Hepatic AMPK signaling dynamic activation in response to REDOX balance are sentinel biomarkers of exercise and antioxidant intervention to improve blood glucose control
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
There is a debate whether ROS (reactive oxygen species) generated through redox signaling could be a friend or foe. There are several paradoxical studies (both animal and human) wherein exercise health benefits were reported to be accompanied by increases in ROS generation. Utilizing the in-vitro studies as well as mice model work, this manuscript illustrates the different regulatory mechanisms of exercise and antioxidant intervention on redox balance and blood glucose level in diabetes. The manuscript does address some advancements in the area of research specialization.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Antioxidant intervention is considered to inhibit reactive oxygen species (ROS) and alleviate hyperglycemia. Paradoxically, moderate exercise can produce ROS to improve diabetes. The exact redox mechanism of these two different approaches remains largely unclear. Here, by comparing exercise and antioxidant intervention on type 2 diabetic rats, we found moderate exercise upregulated compensatory antioxidant capability and reached a higher level of redox balance in the liver. In contrast, antioxidant intervention achieved a low-level redox balance by inhibiting oxidative stress. Both of these two interventions could promote glucose catabolism and inhibit gluconeogenesis through activation of hepatic AMP-activated protein kinase (AMPK) signaling; therefore, ameliorating diabetes. During exercise, different levels of ROS generated by exercise have differential regulations on the activity and expression of hepatic AMPK. Moderate exercise-derived ROS promoted hepatic AMPK glutathionylation activation. However, excessive exercise increased oxidative damage and inhibited the activity and expression of AMPK. Overall, our results illustrate that both exercise and antioxidant intervention improve blood glucose control in diabetes by promoting redox balance, despite different levels of redox state(s). These results indicate that the AMPK signaling activation, combined with oxidative damage markers, could act as sentinel biomarkers, reflecting the threshold of redox balance that is linked to effective glucose control in diabetes. These findings provide theoretical evidence for the precise management of diabetes by antioxidants and exercise.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
Redox signaling is a dynamic and concerted orchestra of inter-connected cellular pathways. There is always a debate whether ROS (reactive oxygen species) could be a friend or foe. Continued research is needed to dissect out how ROS generation and progression could diverge in physiological versus pathophysiological states. Similarly, there are several paradoxical studies (both animal and human) wherein exercise health benefits were reported to be accompanied by increases in ROS generation. It is in this context, that the present manuscript deserves attention.
Utilizing the in-vitro studies as well as mice model work, this manuscript illustrates the different regulatory mechanisms of exercise and antioxidant intervention on redox balance and blood glucose level in diabetes. The manuscript …
Author Response
Reviewer #1 (Public Review):
Redox signaling is a dynamic and concerted orchestra of inter-connected cellular pathways. There is always a debate whether ROS (reactive oxygen species) could be a friend or foe. Continued research is needed to dissect out how ROS generation and progression could diverge in physiological versus pathophysiological states. Similarly, there are several paradoxical studies (both animal and human) wherein exercise health benefits were reported to be accompanied by increases in ROS generation. It is in this context, that the present manuscript deserves attention.
Utilizing the in-vitro studies as well as mice model work, this manuscript illustrates the different regulatory mechanisms of exercise and antioxidant intervention on redox balance and blood glucose level in diabetes. The manuscript does have some limitations and might need additional experiments and explanation.
The authors should consider addressing the following comments with additional experiments.
- Although hepatic AMPK activation appears to be a central signaling element for the benefits of moderate exercise and glucose control, additional signals (on hepatic tissue) related to hepatic gluconeogenesis such as Forkhead box O1 (FoxO1), phosphoenolpyruvate carboxykinase (PEPCK), and GLUT2 needs to be profiled to present a holistic approach. Authors should consider this and revise the manuscript.
We appreciate the constructive suggestion. Besides glycolysis, gluconeogenesis and glucose uptake are critical in maintaining liver and blood glucose homeostasis.
