiration and ATP generation. These aspects were major strengths of the study.
The authors also show that connexin43 is recruited to mitochondria in response to oxidant stress, as a cell protective mechanism. This was primarily done using hydrogen peroxide to generate oxidant stress; primary osteocytes from Csf-1+/- mice, which are prone to Nox4 induced oxidant stress, also show enhanced mitochondrial connexin43 when compared with wild type osteocytes.
Several approaches were used to demonstrate that connexin43 interacts with the ATP synthase subunit, ATP5J2, suggesting a direct role for connexin43 in the control of ATP synthesis by mediating mitochondrial ion homeostasis. Several experiments were done using a series of pHluorin fusion protein constructs as a proton sensor, these experiments hint at a potential role for connexin43 in regulating H+ permeability to support ATP production. However, the effects of inhibiting connexin43 on pH were modest, suggesting that additional roles for mitochondrial connexin43 in ATP generation should be considered.
Thank you for your positive and thoughtful comments. We agree that additional roles for mitochondrial Cx43 may be possible. As an example, we consider that there may be a change in the stability of ATP synthase that occurs after mtCx43 deficiency. This and other possible roles of mtCx43 ought to be investigated in the future.
Reviewer #3 (Public Review):
This manuscript should be of broad interest to readers not only in the field of gap junction (GJ) mediated cell-to-cell communication but also to scientists and clinicians working on the function of mitochondria and metabolism. Their data elucidates a new function of Cx43 in regulating the energy (ATP) generation of mitochondria, e.g., under oxidative stress.
The canonical function of gap junctions is in direct cell-to-cell communication by forming plasma membrane traversing channels that electrically and chemically connect the cytoplasms of adjacent cells. These channels are assembled from connexin proteins, connexin 43 (Cx43). However, more recently new, non-canonical cellular locations and functions of Cx43 have been discovered, e.g. mitochondrial Cx43 (mtCx43). However, very little is known about where Cx43 transported into mitochondria is derived from, how Cx43 is transported into mitochondria, where it is located in mitochondria, in which form Cx43 is present in mitochondria, (polypeptides, hemi-channels (HCs), complete GJ channels), and what the function of mtCx43 is. The authors addressed the latter question. The authors provide convincing evidence that mtCx43 modulates mitochondrial homeostasis and function in bone osteocytes under oxidative stress. Together, their study suggests that mtCx43 hemi-channels regulate mitochondrial ATP generation by mediating K+, H+, and ATP transfer across the mitochondrial inner membrane by directly interacting with mitochondrial ATP synthase (ATP5J2), leading to an enhanced protection of osteocytes against oxidative insult. These findings provide important information of a role of Cx43 functioning directly in mitochondria and not at the canonical location in the plasma membrane. While most of the functional assays presented in Figures 2-8 appear solid, the mitochondrial localization of Cx43, its translocation into mitochondria under oxidative stress, and its configuration as hemi-channels (Figure 1) is less convincing. I have five general comments that should be addressed:
- This study was performed in MLO-Y4 osteocyte cells. Is the H2O2 induced increase of mitochondrial Cx43 MLO-Y4 cell type or osteocyte specific, or is Cx43 playing a more general role in mitochondrial function, e.g. under oxidative stress? Osteoblasts such as MC3T3-E1 and MG63, and many other cell types endogenously express Cx43, and oxidative stress is a general physiological stressor, not only for osteocytes and bone cells. Attending to this question would address the generality of the findings for mitochondrial function.
We thank the reviewer for bringing up these valid points; seeing the phenotype displayed in secondary cell types, such as osteoblasts, would be of great relevance and interest. To address this, we conducted new experiments on MC3T3-E1 cells (Figure 1-figure supplement 2). After 2 hrs of H2O2 treatment, Cx43 accumulated on the mitochondria, marked by Mitotracker. Statistical analysis also showed a significant increase of the localization between Cx43 and Mitotracker (Figure 1-figure supplement 2B). The colocalization coefficient is higher in the Ctrl group in MC3T3-E1 cells when compared with the MLO-Y4 Ctrl group, indicating a different response level in other cell lines. Osteoblasts seemed to be more sensitive to redox interference. Overall, proving the point that under oxidative stress, mtCx43 may display a similar phenotype, across multiple cell lines, although the degree of sensitivity may differ.
