Rabphilin 3A binds the N-peptide of SNAP-25 to promote SNARE complex assembly in exocytosis
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Li et al. use biochemical binding analysis to explore the role of Rabphilin 3A in dense-core vesicle exocytosis in neuroendocrine PC12 cells and in an in vitro SNARE assembly assay. They propose that the Rph3A binding to SNAP25 pre-structures the protein to efficiently assemble with Syntaxin and VAMP2, and thus, promoting the vesicle docking and priming process. This work will be of interest to scientists studying the molecular basis of synaptic vesicle release.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Exocytosis of secretory vesicles requires the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins and small GTPase Rabs. As a Rab3/Rab27 effector protein on secretory vesicles, Rabphilin 3A was implicated to interact with SNAP-25 to regulate vesicle exocytosis in neurons and neuroendocrine cells, yet the underlying mechanism remains unclear. In this study, we have characterized the physiologically relevant binding sites between Rabphilin 3A and SNAP-25. We found that an intramolecular interplay between the N-terminal Rab-binding domain and C-terminal C 2 AB domain enables Rabphilin 3A to strongly bind the SNAP-25 N-peptide region via its C 2 B bottom α-helix. Disruption of this interaction significantly impaired docking and fusion of vesicles with the plasma membrane in rat PC12 cells. In addition, we found that this interaction allows Rabphilin 3A to accelerate SNARE complex assembly. Furthermore, we revealed that this interaction accelerates SNARE complex assembly via inducing a conformational switch from random coils to α-helical structure in the SNAP-25 SNARE motif. Altogether, our data suggest that the promotion of SNARE complex assembly by binding the C 2 B bottom α-helix of Rabphilin 3A to the N-peptide of SNAP-25 underlies a pre-fusion function of Rabphilin 3A in vesicle exocytosis.
Article activity feed
-

