Csf1 from marrow adipogenic precursors is required for osteoclast formation and hematopoiesis in bone
Curation statements for this article:-
Curated by eLife
eLife assessment
This paper is of potential interest for scientists who study bone marrow stem/progenitor cells, bone remodeling, and metabolism. Using Adipoq-Cre-based conditional deletion of Csf1 and scRNA-seq approaches, the authors provide compelling evidence that a subset of cells in murine bone marrow, characterized by AdipoQ expression, are a major source of M-CSF to regulate osteoclast formation and bone remodeling. This is a well-written, well-executed set of studies, the data from which largely support the above key claim.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Colony-stimulating factor 1 (Csf1) is an essential growth factor for osteoclast progenitors and an important regulator for bone resorption. It remains elusive which mesenchymal cells synthesize Csf1 to stimulate osteoclastogenesis. We recently identified a novel mesenchymal cell population, marrow adipogenic lineage precursors (MALPs), in bone. Compared to other mesenchymal subpopulations, MALPs expressed Csf1 at a much higher level and this expression was further increased during aging. To investigate its role, we constructed MALP-deficient Csf1 CKO mice using Adipoq Cre . These mice had increased femoral trabecular bone mass, but their cortical bone appeared normal. In comparison, depletion of Csf1 in the entire mesenchymal lineage using Prrx1 Cre led to a more striking high bone mass phenotype, suggesting that additional mesenchymal subpopulations secrete Csf1. TRAP staining revealed diminished osteoclasts in the femoral secondary spongiosa region of Csf1 CKO Adipoq mice, but not at the chondral-osseous junction nor at the endosteal surface of cortical bone. Moreover, Csf1 CKO Adipoq mice were resistant to LPS-induced calvarial osteolysis. Bone marrow cellularity, hematopoietic progenitors, and macrophages were also reduced in these mice. Taken together, our studies demonstrate that MALPs synthesize Csf1 to control bone remodeling and hematopoiesis.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
Weaknesses:
- The relevance of the LPS-induced calvarial osteolysis model is not clear. Calvaria is mostly composed of cortical bone-like structures lacking marrow space, though small marrow space exists near the suture. Osteolysis appears to occur in areas apart from where marrow is located. The authors did not show in the manuscript which cells Adipoq-Cre marks in the calvaria.
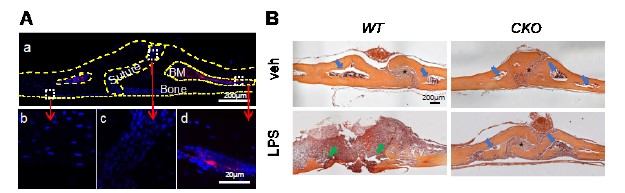
We have shown in a recent publication that MALPs exist in the calvarial bone marrow (2). As shown in Fig. R1A, Td+ cells are layer of cortical bone (Fig. R1B, blue arrows). In WT mice, after LPS injection, the normal bone structure, including suture and cortical bone, were mostly eroded, and filled with inflammatory cells (green arrows). Thus, osteolysis does occur at the area where bone marrow is originally …
Author Response
Reviewer #2 (Public Review):
Weaknesses:
- The relevance of the LPS-induced calvarial osteolysis model is not clear. Calvaria is mostly composed of cortical bone-like structures lacking marrow space, though small marrow space exists near the suture. Osteolysis appears to occur in areas apart from where marrow is located. The authors did not show in the manuscript which cells Adipoq-Cre marks in the calvaria.
We have shown in a recent publication that MALPs exist in the calvarial bone marrow (2). As shown in Fig. R1A, Td+ cells are layer of cortical bone (Fig. R1B, blue arrows). In WT mice, after LPS injection, the normal bone structure, including suture and cortical bone, were mostly eroded, and filled with inflammatory cells (green arrows). Thus, osteolysis does occur at the area where bone marrow is originally located. On the contrary, calvarial bone structure was preserved in the CKO mice, demonstrating that Csf1 deficiency in MALPs suppresses LPS-induced osteolysis. We included the H&E staining data in the revised manuscript:
"H&E staining showed that calvarial bone marrow is surrounded by a thin layer of cortical bone (Fig. 5C). After the LPS injection, normal calvarial structure, including suture and cortical bone, were mostly eroded and filled with inflammatory cells in WT mice, but unaltered in CKO mice."