- The images of MLO-Y4 cells (Figure 1A) and the primary osteocytes isolated from Csf-1+/- and control mice (Figure 8) do not show visible gap junctions. I guess this is due to the fact that slides were stained with the Cx43(E2) antibody. I feel, staining of these cells in addition with the Cx43(CT) antibody would be helpful to get a better understanding on the distribution of Cx43 in gap junctions and undocked/un-oligomerized Cx43 in these cells.
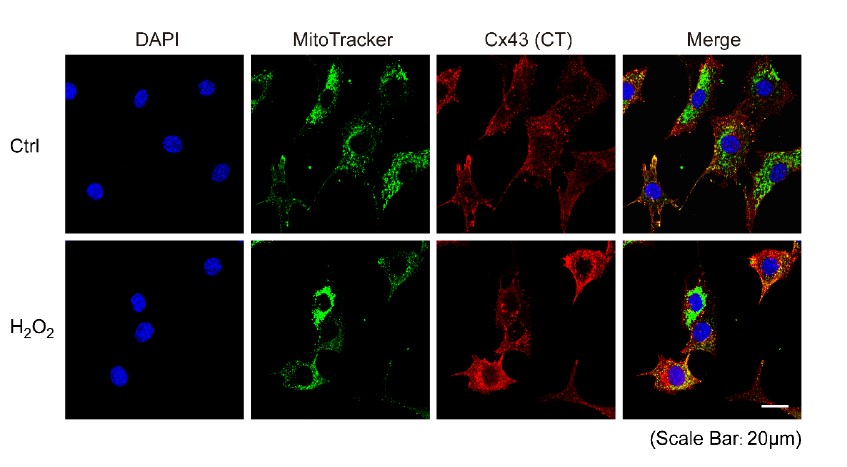
Thank you for the suggestion. To get a better understanding of the distribution of Cx43, either in GJ or HC form, we performed additional experiments in MLO-Y4 cells using the Cx43(CT) antibody and data are shown below. With Cx43(CT) staining, we observed more signals in the cells and on the plasma membrane. After H2O2 treatment, we observed increased and stronger signals localized on the mitochondria compared with the untreated control group. Stronger signals observed in the plasma membrane indicate the gap junction stained by Cx43(CT) antibody.
- The images of cells presented in Figure 1A are quite fussy. No mitochondria are visible, and the Cx43 staining is hazy and does not localize to any subcellular structures. Also, it is not clear if the higher resolution image presented in Figure 1C actually represents a mitochondrion. A good DIC image, or co-staining with another mitochondrial marker such as MitoTracker (as shown in Figure 4-S1) would make the localization and translocation of Cx43 into mitochondria upon oxidative stress more convincing. This is especially important as the translocation, although statistically significant, increases only by about 10% or less (Figure 1B). Such a small difference (also represented in the Western analyses presented in Figure 1D) could easily be artefactual, depending on how the correlation coefficient was generated. Of note in this respect is that control cells in Figure 1A appear larger (compare the size of the nuclei) and are spread out more than the H2O2 treated cells. Better, more clear images would make the mitochondrial localization/translocation more convincing.
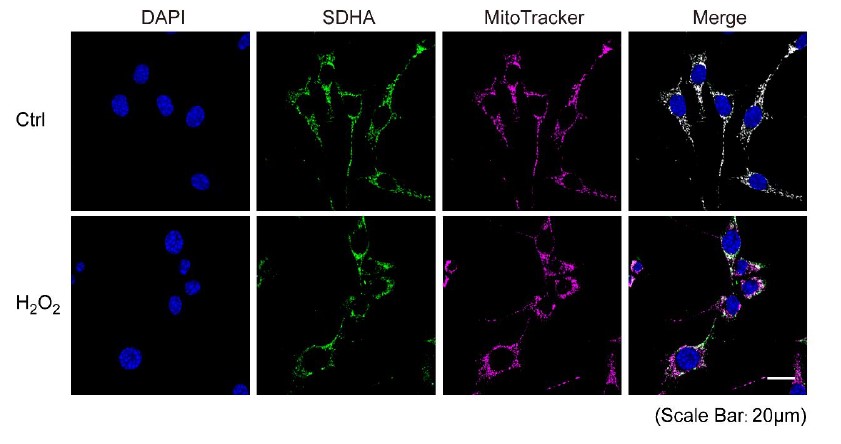
The reviewer made great points. To improve the image clarity, we redid the staining/imaging and determined the colocalization of SDHA and MitoTracker Deepred. The result (shown below) suggested that under normal conditions without H2O2 treatment, SDHA and MitoTracker merged perfectly, while after H2O2 treatment for 2 hrs, mitochondria became fragmented and the SDHA signal exhibited a more dotted pattern compared to the MitoTracker. Overall, we feel that MitoTracker represents the distribution of mitochondria better. SDHA is a subunit of mitochondrial complex II, and the images we presented in Figure 1C were captured from isolated mitochondria under a confocal microscope with SDHA and Cx43(CT) co-staining. Considering the specificity of SDHA (see images below), we believe the Cx43 signal we captured demonstrates the mitochondrial localization/translocation. After using MitoTracker as a mitochondrial marker and higher magnificent images, the correlation coefficient increased from 0.35 to 0.47, a 32% increment with statistical significance. As to the nuclei size, some cells indeed have smaller sizes, which may be affected by varied local cell density. The new images represented in Figure 1A are much more consistent in the nuclei size.
