Optogenetics and electron tomography for structure-function analysis of cochlear ribbon synapses
Curation statements for this article:-
Curated by eLife
eLife assessment
This is an important and methodologically compelling paper that reports the first application of optogenetics to inner hair cell ribbon exocytosis mechanisms in the inner ear and rapid flash-and-freeze techniques to a ribbon synapse. The conclusions of the paper are mostly well supported by the data and it will capture the interests of a broad audience of neurobiologists and sensory physiologists. Paired recordings of inner hair cells and afferents validate the optogenetic protocols of stimulation. A surprising finding is the nearly complete absence of docked vesicles at rest and after stimulation, but upon stimulation vesicles rapidly associate with the ribbon. The reviewers agreed that this is a high-quality study, but that some additional work is needed to address certain pitfalls of the methods used and to rule out alternative explanations of the data.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Ribbon synapses of cochlear inner hair cells (IHCs) are specialized to indefatigably transmit sound information at high rates. To understand the underlying mechanisms, structure-function analysis of the active zone (AZ) of these synapses is essential. Previous electron microscopy studies of synaptic vesicle (SV) dynamics at the IHC AZ used potassium stimulation, which limited the temporal resolution to minutes. Here, we established optogenetic IHC stimulation followed by quick freezing within milliseconds and electron tomography to study the ultrastructure of functional synapse states with good temporal resolution in mice. We characterized optogenetic IHC stimulation by patch-clamp recordings from IHCs and postsynaptic boutons revealing robust IHC depolarization and neurotransmitter release. Ultrastructurally, the number of docked SVs increased upon short (17–25 ms) and long (48–76 ms) light stimulation paradigms. We did not observe enlarged SVs or other morphological correlates of homotypic fusion events. Our results indicate a rapid recruitment of SVs to the docked state upon stimulation and suggest that univesicular release prevails as the quantal mechanism of exocytosis at IHC ribbon synapses.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
Chakrabarti et al study inner hair cell synapses using electron tomography of tissue rapidly frozen after optogenetic stimulation. Surprisingly, they find a nearly complete absence of docked vesicles at rest and after stimulation, but upon stimulation vesicles rapidly associate with the ribbon. Interestingly, no changes in vesicle size were found along or near the ribbon. This would have indicated a process of compound fusion prior to plasma membrane fusion, as proposed for retinal bipolar cell ribbons. This lack of compound fusion is used to argue against MVR at the IHC synapse. However, that is only one form of MVR. Another form, coordinated and rapid fusion of multiple docked vesicles at the bottom of the ribbon, is not ruled out. Therefore, I agree that the data set provides good …
Author Response:
Reviewer #1 (Public Review):
Chakrabarti et al study inner hair cell synapses using electron tomography of tissue rapidly frozen after optogenetic stimulation. Surprisingly, they find a nearly complete absence of docked vesicles at rest and after stimulation, but upon stimulation vesicles rapidly associate with the ribbon. Interestingly, no changes in vesicle size were found along or near the ribbon. This would have indicated a process of compound fusion prior to plasma membrane fusion, as proposed for retinal bipolar cell ribbons. This lack of compound fusion is used to argue against MVR at the IHC synapse. However, that is only one form of MVR. Another form, coordinated and rapid fusion of multiple docked vesicles at the bottom of the ribbon, is not ruled out. Therefore, I agree that the data set provides good evidence for rapid replenishment of the ribbon-associated vesicles, but I do not find the evidence against MVR convincing. The work provides fundamental insight into the mechanisms of sensory synapses.
We thank the reviewer for the appreciation of our work and the constructive comments. As pointed out below, we now included this discussion (from line 679 onwards).