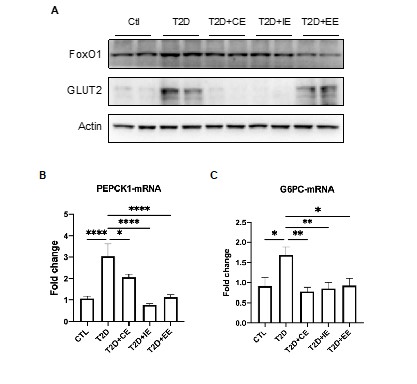
FoxO1 has been tightly linked with hepatic gluconeogenesis through inhibiting the transcription of gluconeogenesis-related PEPCK and G6Pase expression (1, 2). Herein, we found the expression of FoxO1 increased in the diabetic group but reduced in the CE, IE and EE groups (Fig. X1A, Fig.5E-F in manuscript). Meanwhile, the mRNA level of Pepck and G6PC (one of the three G6Pase catalytic-subunit-encoding genes) also decreased in the CE, IE, and EE groups (Fig. X1B-1C, Fig.5H-I in manuscript). These results indicates that these three modes of exercise all inhibited gluconeogenesis through down-regulating FoxO1.
For the glucose uptake, we detected the protein expression of GLUT2 in the liver tissue. Glut2 helps in the uptake of glucose by the hepatocytes for glycolysis and glycogenesis. Accordingly, we found GLUT2,a glucose sensor in liver, was up-regulated in diabetic rats, but down-regulated by the CE and IE intervention. However, GLUT2 didn’t decrease in the EE group, which is consistent with the results of the unimproved blood glucose by EE intervention (Figure X1A, Fig.5E and 5G in manuscript).
Taken together, moderate exercise could benefits glucose control through increasing glycolysis and decreasing gluconeogenesis. We added this part in Page 9 line 251-263 and Figure 5E-5I in this version.
Figure X1. A. Representative protein level and quantitative analysis of FOXO1 (82 kDa), GLUT2 (60-70 kDa) and Actin (45 kDa) in the rats in the Ctl, T2D, T2D + CE, T2D + IE and T2D + EE groups. C-D. Expression of hepatic Pepck and G6PC mRNA in the Ctl, T2D, T2D + CE, T2D + IE and T2D + EE groups were evaluated by real-time PCR analysis. Values represent mean ratios of Pepck and G6PC transcripts normalized to GAPDH transcript levels.
- Very recently sestrin2 signaling is assumed significant attention in relation to exercise and antioxidant responses. Therefore, authors should profile the sestrin2 levels as it is linked to several targets such as mTOR, AMPK and Sirt1. Additionally, the levels of Nrf2 should be reported as this is the central regulator of the threshold mechanisms of oxidative stress and ROS generation.
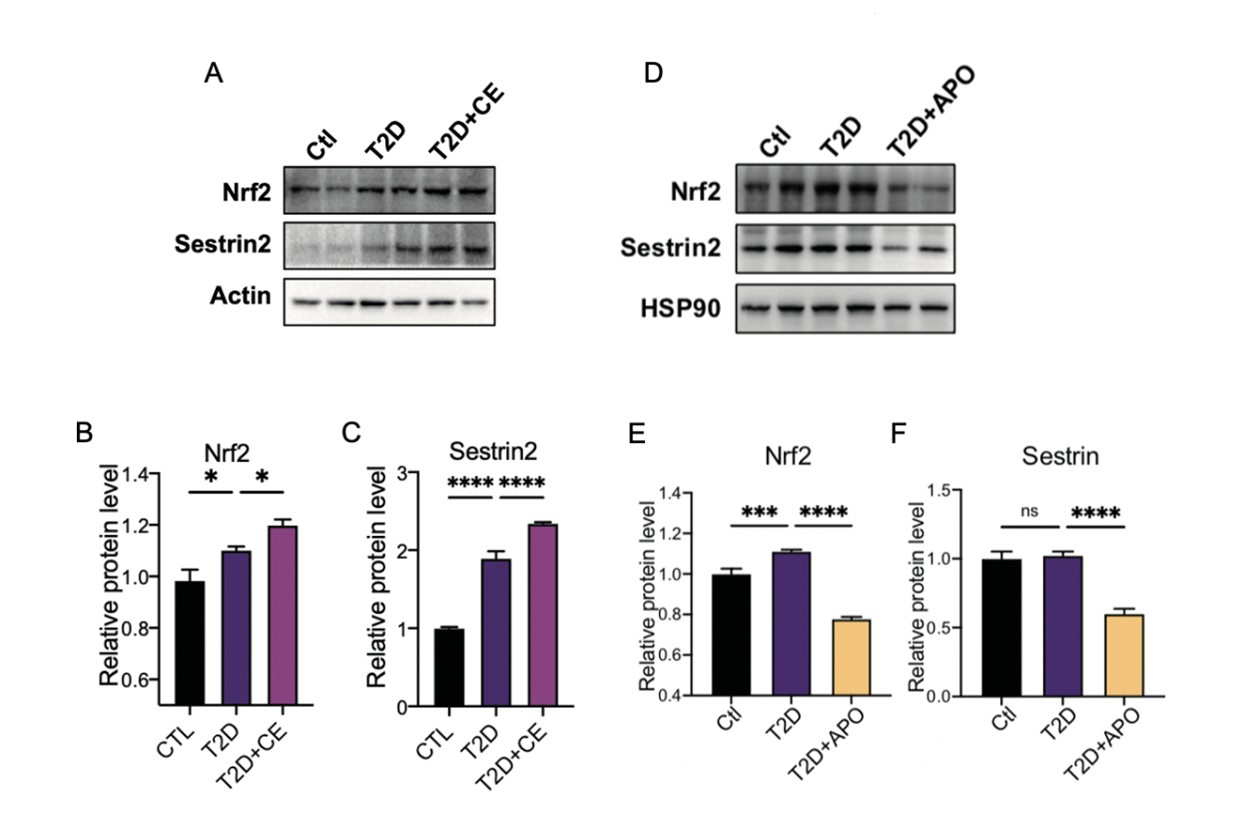