-

Author Response
Reviewer #3: (Public Review):
In this ms Li et al. examine the molecular interaction of Rabphilin 3A with the SNARE complex protein SNAP25 and its potential impact in SNARE complex assembly and dense core vesicle fusion.
Overall the literature of rabphilin as a major rab3/27effector on synaptic function has been quite enigmatic. After its cloning and initial biochemical analysis, rather little new has been found about rabphilin, in particular since loss of function analysis has shown rather little synaptic phenotypes (Schluter 1999, Deak 2006), arguing against that rabphilin plays a crucial role in synaptic function.
While the interaction of rabphilin to SNAP25 via its bottom part of the C2 domain has been already described biochemically and structurally in the Deak et al. 2006, and others, the authors make …
Author Response
Reviewer #3: (Public Review):
In this ms Li et al. examine the molecular interaction of Rabphilin 3A with the SNARE complex protein SNAP25 and its potential impact in SNARE complex assembly and dense core vesicle fusion.
Overall the literature of rabphilin as a major rab3/27effector on synaptic function has been quite enigmatic. After its cloning and initial biochemical analysis, rather little new has been found about rabphilin, in particular since loss of function analysis has shown rather little synaptic phenotypes (Schluter 1999, Deak 2006), arguing against that rabphilin plays a crucial role in synaptic function.
While the interaction of rabphilin to SNAP25 via its bottom part of the C2 domain has been already described biochemically and structurally in the Deak et al. 2006, and others, the authors make significant efforts to further map the interactions between SNAP25 and rabphilin and indeed identified additional binding motifs in the first 10 amino acids of SNAP25 that appear critical for the rabphilin interaction.
Using KD-rescue experiments for SNAP25, in TIRF based imaging analysis of labeled dense core vesicles showed that the N-terminus of SN25 is absolutely essential for SV membrane proximity and release. Similar, somewhat weaker phenotypes were observed when binding deficient rabphilin mutants were overexpressed in PC12 cells coexpressing WT rabphilin. The loss of function phenotypes in the SN25 and rabphilin interaction mutants made the authors to claim that rabphilin-SN25 interactions are critical for docking and exocytosis. The role of these interaction sites were subsequently tested in SNARE assembly assays, which were largely supportive of rabphilin accelerating SNARE assembly in a SN25 -terminal dependent way.
Regarding the impact of this work, the transition of synaptic vesicles to form fusion competent trans-SNARE complex is very critical in our understanding of regulated vesicle exocytosis, and the authors put forward an attractive model forward in which rabphilin aids in catalyzing the SNARE complex assembly by controlling SNAP25 a-helicalicity of the SNARE motif. This would provide here a similar regulatory mechanism as put forward for the other two SNARE proteins via their interactions with Munc18 and intersection, respectively.
We thank the reviewer #3 for the summary of the paper and for the praise of our work. The point-to-point replies are as follow:
While discovery of the novel interaction site of rabphilin with the N-Terminus of SNAP25 is interesting, I have issues with the functional experiments. The key reliance of the paper is whether it provides convincing data on the functional role of the interactions, given the history of loss of function phenotypes for Rabphilin. First, the authors use PC12 cells and dense core vesicle docking and fusion assays. Primary neurons, where rabphilin function has been tested before, has unfortunately not been utilized, reducing the impact of docking and fusion phenotype.
We have discussed these questions as mentioned in our response to Essential Revisions 3 and added this corresponding passage to the Discussion section (pp.18-19, lines 407-427).
In particular the loss of function phenotype in figure 3 of the n-terminally deleted SNAP25 in docking and fusion is profound, and at a similar level than the complete loss of the SNARE protein itself. This is of concern as this is in stark contrast to the phenotype of rabphilin loss in mammalian neurons where the phenotype of SNAP25 loss is very severe while rabphilin loss has almost no effect on secretion. This would argue that the N-terminal of SNAPP25 has other critical functions besides interacting with rabphilin. In addition, it could argue that the n-Terminal SNAP25 deletion mutant may be made in the cell (as indicated from the western blot) but may not be properly trafficked to the site of release
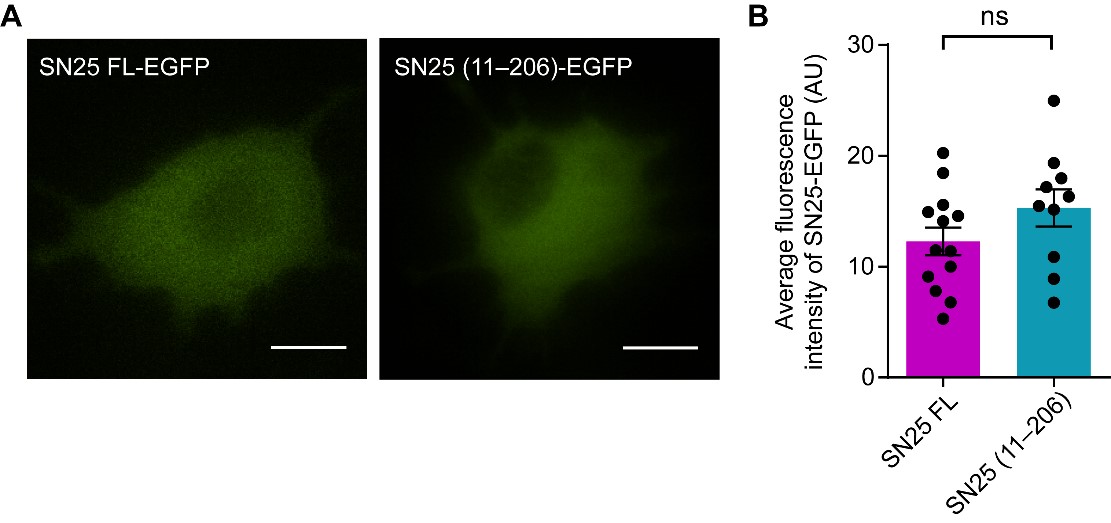
To test whether the N-peptide deletion mutant of SN25 can properly target to the plasma membrane, we overexpressed the SN25 FL or SN25 (11–206) with C-terminal EGFP-tag in PC12 cells and monitored the localization of SN25 FL-EGFP and SN25 (11–206)-EGFP near the plasma membrane by TIRF microscopy. We observed that the average fluorescence intensity of SN25 (11–206)-EGFP showed no significant difference with SN25 FL-EGFP as below, suggesting that the N-peptide deletion mutant may not influence the trafficking of SN25 to plasma membrane.
(A) TIRF imaging assay to monitor the localization of SN25-EGFP near the plasma membrane. Overexpression of SN25 FL-EGFP (left) and SN25 (11–206)-EGFP (right) using pEGFP-N3 vector in PC12 cells. Scale bars, 10 μm. (B) Quantification of the average fluorescence intensity of SN25-EGFP near the plasma membrane in (A). Data are presented as mean ± SEM (n ≥ 10 cells in each). Statistical significance and P values were determined by Student’s t-test. ns, not significant.
-

Evaluation Summary:
Li et al. use biochemical binding analysis to explore the role of Rabphilin 3A in dense-core vesicle exocytosis in neuroendocrine PC12 cells and in an in vitro SNARE assembly assay. They propose that the Rph3A binding to SNAP25 pre-structures the protein to efficiently assemble with Syntaxin and VAMP2, and thus, promoting the vesicle docking and priming process. This work will be of interest to scientists studying the molecular basis of synaptic vesicle release.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
Li et al. use biochemical binding analysis combined with deletions/mutations to demonstrate that the bottom helix of the Rph3A C2B domain directly interacts with the first 10 residues (N-peptide region) on SNAP25, and this interaction is amplified by the intramolecular interaction of the C2B domain with RAB-binding domain. They establish the functional relevance of this interaction using live-cell imaging of dense-core vesicle exocytosis in neuroendocrine PC12 cells and in vitro SNARE assembly assay. They propose that the Rph3A binding to SNAP25 pre-structures the protein to efficiently assemble with Syntaxin and VAMP2, and thus, promoting the vesicle docking and priming process. This is a systematic analysis that clarifies the role of Rph3A in regulated exocytosis and provides novel insight into the …
Reviewer #1 (Public Review):
Li et al. use biochemical binding analysis combined with deletions/mutations to demonstrate that the bottom helix of the Rph3A C2B domain directly interacts with the first 10 residues (N-peptide region) on SNAP25, and this interaction is amplified by the intramolecular interaction of the C2B domain with RAB-binding domain. They establish the functional relevance of this interaction using live-cell imaging of dense-core vesicle exocytosis in neuroendocrine PC12 cells and in vitro SNARE assembly assay. They propose that the Rph3A binding to SNAP25 pre-structures the protein to efficiently assemble with Syntaxin and VAMP2, and thus, promoting the vesicle docking and priming process. This is a systematic analysis that clarifies the role of Rph3A in regulated exocytosis and provides novel insight into the underlying molecular mechanisms.
-