We wrote:
“This might reflect spontaneous univesicular release (UVR) via a dynamic fusion pore (i.e. ‘kiss and run’, (Ceccarelli et al., 1979), which was suggested previously for IHC ribbon synapses (Chapochnikov et al., 2014; Grabner and Moser, 2018; Huang and Moser, 2018; Takago et al., 2019) and/or and rapid undocking of vesicles (e.g. Dinkelacker et al., 2000; He et al., 2017; Nagy et al., 2004; Smith et al., 1998). In the UVR framework, stimulation by ensuing Ca2+ influx triggers the statistically independent release of several SVs. Coordinated multivesicular release (MVR) has been indicated to occur at hair cell synapses (Glowatzki and Fuchs, 2002; Goutman and Glowatzki, 2007; Li et al., 2009) and retinal ribbon synapses (Hays et al., 2020; Mehta et al., 2013; Singer et al., 2004) during both spontaneous and evoked release. We could not observe structures which might hint towards compound or cumulative fusion, neither at the ribbon nor at the AZ membrane under our experimental conditions. Upon short and long stimulation, RA-SVs as well as docked SVs even showed a slightly reduced size compared to controls. However, since some AZs harbored more than one docked SV per AZ in stimulated conditions, we cannot fully exclude the possibility of coordinated release of few SVs upon depolarization.”
Reviewer #2 (Public Review):
Chakrabarti et al. aimed to investigate exocytosis from ribbon synapses of cochlear inner hair cells with high-resolution electron microscopy with tomography. Current methods to capture the ultrastructure of the dynamics of synaptic vesicle release in IHCs rely on the application of potassium for stimulation, which constrains temporal resolution to minutes rather than the millisecond resolution required to analyse synaptic transmission. Here the authors implemented a high-pressure freezing method relying on optogenetics for stimulation (Opto-HPF), granting them both high spatial and temporal resolutions. They provide an extremely well-detailed and rigorously controlled description of the method, falling in line with previously use of such "Opto-HPF" studies. They successfully applied Opto-HPF to IHCs and had several findings at this highly specialised ribbon synapse. They observed a stimulation-dependent accumulation of docked synaptic vesicles at IHC active-zones, and a stimulation-dependent reduction in the distance of non-docked vesicles to the active zone membrane; while the total number of ribbon-associated vesicles remained unchanged. Finally, they did not observe increases in diameter of synaptic vesicles proximal to the active zone, or other potential correlates to compound fusion - a potential mode of multivesicular release. The conclusions of the paper are mostly well supported by data, but some aspects of their findings and pitfalls of the methods should be better discussed.
We thank the reviewer for the appreciation of our work and the constructive comments.
Strengths:
While now a few different groups have used "Opto-HPF" methods (also referred to as "Flash and Freeze) in different ways and synapses, the current study implemented the method with rigorous controls in a novel way to specifically apply to cochlear IHCs - a different sample preparation than neuronal cultures, brain slices or C. elegans, the sample preparations used so far. The analysis of exocytosis dynamics of IHCs with electron microscopy with stimulation has been limited to being done with the application of potassium, which is not physiological. While much has been learned from these methods, they lacked time resolution. With Opto-HPF the authors were successfully able to investigate synaptic transmission with millisecond precision, with electron tomography analysis of active zones. I have no overall questions regarding the methodology as they were very thoroughly described. The authors also employed electrophysiology with optogenetics to characterise the optical simulation parameters and provided a well described analysis of the results with different pulse durations and irradiance - which is crucial for Opto-HPF.
Thank you very much.
Further, the authors did a superb job in providing several tables with data and information across all mouse lines used, experimental conditions, and statistical tests, including source code for the diverse analysis performed. The figures are overall clear and the manuscript was well written. Such a clear representation of data makes it easier to review the manuscript.
Thank you very much.
Weaknesses:
There are two main points that I think need to be better discussed by the authors.
The first refers to the pitfalls of using optogenetics to analyse synaptic transmission. While ChR2 provides better time resolution than potassium application, one cannot discard the possibility that calcium influx through ChR2 alters neurotransmitter release. This important limitation of the technique should be properly acknowledged by the authors and the consequences discussed, specifically in the context in which they applied it: a single sustained pulse of light of ~20ms (ShortStim) and of ~50ms (LongStim). While longer, sustained stimulation is characteristic for IHCs, these are quite long pulses as far as optogenetics and potential consequences to intrinsic or synaptic properties.