Figure R1. Calvarial bone marrow structure. (A) Representative coronal section of 1.5-month-old Adipoq/Td mouse calvaria. Bone surfaces are outlined by dashed lines. Boxed areas in the low magnification image (top) are enlarged to show periosteum (bottom left), suture (bottom middle), and bone marrow (BM, bottom right) regions. Red: Td; Blue: DAPI. Adopted from our previous publication (2). (B) H&E staining of coronal sections of WT and Csf1 CKOAdipoq mice after LPS injection. Blue arrows point to bone marrow space close to suture (indicated by *). Green arrows point to the osteolytic lesion where cortical bone was eroded, and the space were filled with inflammatory cells.
- Although the contrast between the two Csf1 conditional deletion models (Adipoq-Cre and Prx1-Cre) is very interesting, the relationship between these two cell populations are not well described. The authors did not clarify if MALPs are also targeted by Prx1-Cre, or these two cell types are from different cell lineages. "Other mesenchymal lineage cells" in the subtitle is not extremely helpful to place this finding in context.
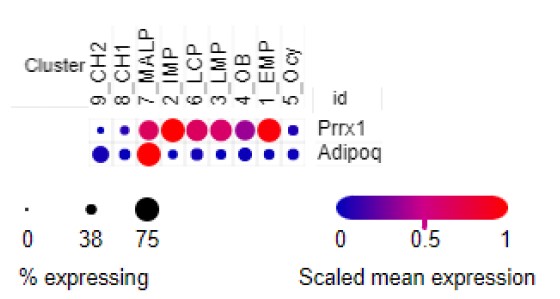
We thank the Reviewer for this comment. The original article constructing Prx1-Cre mouse line demonstrates that Prx1-Cre targets all mesenchymal cells in the limb bud at early as 10.5 dpc (10). This early expression pattern ensures that all bone marrow mesenchymal lineage cells, including MALPs, are targeted by Prx1-Cre. In addition, based on our scRNA-seq data (1), Adipoq is mainly expressed in MALPs, while Prrx1 (Prx1) is highly expressed not only in MALPs but also in EMPs, IMPs, LMPs, LCPs, and OBs (Fig. R2). Thus, the fact that Prx1-Cre driven CKO mice have much more severer bone phenotypes than AdipoqCre driven CKO mice indicates that mesenchymal lineage cells other than MALPs also contribute Csf1 to regulate bone resorption. To avoid confusion, we changed the title and the first sentence in the Result session about Prx1 mice to the following:
"Csf1 from mesenchymal lineage cells other than MALPs regulate bone structure.
To explore whether Csf1 from MALPs plays a dominant role in regulating bone structure, we generated Prx1-Cre Csf1flox/flox (Csf1 CKOPrx1) mice to knockout Csf1 in all mesenchymal lineage cells in bone (10), including MALPs."
Figure R2. Dotplot of Prrx1 and Adipoq expression in bone marrow mesenchymal lineage cells based on our scRNA-seq analysis of 1-month-old mice.
- The data supporting defective bone marrow hematopoiesis in Csf1 CKO mice are not particularly strong. They observed a reduction in bone marrow cellularity, but this was only associated with an expected reduction in macrophages and a mild reduction in overall HSPC populations. More in-depth analyses might be required to define mechanisms underlying reduced bone marrow cellularity in CKO mice.
We thank the Reviewer for this constructive comment. Accordingly, we performed a thorough analysis of bone marrow hematopoietic compartments and observed significant decreases of monocytes and erythroid progenitors in CKO mice compared to WT mice. These results are now included as Fig. 6E.