Reviewer #2 (Public Review):
In this manuscript, the authors investigated the mechanism of the Rab3/Rab27 effector Rabphilin 3A in exocytosis. Previous research using truncated Rabphilin 3A led to conflicting conclusions. Here, the authors prepared full-length Rabphilin 3A proteins and discovered that Rabphilin 3A interacts with the t-SNARE SNAP-25 and promotes the helical transition of the latter. They mapped the binding interface to the N peptide of SNAP-25 and an alpha-helical region in the C2B domain of Rabphilin 3A. Mutations of these motifs impair DVC exocytosis in PC12 cells and also in reconstituted SNARE assembly assays. This is a thorough and elegant study that fills a notable gap in our understanding of neuronal exocytosis.
-

Reviewer #3 (Public Review):
In this ms Li et al. examine the molecular interaction of Rabphilin 3A with the SNARE complex protein SNAP25 and its potential impact in SNARE complex assembly and dense core vesicle fusion.
Overall the literature of rabphilin as a major rab3/27effector on synaptic function has been quite enigmatic. After its cloning and initial biochemical analysis, rather little new has been found about rabphilin, in particular since loss of function analysis has shown rather little synaptic phenotypes (Schluter 1999, Deak 2006), arguing against that rabphilin plays a crucial role in synaptic function.
While the interaction of rabphilin to SNAP25 via its bottom part of the C2 domain has been already described biochemically and structurally in the Deak et al. 2006, and others, the authors make significant efforts to further …
Reviewer #3 (Public Review):
In this ms Li et al. examine the molecular interaction of Rabphilin 3A with the SNARE complex protein SNAP25 and its potential impact in SNARE complex assembly and dense core vesicle fusion.
Overall the literature of rabphilin as a major rab3/27effector on synaptic function has been quite enigmatic. After its cloning and initial biochemical analysis, rather little new has been found about rabphilin, in particular since loss of function analysis has shown rather little synaptic phenotypes (Schluter 1999, Deak 2006), arguing against that rabphilin plays a crucial role in synaptic function.
While the interaction of rabphilin to SNAP25 via its bottom part of the C2 domain has been already described biochemically and structurally in the Deak et al. 2006, and others, the authors make significant efforts to further map the interactions between SNAP25 and rabphilin and indeed identified additional binding motifs in the first 10 amino acids of SNAP25 that appear critical for the rabphilin interaction.
Using KD-rescue experiments for SNAP25, in TIRF based imaging analysis of labeled dense core vesicles showed that the N-terminus of SN25 is absolutely essential for SV membrane proximity and release. Similar, somewhat weaker phenotypes were observed when binding deficient rabphilin mutants were overexpressed in PC12 cells coexpressing WT rabphilin. The loss of function phenotypes in the SN25 and rabphilin interaction mutants made the authors to claim that rabphilin-SN25 interactions are critical for docking and exocytosis. The role of these interaction sites were subsequently tested in SNARE assembly assays, which were largely supportive of rabphilin accelerating SNARE assembly in a SN25 -terminal dependent way.
Regarding the impact of this work, the transition of synaptic vesicles to form fusion competent trans-SNARE complex is very critical in our understanding of regulated vesicle exocytosis, and the authors put forward an attractive model forward in which rabphilin aids in catalyzing the SNARE complex assembly by controlling SNAP25 a-helicalicity of the SNARE motif. This would provide here a similar regulatory mechanism as put forward for the other two SNARE proteins via their interactions with Munc18 and intersection, respectively.
While discovery of the novel interaction site of rabphilin with the N-Terminus of SNAP25 is interesting, I have issues with the functional experiments. The key reliance of the paper is whether it provides convincing data on the functional role of the interactions, given the history of loss of function phenotypes for Rabphilin. First, the authors use PC12 cells and dense core vesicle docking and fusion assays. Primary neurons, where rabphilin function has been tested before, has unfortunately not been utilized, reducing the impact of docking and fusion phenotype.
In particular the loss of function phenotype in figure 3 of the n-terminally deleted SNAP25 in docking and fusion is profound, and at a similar level than the complete loss of the SNARE protein itself. This is of concern as this is in stark contrast to the phenotype of rabphilin loss in mammalian neurons where the phenotype of SNAP25 loss is very severe while rabphilin loss has almost no effect on secretion. This would argue that the N-terminal of SNAPP25 has other critical functions besides interacting with rabphilin. In addition, it could argue that the n-Terminal SNAP25 deletion mutant may be made in the cell (as indicated from the western blot) but may not be properly trafficked to the site of release.
-