We appreciate reviewer’s expert comments. Nrf2 is an important mediator of antioxidant signaling, playing a fundamental role in maintaining the redox homeostasis of the cell. Under unstressed conditions, Nrf2 activity is suppressed by its innate repressor Kelch-like ECH-associated protein 1 (Keap1) (3). With the increase of ROS level in the development of diabetes, Nrf2 was activated to induce the transcription of several antioxidant enzymes (4, 5).
Nrf2 expression level has been reported to increase in HFD mice or diabetic patients (6, 7). It has been found from in vitro studies that NRF2 activation is achieved with acute exposure to high glucose, whereas longer incubation times or oscillating glucose concentration failed to activate Nrf2 (8, 9). These suggest that the increase of ROS in diabetes can cause compensatory upregulation of Nrf2. In our study, we found that Nrf2 increased in diabetic rats, which can further initiate the expression of antioxidant enzymes. As shown in Fig.X2A (Fig.2H-2K in manuscript), Grx and Trx involved in thioredoxin metabolism were up-regulated accordingly like Nrf2. After CE intervention, the level of Nrf2 increased further more (Fig.2E-2F), suggesting that CE intervention could activate antioxidant system to achieve a high-level redox balance. We have added these new results into Figure 2.
On the other hand, the expression level of Sestrin2 and Nrf2 decreased after antioxidant supplement. Our results suggest that the antioxidant treatment improved the diabetes through inhibiting ROS level to achieve a low-level redox balance, but moderate exercise enhanced ROS tolerance to achieve a high-level balance (Fig.X2D-F, Fig.3E-3G in manuscript).
We added the new data in “Page 5 line 147-153 and Page 7 line 183-186” and Figure 2-3 in current version.
Figure X2. A-C. Representative protein level and quantitative analysis of Nrf2 (97 kDa), Sestrin2 (57 kDa) and Actin (45 kDa) in the rats in the Ctl, T2D and T2D + CE groups. D-F. Representative protein level and quantitative analysis of Nrf2 (97 kDa), Sestrin2 (57 kDa) and HSP90 (90 kDa) in the rats in the Ctl, T2D and T2D + APO groups.
- Authors should discuss the exercise-associated hormesis curve. They should discuss whether moderate exercise could decrease the sensitivity to oxidative stress by altering the bell-shaped dose-response curve.