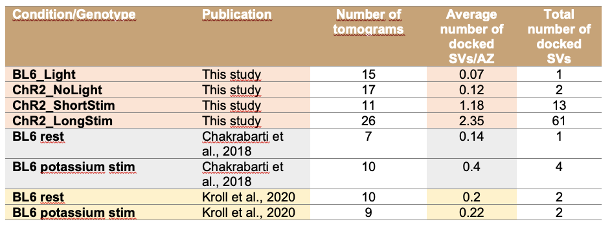
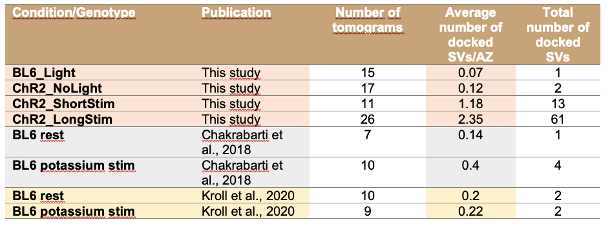
We thank the reviewer for pointing this out. We would like to mention that upon 15 min high potassium depolarization, the number of docked SVs only slightly increased as shown in Chakrabarti et al., 2018, EMBO rep and Kroll et al. 2020 JCS, but it was not statistically significant. In the current study, we report a similar phenomenon, but here light induced depolarization resulted in a more robust increase in the number of docked SVs.
To compare the data from the previous studies with the current study, we included an additional table 3 (line 676) now in the discussion with all total counts (and average per AZ) of docked SVs.
Furthermore, in response to the reviewers’ concern, we now discuss the Ca2+ permeability of ChR2 in addition to the above comparison to our previous studies that demonstrated very few docked SVs in the absence of K+ channel blockers and ChR2 expression in IHCs. We are not entirely certain, if the reviewer refers to potential dark currents of ChR2 (e.g. as an explanation for a depletion of docked vesicles under non-stimulated conditions) or to photocurrents, the influx of Ca2+ through ChR2 itself, and their contribution to Ca2+ concentration at the active zone.
However, regardless this, we consider it unlikely that a potential contribution of Ca2+ influx via ChR2 evokes SV fusion at the hair cell active zone.
First of all, we note that the Ca2+ affinity of IHC exocytosis is very low. As first shown in Beutner et al., 2001 and confirmed thereafter (e.g. Pangrsic et al., 2010), there is little if any IHC exocytosis for Ca2+ concentrations at the release sites below 10 µM. Two studies using CatCh (a ChR2 mutant with higher Ca2+ permeability than wildtype ChR2 (Kleinlogel et al., 2011; Mager et al., 2017) estimated a max intracellular Ca2+ increase below 10 µM, even at very negative potentials that promote Ca2+ influx along the electrochemical gradient or at high extracellular Ca2+ concentrations of 90 mM. In our experiments, IHCs were depolarized, instead, to values for which extrapolation of the data of Mager et al., 2017 indicate a submicromolar Ca2+ concentration. In addition, we and others have demonstrated powerful Ca2+ buffering and extrusion in hair cells (e.g. Tucker and Fettiplace, 1995; Issa and Hudspeth., 1996; Frank et al., 2009 Pangrsic et al., 2015). As a result, the hair cells efficiently clear even massive synaptic Ca2+ influx and establish a low bulk cytosolic Ca2+ concentration (Beutner and Moser, 2001; Frank et al., 2009). We reason that these clearance mechanisms efficiently counter any Ca2+ influx through ChR2. This will likely limit potential effects of ChR2 mediated Ca2+ influx on Ca2+ dependent replenishment of synaptic vesicles during ongoing stimulation.
We have now added the following in the discussion (starting in line 620):
“We note that ChR2, in addition to monovalent cations, also permeates Ca2+ ions and poses the question whether optogenetic stimulation of IHCs could trigger release due to direct Ca2+ influx via the ChR2. We do not consider such Ca2+ influx to trigger exocytosis of synaptic vesicles in IHCs. Optogenetic stimulation of HEK293 cells overexpressing ChR2 (wildtype version) only raises the intracellular Ca2+ concentration up to 90 nM even with an extracellular Ca2+ concentration of 90 mM (Kleinlogel et al., 2011). IHC exocytosis shows a low Ca2+ affinity (~70 µM, Beutner et al., 2001) and there is little if any IHC exocytosis for Ca2+ concentrations below 10 µM, which is far beyond what could be achieved even by the highly Ca2+ permeable ChR2 mutant (CatCh: Ca2+ translocating channelrhodopsin, Mager et al., 2017). In addition, we reason that the powerful Ca2+ buffering and extrusion by hair cells (e.g., Frank et al., 2009; Issa and Hudspeth, 1996; Pangršič et al., 2015; Tucker and Fettiplace, 1995) will efficiently counter Ca2+ influx through ChR2 and, thereby limit potential effects on Ca2+ dependent replenishment of synaptic vesicles during ongoing stimulation. “
The second refers to the finding that the authors did not observe evidence of compound fusion (or homotypic fusion) in their data. This is an interesting finding in the context of multivesicular release in general, as well as specifically for IHCs. While the authors discussed the potential for "kiss-and-run" and/or "kiss-and-stay", it would be valuable if they could discuss their findings further in the context of the field for multivesicular release. For example, the evidence in support of the potential of multiple independent release events. Further, as far as such function-structure optical-quick-freezing methods, it is not unusual to not capture fusion events (so-called omega-shapes or vesicles with fusion pores); this is largely because these are very fast events (less than 10 ms), and not easily captured with optical stimulation.