- Some of the phenotypic analyses are still incomplete. The authors did not report whether CHet (Adipoq-Cre Csf1(flox/+)) showed any bone phenotype. Further, the authors did not report whether Csf1 mRNA or M-Csf protein is indeed expressed by MALPs, with current evidence solely reliant on scRNAseq and qPCR data of bulk-isolated cells. More specific histological methods will be helpful to support the premise of the study.
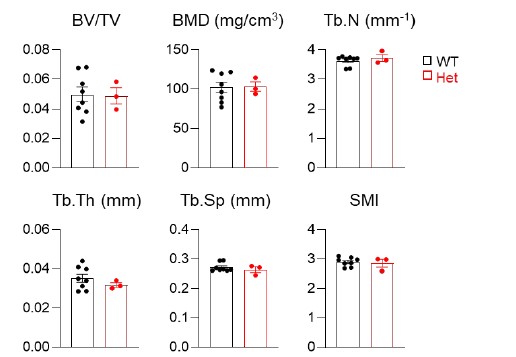
A pilot microCT study revealed the same femoral trabecular bone structure in WT and Adipoq-Cre Csf1flox/+ (Csf1 Het) mice at 3 months of age (Fig. R3). While the sample number for Het is low, we are confident about this conclusion.
Figure R3. MicroCT measurement of trabecular bone structural parameters from WT and Csf1 Het mice. BV/TV: bone volume fraction; BMD: bone mineral density; Tb.N: trabecular number; Tb.Th: trabecular thickness; Tb.Sp: trabecular separation; SMI: structural model index. n=3-8 mice/group.
-

eLife assessment
This paper is of potential interest for scientists who study bone marrow stem/progenitor cells, bone remodeling, and metabolism. Using Adipoq-Cre-based conditional deletion of Csf1 and scRNA-seq approaches, the authors provide compelling evidence that a subset of cells in murine bone marrow, characterized by AdipoQ expression, are a major source of M-CSF to regulate osteoclast formation and bone remodeling. This is a well-written, well-executed set of studies, the data from which largely support the above key claim.
-

Reviewer #1 (Public Review):
This study demonstrates that MALPs (Marrow Adipogenic Lineage Progenitors), which were previously described by these authors and constitute approximately 0.5% of bone marrow mesenchymal cells, are major producers of Csf1 (M-CSF) in murine bone marrow. The initial discovery of Csf1 in MALPs occurred during review of scRNA-seq datasets. Here the authors show that deletion of Csf1 in MALPs with AdipoQ-Cre increased trabecular bone mass in long bones, but not vertebrae, and reduced the number of osteoclasts on trabecular bone surfaces. Cortical bone was not altered. Deletion of Csf1 with Adipo-Cre also prevented LPS-induces osteolysis and reduced numbers of hematopoietic progenitors and F4/80+ macrophages. Strengths of this study include use of two CKO lines (Adipo-Cre and Prx-Cre) to understand the relative …
Reviewer #1 (Public Review):
This study demonstrates that MALPs (Marrow Adipogenic Lineage Progenitors), which were previously described by these authors and constitute approximately 0.5% of bone marrow mesenchymal cells, are major producers of Csf1 (M-CSF) in murine bone marrow. The initial discovery of Csf1 in MALPs occurred during review of scRNA-seq datasets. Here the authors show that deletion of Csf1 in MALPs with AdipoQ-Cre increased trabecular bone mass in long bones, but not vertebrae, and reduced the number of osteoclasts on trabecular bone surfaces. Cortical bone was not altered. Deletion of Csf1 with Adipo-Cre also prevented LPS-induces osteolysis and reduced numbers of hematopoietic progenitors and F4/80+ macrophages. Strengths of this study include use of two CKO lines (Adipo-Cre and Prx-Cre) to understand the relative contribution of MALPs to Csf1 levels in the bone marrow, examination of bone mass in both long bones and vertebrae at several ages, challenging bone responses in Csf1 CKOAdipoQ mice with LPS-induced osteolysis, and studying the effect of Csf1 deletion in MALPs on hematopoiesis and vasculature. Mechanical studies of bone strength were not included but would be necessary to determine if deletion of Csf1 in MALPs is sufficient to cause osteopetrosis. Additional information on other molecular changes Csf1 CKOAdipoQ mice would provide insights into how deletion of Csf1 in MALPs affects bone remodeling. Overall, this is a very important study that will have a high impact on the field because it is challenging the paradigm that osteoblasts and osteocytes are the major sources of M-CSF in the bone marrow.