We thank the reviewer’s valuable comments. According to literatures, Zsolt Radak et al proposed a bell-shaped dose-response curve between normal physiological function and level of ROS in healthy individuals, and suggested that moderate exercise can extend or stretch the levels of ROS while increases the physiological function (10). Our results validated this hypothesis and further proposed that moderate exercise could produce ROS meanwhile increase antioxidant enzyme activity to maintain high level redox balance according to the Bell-shaped curve, whereas excessive exercise would generate a higher level of ROS, leading to reduced physiological function. In this study, we found the state of diabetic individuals is more applicable to the description of a S-shaped curve, due to the high level of oxidative stress and decreased reduction level in diabetic individuals (Fig.8B). With the increase of ROS, the physiological function of diabetic individuals gradually decreases and enters a state of redox imbalance. Moderate exercise shifts the S-shaped curve into a bell-shaped dose-response curve, thus reducing the sensitivity to oxidative stress in diabetic individuals and restoring redox homeostasis. However, with excessive exercise, ROS production increases beyond the threshold range of redox balance, resulting in decreased physiological function (Fig.8B, see the decreasing portion of the bell curve to the right of the apex).
Nevertheless, the antioxidant intervention increased physiological activity by reducing ROS levels in diabetic individuals, restoring a bell-shaped dose-response curve at low level of ROS (Fig.8B). Therefore, redox balance could be achieved either at low level of ROS mediated by antioxidant intervention or at high level of ROS mediated by moderate exercise, both of which were regulated by AMPK activation. Therefore, both high and low levels of redox balance can lead to high physiological function as long as they are in the redox balance threshold range. Then, the activation of AMPK is an important sign of exercise or antioxidant intervention to obtain redox dynamic balance which helps restore physiological function. Accordingly, we speculate that the antioxidant intervention based on moderate exercise might offset the effect of exercise, but antioxidants could be beneficial during excessive exercise. The human study also supports that supplementation with antioxidants may preclude the health-promoting effects of exercise (11). Therefore, personalized intervention with respect to redox balance will be crucial for the effective treatment of diabetes patients.
We added this part into “Discussion” in this version (Page 13-14 line 389-418).
- It would not be ideal to single-out AMPK as a sole biomarker in this manuscript. Instead, authors should consider AMPK activation and associated signaling in relation to redox balance. This should also be presented in Fig 7.
We thank reviewer’s critical comments. According to the comments, we have discussed the AMPK signaling in the discussion part (Page 13, line 373-384) and added the AMPK signaling in Fig.8A.
Reference:
- R. A. Haeusler, K. H. Kaestner, D. Accili, FoxOs function synergistically to promote glucose production. J Biol Chem 285, 35245-35248 (2010).
- J. Nakae, T. Kitamura, D. L. Silver, D. Accili, The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 108, 1359-1367 (2001).
- M. McMahon, K. Itoh, M. Yamamoto, J. D. Hayes, Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 278, 21592-21600 (2003).
- R. S. Arnold et al., Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A 98, 5550-5555 (2001).
- J. M. Lee, M. J. Calkins, K. Chan, Y. W. Kan, J. A. Johnson, Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem 278, 12029-12038 (2003).
- T. Jiang et al., The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59, 850-860 (2010).
- X. H. Wang et al., High Fat Diet-Induced Hepatic 18-Carbon Fatty Acids Accumulation Up-Regulates CYP2A5/CYP2A6 via NF-E2-Related Factor 2. Front Pharmacol 8, 233 (2017).
- T. S. Liu et al., Oscillating high glucose enhances oxidative stress and apoptosis in human coronary artery endothelial cells. J Endocrinol Invest 37, 645-651 (2014).
- Z. Ungvari et al., Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol 300, H1133-1140 (2011).
- Z. Radak et al., Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol 12, 285-290 (2017).
- M. Ristow et al., Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106, 8665-8670 (2009).