We agree with the reviewer that the discussion on MVR and UVR should be extended. We now added the following paragraph to the discussion from line 679 on:
“This might reflect spontaneous univesicular release (UVR) via a dynamic fusion pore (i.e. ‘kiss and run’, (Ceccarelli et al., 1979), which was suggested previously for IHC ribbon synapses (Chapochnikov et al., 2014; Grabner and Moser, 2018; Huang and Moser, 2018; Takago et al., 2019) and/or and rapid undocking of vesicles (e.g. Dinkelacker et al., 2000; He et al., 2017; Nagy et al., 2004; Smith et al., 1998). In the UVR framework, stimulation by ensuing Ca2+ influx triggers the statistically independent release of several SVs. Coordinated multivesicular release (MVR) has been indicated to occur at hair cell synapses (Glowatzki and Fuchs, 2002; Goutman and Glowatzki, 2007; Li et al., 2009) and retinal ribbon synapses (Hays et al., 2020; Mehta et al., 2013; Singer et al., 2004) during both spontaneous and evoked release. We could not observe structures which might hint towards compound or cumulative fusion, neither at the ribbon nor at the AZ membrane under our experimental conditions. Upon short and long stimulation, RA-SVs as well as docked SVs even showed a slightly reduced size compared to controls. However, since some AZs harbored more than one docked SV per AZ in stimulated conditions, we cannot fully exclude the possibility of coordinated release of few SVs upon depolarization.”
Reviewer #3 (Public Review):
Precise methods were developed to validate the expression of channelrhodopsin in inner hair cells of the Organ of Corti, to quantify the relationship between blue light irradiance and auditory nerve fiber depolarization, to control light stimulation within the chamber of a high-pressure freezing device, and to measure with good precision the delay between stimulation and freezing of the specimen. These methods represent a clear advance over previous experimental designs used to study this synaptic system and are an initial application of rapid high-pressure freezing with freeze substitution, followed by high-resolution electron tomography (ET), to sensory cells that operate via graded potentials.
Short-duration stimuli were used to assess the redistribution of vesicles among pools at hair cell ribbon synapses. The number of vesicles linked to the synaptic ribbon did not change, but vesicles redistributed within the membrane-proximal pool to docked locations. No evidence was found for vesicle-to-vesicle fusion prior to vesicle fusion to the membrane, which is an important, ongoing question for this synapse type. The data for quantifying numbers of vesicles in membrane-tethered, non-tethered, and docked vesicle pools are compelling and important.
We thank the reviewer for the appreciation of our work and the constructive comments.
These quantifications would benefit from additional presentation of raw images so that the reader can better assess their generality and variability across synaptic sites.
The images shown for each of the two control and two experimental (stimulated) preparation classes should be more representative. Variation in synaptic cleft dimensions and numbers of ribbon-associated and membrane-proximal vesicles do not track the averaged data. Since the preparation has novel stimulus features, additional images (as the authors employed in previous publications) exhibiting tethered vesicles, non-tethered vesicles, docked vesicles, several sections through individual ribbons, and the segmentation of these structures, will provide greater confidence that the data reflect the images.
Thank you very much for pointing this out. We now included more details in supplemental figures and in the text.