-

Reviewer #2 (Public Review):
This is a well-written manuscript in which the authors' conclusions are supported by well-established mouse genetic conditional approaches and phenotypic analyses.
Strengths:
1. The authors utilized well-established genetic tools, Adipoq-Cre to target MALPs and Prx1-Cre to globally target limb skeletal cells, and combined these drivers with Csf1 floxed alleles. This double-angled approach helps the authors determine the importance of Csf1 secreted by different skeletal cell populations in regulating bone mass.
2. The scRNA-seq analysis and the in vivo phenotypic analyses (3DmicroCT, histology, dynamic histomorphometry, serum bone resorption and formation markers) of the Csf1 CKO models are well-conducted. The authors convincingly show that cortical bone parameters are unaffected in MALP-specific Csf1 CKO …Reviewer #2 (Public Review):
This is a well-written manuscript in which the authors' conclusions are supported by well-established mouse genetic conditional approaches and phenotypic analyses.
Strengths:
1. The authors utilized well-established genetic tools, Adipoq-Cre to target MALPs and Prx1-Cre to globally target limb skeletal cells, and combined these drivers with Csf1 floxed alleles. This double-angled approach helps the authors determine the importance of Csf1 secreted by different skeletal cell populations in regulating bone mass.
2. The scRNA-seq analysis and the in vivo phenotypic analyses (3DmicroCT, histology, dynamic histomorphometry, serum bone resorption and formation markers) of the Csf1 CKO models are well-conducted. The authors convincingly show that cortical bone parameters are unaffected in MALP-specific Csf1 CKO mice, while serum CTX-1 is reduced in these mice. The confidence for the reported phenotypes is high.
3. The data presented in this manuscript are of very high quality. Particularly, the authors detected no changes in osteoclasts at the chondral-osseous junction and the endosteal surface, emphasizing the uniqueness of the CKO model.Weaknesses:
1. The relevance of the LPS-induced calvarial osteolysis model is not clear. Calvaria is mostly composed of cortical bone-like structures lacking marrow space, though small marrow space exists near the suture. Osteolysis appears to occur in areas apart from where marrow is located. The authors did not show in the manuscript which cells Adipoq-Cre marks in the calvaria.
2. Although the contrast between the two Csf1 conditional deletion models (Adipoq-Cre and Prx1-Cre) is very interesting, the relationship between these two cell populations are not well described. The authors did not clarify if MALPs are also targeted by Prx1-Cre, or these two cell types are from different cell lineages. "Other mesenchymal lineage cells" in the subtitle is not extremely helpful to place this finding in context.
3. The data supporting defective bone marrow hematopoiesis in Csf1 CKO mice are not particularly strong. They observed a reduction in bone marrow cellularity, but this was only associated with an expected reduction in macrophages and a mild reduction in overall HSPC populations. More in-depth analyses might be required to define mechanisms underlying reduced bone marrow cellularity in CKO mice.
4. Some of the phenotypic analyses are still incomplete. The authors did not report whether CHet (Adipoq-Cre Csf1(flox/+)) showed any bone phenotype. Further, the authors did not report whether Csf1 mRNA or M-Csf protein is indeed expressed by MALPs, with current evidence solely reliant on scRNAseq and qPCR data of bulk-isolated cells. More specific histological methods will be helpful to support the premise of the study. -