- How pure are the mitochondria that were probed for Cx43 by Western shown in Figure 1D? The preparation method described is relatively simple, collecting the 10,000xg supernatant (here 9,000xg supernatant) as mitochondrial fraction. Is it possible that the Cx43 signal, at least in part, is derived from other, contaminating membranes, such as PM, Golgi, or ER? Testing the mitochondrial preparation by Western with marker proteins specific for these compartments would strengthen the author's results.
The reviewer made a great suggestion. To address this, we did a western blot to test the mitochondrial purity. Indeed, this method using centrifugation is simple, and as expected there were some contamination of ER (marked by PDI) and Golgi (marked by STX6). However, to further confirm the purity of the mitochondrial fraction, fluorescent dyes for mitochondria (MitoTracker Deepred), ER (ER-Tracker Blue-White), and nuclei (Hochest) were used. The organelle-specific dyes indicated most parts of the fraction were mitochondria. There were some contaminations with ER fragments and minimal nuclear contamination. Combining our western blot and immunofluorescence data, it can be concluded that our Cx43 signal is primarily derived from mitochondria.
- The authors rely on previous studies to postulate that Cx43 in mitochondria forms hemichannels in their system, is localized in the inner membrane, and is oriented with the Cx43 C-termini facing the inter-membrane space (as schemed in Figure 8C). The authors use lucifer yellow (LY) dye transfer and carbenoxolone, but both are not hemi-channel specific probes. They are transferred by, and block GJ channels as well. Experiments, using hemi-channel specific probes would be more convincing. This is important, as the information cited is based on only two references (Boengler et al., 2009; Miro-Casas et al., 2009), and it still is highly unclear how a membrane protein that is co-translationally inserted into the ER membrane, then traffics through the Golgi to be inserted into the plasma membrane is actually imported into mitochondria and in which state (monomeric, hexameric). Why the Cx43(CT) specific antibody traverses the outer mitochondrial membrane and reaches the Cx43CT while the Cx43(E2) specific antibody is not described and clear either. Where are these mitochondria permeabilized with Triton X-100 as described in M&M?
We edited the Methods section. We did not use Triton X-100 to permeate mitochondria. PMP appeared to preserve mitochondrial inner membrane integrity allowing us to assess the localization of Cx43(CT) antibody on mitochondria. We showed these new immunofluorescence images in Figure 5- figure supplement 2. PMP used as a plasma membrane permeabilizer has a 6x affinity with MOM compared with MIM. Meanwhile, no Cx43(E2) Ab signal was detected in mitochondria, suggesting the extracellular loop of Cx43 faces the matrix and cannot be accessed by Cx43(E2) antibody.
The translocation of Cx43 to mitochondria was reported to involve the chaperone Hsp90-dependent TOM complex pathway (Rodriguez-Sinovas et al., 2006). After the translocation, if mtCx43 forms gap junctions in mitochondria is unclear. Lucifer yellow is widely used in hemichannel-mediated dye uptake or gap junction-mediated dye transfer. In our case, considering the channel orientation, mtCx43 should form hemichannels, and Cx43(CT) Ab could be used as a specific Cx43 HCs blocker like the study reported in cardiomyocytes (Lillo et al., 2019).