Precisely, we added:
More details about the morphological sub-pools (analysis and images):
-We now show a sequence of images with different tethering states of membrane proximal SVs together with examples for docked and non-tethered SVs as we did in Chakrabarti et al., 2018 for each condition (Fig. 6-figure supplement 2, line 438). Moreover, we included for each condition additional information, we selected further tomograms, one per condition, and depict two additional virtual sections: Fig. 6-figure supplement 2.
-Moreover, we present a more detailed quantification for the different morphological sub-pools: For the MP-SV pool, we analyzed the SV diameters and the distances to the AZ membrane and PD of different SV sub-pools separately, we now included this information in Fig. 7 For the RA-SVs, we analyzed in addition the morphological sub-pools and the SV diameters in the distal and the proximal ribbon part as done in Chakrabarti et al. 2018. We now added a new supplement figure (Fig. 7-figure supplement 2, line 558 and a supplementary file 2).
We replaced the virtual section in panel 6D: In the old version, it appeared that the ribbon was contacting the membrane and we realized that this virtual section was not representative: actually, the ribbon was not directly contacting the AZ membrane, a presynaptic density was still visible adjacent to the docked SVs. To avoid potential confusion, we selected a different virtual section of the same tomogram and now indicated the presynaptic density also as graphical aid in Fig. 6.
The introduction raises questions about the length of membrane tethers in relation to vesicle movement toward the active zone, but this topic was not addressed in the manuscript.
We apologize for not stating it sufficiently clear, we now rephrased this sentence. We now wrote:
“…and seem to be organized in sub-pools based on the number of tethers and to which structure these tethers are connected. “
Seemingly quantification of this metric, and the number of tethers especially for vesicles near the membrane, is straightforward. The topic of EPSC amplitude as representing unitary events due to variation in vesicle volume, size of the fusion pore, or vesicle-vesicle fusion was partially addressed. Membrane fusion events were not evident in the few images shown, but these presumably occurred and could be quantified. Likewise, sites of membrane retrieval could also be marked. These analyses will broaden the scope of the presentation, but also contribute to a more complete story.
Regarding the presence/absence of membrane fusion events we agree with the reviewer that this should be clearly addressed in the MS. We would like to point out that we
(i) did not observe any omega shapes at the AZ membrane, which we also mention in the MS. We can also report that we could not see them in data sets from previous publications (Vogl et al., 2015, JCS; Jung et al., 2015, PNAS).
(ii) To be clear on our observations on potential SV-SV fusion events we now point out in the discussion from line 688ff:
“We could not observe structures which might hint towards compound or cumulative fusion, neither at the ribbon nor at the AZ membrane under our experimental conditions. Upon short and long stimulation, RA-SVs as well as docked SVs even showed a slightly reduced size compared to controls. However, since some AZs harbored more than one docked SV per AZ in stimulated conditions, we cannot fully exclude the possibility of coordinated release of few SVs upon depolarization.”
Furthermore, we agree with the reviewer that a complete presentation of endo-exocytosis structural correlates is very important. However, we focused our study on exocytosis events and therefore mainly analyzed membrane proximal SVs at active zones.
Nonetheless, in response to the reviewer’s comment, we now included a quantification of clathrin-coated (CC) structures. We determined the appearance of CC vesicles (V) and CC invaginations within 0-500 nm away from the PD. We measured the diameter of the CCV, and their distance to the membrane and the PD. We only found very few CC structures in our tomograms (now added in a table to the result section (Supplementary file 1). Sites for endocytic membrane retrieval likely are in the peri-active zone area or even beyond. We did not observe obvious bulk endocytosis events that were connected to the AZ membrane. However, we do observe large endosomal like vesicles that we did not quantify in this study. More details were presented in two of our previous studies: Kroll et al., 2019 and 2020, however, under different stimulation conditions.
Overall, the methodology forms the basis for future studies by this group and others to investigate rapid changes in synaptic vesicle distribution at this synapse.
Reviewer #4 (Public Review):
This manuscript investigates the process of neurotransmitter release from hair cell synapses using electron microscopy of tissue rapidly frozen after optogenetic stimulation. The primary finding is that in the absence of a stimulus very few vesicles appear docked at the membrane, but upon stimulation vesicles rapidly associate with the membrane. In contrast, the number of vesicles associated with the ribbon and within 50 nm of the membrane remains unchanged. Additionally, the authors find no changes in vesicle size that might be predicted if vesicles fuse to one-another prior to fusing with the membrane. The paper claims that these findings argue for rapid replenishment and against a mechanism of multi-vesicular release, but neither argument is that convincing. Nonetheless, the work is of high quality, the results are intriguing, and will be of interest to the field.