-

Evaluation Summary:
There is a debate whether ROS (reactive oxygen species) generated through redox signaling could be a friend or foe. There are several paradoxical studies (both animal and human) wherein exercise health benefits were reported to be accompanied by increases in ROS generation. Utilizing the in-vitro studies as well as mice model work, this manuscript illustrates the different regulatory mechanisms of exercise and antioxidant intervention on redox balance and blood glucose level in diabetes. The manuscript does address some advancements in the area of research specialization.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
Redox signaling is a dynamic and concerted orchestra of inter-connected cellular pathways. There is always a debate whether ROS (reactive oxygen species) could be a friend or foe. Continued research is needed to dissect out how ROS generation and progression could diverge in physiological versus pathophysiological states. Similarly, there are several paradoxical studies (both animal and human) wherein exercise health benefits were reported to be accompanied by increases in ROS generation. It is in this context, that the present manuscript deserves attention.
Utilizing the in-vitro studies as well as mice model work, this manuscript illustrates the different regulatory mechanisms of exercise and antioxidant intervention on redox balance and blood glucose level in diabetes. The manuscript does have some …
Reviewer #1 (Public Review):
Redox signaling is a dynamic and concerted orchestra of inter-connected cellular pathways. There is always a debate whether ROS (reactive oxygen species) could be a friend or foe. Continued research is needed to dissect out how ROS generation and progression could diverge in physiological versus pathophysiological states. Similarly, there are several paradoxical studies (both animal and human) wherein exercise health benefits were reported to be accompanied by increases in ROS generation. It is in this context, that the present manuscript deserves attention.
Utilizing the in-vitro studies as well as mice model work, this manuscript illustrates the different regulatory mechanisms of exercise and antioxidant intervention on redox balance and blood glucose level in diabetes. The manuscript does have some limitations and might need additional experiments and explanation.
The authors should consider addressing the following comments with additional experiments.
1. Although hepatic AMPK activation appears to be a central signaling element for the benefits of moderate exercise and glucose control, additional signals (on hepatic tissue) related to hepatic gluconeogenesis such as Forkhead box O1 (FoxO1), phosphoenolpyruvate carboxykinase (PEPCK), and GLUT2 needs to be profiled to present a holistic approach. Authors should consider this and revise the manuscript.
2. Very recently sestrin2 signaling is assumed significant attention in relation to exercise and antioxidant responses. Therefore, authors should profile the sestrin2 levels as it is linked to several targets such as mTOR, AMPK and Sirt1. Additionally, the levels of Nrf2 should be reported as this is the central regulator of the threshold mechanisms of oxidative stress and ROS generation.
3. Authors should discuss the exercise-associated hormesis curve. They should discuss whether moderate exercise could decrease the sensitivity to oxidative stress by altering the bell-shaped dose-response curve.
4. It would not be ideal to single-out AMPK as a sole biomarker in this manuscript. Instead, authors should consider AMPK activation and associated signaling in relation to redox balance. This should also be presented in Fig 7. -

Reviewer #2 (Public Review):
The authors compared the different redox mechanisms between exercise and antioxidant interventions, as two opposite approaches, to achieve the regulation of diabetes. This study presents a combination of in vitro and in vivo experiments to support the redox- activation of AMPK could be the potential explanation for both exercise and antioxidant intervention in improving diabetes. Considering that excessive exercise is hard to define, identifying an endogenous indicator that can reflect the range of appropriate exercise is novel and interesting. In addition, these are important findings that shed light on the effect of exercise and redox balance in the liver, which is not getting enough attention in redox biology studies in general (mainly focusing on muscle). Overall, the manuscript is sufficiently concise …
Reviewer #2 (Public Review):
The authors compared the different redox mechanisms between exercise and antioxidant interventions, as two opposite approaches, to achieve the regulation of diabetes. This study presents a combination of in vitro and in vivo experiments to support the redox- activation of AMPK could be the potential explanation for both exercise and antioxidant intervention in improving diabetes. Considering that excessive exercise is hard to define, identifying an endogenous indicator that can reflect the range of appropriate exercise is novel and interesting. In addition, these are important findings that shed light on the effect of exercise and redox balance in the liver, which is not getting enough attention in redox biology studies in general (mainly focusing on muscle). Overall, the manuscript is sufficiently concise and well structured.
-