-

Evaluation Summary:
The manuscript is well organized and clearly written. The discussion provides the required information to easily understand the relevance of each finding. the authors demonstrated using an osteocyte cell model that connexin43 is localized to mitochondria and that this is enhanced in response to oxidative stress. Several lines of evidence were presented showing that mitochondrial connexin43 forms functional hemichannels and that connexin43 is required for optimal mitochondrial respiration and ATP generation. These aspects were major strengths of the study.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
An osteocyte cell line exposed to oxidant stress shows enhanced translocation of connexin43 to mitochondria where it forms hemichannels that favor the ATP synthesis. Moreover, connexin43 hemichannels mediate the K+, H+, and ATP transfer across the mitochondrial inner membrane. This article provides valuable information that explains relevant steps of preconditioning. The authors used ad hoc modern cell biology techniques to unravel the interaction of Cx43 with other critical molecular elements and to demonstrate the functional role of connexin hemichannels.
In general, the manuscript is well organized and clearly written. The discussion provides the required information to easily understand the relevance of each finding.
-

Reviewer #2 (Public Review):
There is emerging evidence that connexin43 hemichannels localized to mitochondria can influence their function. Here the authors demonstrated using an osteocyte cell model that connexin43 is localized to mitochondria and that this is enhanced in response to oxidative stress. Several lines of evidence were presented showing that mitochondrial connexin43 forms functional hemichannels and that connexin43 is required for optimal mitochondrial respiration and ATP generation. These aspects were major strengths of the study.
The authors also show that connexin43 is recruited to mitochondria in response to oxidant stress, as a cell protective mechanism. This was primarily done using hydrogen peroxide to generate oxidant stress; primary osteocytes from Csf-1+/- mice, which are prone to Nox4 induced oxidant stress, …
Reviewer #2 (Public Review):
There is emerging evidence that connexin43 hemichannels localized to mitochondria can influence their function. Here the authors demonstrated using an osteocyte cell model that connexin43 is localized to mitochondria and that this is enhanced in response to oxidative stress. Several lines of evidence were presented showing that mitochondrial connexin43 forms functional hemichannels and that connexin43 is required for optimal mitochondrial respiration and ATP generation. These aspects were major strengths of the study.
The authors also show that connexin43 is recruited to mitochondria in response to oxidant stress, as a cell protective mechanism. This was primarily done using hydrogen peroxide to generate oxidant stress; primary osteocytes from Csf-1+/- mice, which are prone to Nox4 induced oxidant stress, also show enhanced mitochondrial connexin43 when compared with wild type osteocytes.
Several approaches were used to demonstrate that connexin43 interacts with the ATP synthase subunit, ATP5J2, suggesting a direct role for connexin43 in the control of ATP synthesis by mediating mitochondrial ion homeostasis. Several experiments were done using a series of pHluorin fusion protein constructs as a proton sensor, these experiments hint at a potential role for connexin43 in regulating H+ permeability to support ATP production. However, the effects of inhibiting connexin43 on pH were modest, suggesting that additional roles for mitochondrial connexin43 in ATP generation should be considered.
-

Reviewer #3 (Public Review):
This manuscript should be of broad interest to readers not only in the field of gap junction (GJ) mediated cell-to-cell communication but also to scientists and clinicians working on the function of mitochondria and metabolism. Their data elucidates a new function of Cx43 in regulating the energy (ATP) generation of mitochondria, e.g., under oxidative stress.
The canonical function of gap junctions is in direct cell-to-cell communication by forming plasma membrane traversing channels that electrically and chemically connect the cytoplasms of adjacent cells. These channels are assembled from connexin proteins, connexin 43 (Cx43). However, more recently new, non-canonical cellular locations and functions of Cx43 have been discovered, e.g. mitochondrial Cx43 (mtCx43). However, very little is known about where …
Reviewer #3 (Public Review):
This manuscript should be of broad interest to readers not only in the field of gap junction (GJ) mediated cell-to-cell communication but also to scientists and clinicians working on the function of mitochondria and metabolism. Their data elucidates a new function of Cx43 in regulating the energy (ATP) generation of mitochondria, e.g., under oxidative stress.
The canonical function of gap junctions is in direct cell-to-cell communication by forming plasma membrane traversing channels that electrically and chemically connect the cytoplasms of adjacent cells. These channels are assembled from connexin proteins, connexin 43 (Cx43). However, more recently new, non-canonical cellular locations and functions of Cx43 have been discovered, e.g. mitochondrial Cx43 (mtCx43). However, very little is known about where Cx43 transported into mitochondria is derived from, how Cx43 is transported into mitochondria, where it is located in mitochondria, in which form Cx43 is present in mitochondria, (polypeptides, hemi-channels (HCs), complete GJ channels), and what the function of mtCx43 is. The authors addressed the latter question. The authors provide convincing evidence that mtCx43 modulates mitochondrial homeostasis and function in bone osteocytes under oxidative stress. Together, their study suggests that mtCx43 hemi-channels regulate mitochondrial ATP generation by mediating K+, H+, and ATP transfer across the mitochondrial inner membrane by directly interacting with mitochondrial ATP synthase (ATP5J2), leading to an enhanced protection of osteocytes against oxidative insult. These findings provide important information of a role of Cx43 functioning directly in mitochondria and not at the canonical location in the plasma membrane. While most of the functional assays presented in Figures 2-8 appear solid, the mitochondrial localization of Cx43, its translocation into mitochondria under oxidative stress, and its configuration as hemi-channels (Figure 1) is less convincing. I have five general comments that should be addressed:
This study was performed in MLO-Y4 osteocyte cells. Is the H2O2 induced increase of mitochondrial Cx43 MLO-Y4 cell type or osteocyte specific, or is Cx43 playing a more general role in mitochondrial function, e.g. under oxidative stress? Osteoblasts such as MC3T3-E1 and MG63, and many other cell types endogenously express Cx43, and oxidative stress is a general physiological stressor, not only for osteocytes and bone cells. Attending to this question would address the generality of the findings for mitochondrial function.
The images of MLO-Y4 cells (Figure 1A) and the primary osteocytes isolated from Csf-1+/- and control mice (Figure 8) do not show visible gap junctions. I guess this is due to the fact that slides were stained with the Cx43(E2) antibody. I feel, staining of these cells in addition with the Cx43(CT) antibody would be helpful to get a better understanding on the distribution of Cx43 in gap junctions and undocked/un-oligomerized Cx43 in these cells.
The images of cells presented in Figure 1A are quite fussy. No mitochondria are visible, and the Cx43 staining is hazy and does not localize to any subcellular structures. Also, it is not clear if the higher resolution image presented in Figure 1C actually represents a mitochondrion. A good DIC image, or co-staining with another mitochondrial marker such as MitoTracker (as shown in Figure 4-S1) would make the localization and translocation of Cx43 into mitochondria upon oxidative stress more convincing. This is especially important as the translocation, although statistically significant, increases only by about 10% or less (Figure 1B). Such a small difference (also represented in the Western analyses presented in Figure 1D) could easily be artefactual, depending on how the correlation coefficient was generated. Of note in this respect is that control cells in Figure 1A appear larger (compare the size of the nuclei) and are spread out more than the H2O2 treated cells. Better, more clear images would make the mitochondrial localization/translocation more convincing.
How pure are the mitochondria that were probed for Cx43 by Western shown in Figure 1D? The preparation method described is relatively simple, collecting the 10,000xg supernatant (here 9,000xg supernatant) as mitochondrial fraction. Is it possible that the Cx43 signal, at least in part, is derived from other, contaminating membranes, such as PM, Golgi, or ER? Testing the mitochondrial preparation by Western with marker proteins specific for these compartments would strengthen the author's results.
The authors rely on previous studies to postulate that Cx43 in mitochondria forms hemichannels in their system, is localized in the inner membrane, and is oriented with the Cx43 C-termini facing the inter-membrane space (as schemed in Figure 8C). The authors use lucifer yellow (LY) dye transfer and carbenoxolone, but both are not hemi-channel specific probes. They are transferred by, and block GJ channels as well. Experiments, using hemi-channel specific probes would be more convincing. This is important, as the information cited is based on only two references (Boengler et al., 2009; Miro-Casas et al., 2009), and it still is highly unclear how a membrane protein that is co-translationally inserted into the ER membrane, then traffics through the Golgi to be inserted into the plasma membrane is actually imported into mitochondria and in which state (monomeric, hexameric). Why the Cx43(CT) specific antibody traverses the outer mitochondrial membrane and reaches the Cx43CT while the Cx43(E2) specific antibody is not described and clear either. Where are these mitochondria permeabilized with Triton X-100 as described in M&M?
-