We thank the reviewer for the appreciation of our work and the constructive comments.
- The abstract states that their results "argue against synchronized multiquantal release". While I might agree that the lack of larger structures is suggestive that homotypic fusion may not be common, this is far from an argument against any mechanisms of multi-quantal release. At least one definition of synchronized multiquantal release posits that multiple vesicles are fusing at the same time through some coordinated mechanism. Given that they do not report evidence of fusion itself, I fail to see how these results inform us one way or the other.
We agree with the reviewer that the discussion on MVR and UVR should be extended. It is important to point out that we do not claim that the evoked release is mediated by one single SV. As discussed in the paper (line 672), we consider that our optogenetic stimulation of IHCs triggers the release of more than 10 SVs per AZ. This falls in line with the previous reports of several SVs fusing upon stimulation. This type of evoked MVR is probably mediated by the opening of Ca2+ channels in close proximity to each SV Ca2+ sensor. We indeed sometimes observed more than one docked SV per AZ upon long optogenetic stimulation. This could reflect that possibility. However, given the absence of large structures directly at the ribbon or the AZ membrane that could suggest the compound fusion of several SVs prior or during fusion, we argue against compound MVR release at IHCs. As mentioned above, we added to the discussion (from line 679 onwards).
We wrote:
“This might reflect spontaneous univesicular release (UVR) via a dynamic fusion pore (i.e. ‘kiss and run’, (Ceccarelli et al., 1979), which was suggested previously for IHC ribbon synapses (Chapochnikov et al., 2014; Grabner and Moser, 2018; Huang and Moser, 2018; Takago et al., 2019) and/or and rapid undocking of vesicles (e.g. Dinkelacker et al., 2000; He et al., 2017; Nagy et al., 2004; Smith et al., 1998). In the UVR framework, stimulation by ensuing Ca2+ influx triggers the statistically independent release of several SVs. Coordinated multivesicular release (MVR) has been indicated to occur at hair cell synapses (Glowatzki and Fuchs, 2002; Goutman and Glowatzki, 2007; Li et al., 2009) and retinal ribbon synapses (Hays et al., 2020; Mehta et al., 2013; Singer et al., 2004) during both spontaneous and evoked release. We could not observe structures which might hint towards compound or cumulative fusion, neither at the ribbon nor at the AZ membrane under our experimental conditions. Upon short and long stimulation, RA-SVs as well as docked SVs even showed a slightly reduced size compared to controls. However, since some AZs harbored more than one docked SV per AZ in stimulated conditions, we cannot fully exclude the possibility of coordinated release of few SVs upon depolarization.”
- The complete lack of docked vesicles in the absence of a stimulus followed by their appearance with a stimulus is a fascinating result. However, since there are no docked vesicles prior to a stimulus, it is really unclear what these docked vesicles represent - clearly not the RRP. Are these vesicles that are fusing or recently fused or are they ones preparing to fuse? It is fine that it is unknown, but it complicates their interpretation that the vesicles are "rapidly replenished". How does one replenish a pool of docked vesicles that didn't exist prior to the stimulus?
In response to the reviewers’ comment, we would like to note that we indeed reported very few docked SVs in wild type IHCs at resting conditions without K+ channel blockers in Chakrabarti et al. EMBO Rep 2018 and in Kroll et al., 2020, JCS. In both studies, a solution without TEA and Cs was used for the experiments (resting solution Chakrabarti: 5 mM KCl, 136.5 mM NaCl, 1 mM MgCl2, 1.3 mM CaCl2, 10 mM HEPES, pH 7.2, 290 mOsmol; control solution Kroll: 5.36 mM KCl, 139.7 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 0.5 mM MgSO4, 10 mM HEPES, 3.4 mM L-glutamine, and 6.9 mM D-glucose, pH 7.4). Similarly, our current study shows very few docked SVs in the resting condition even in the presence of TEA and Cs. Based on the results presented in ‘Response to reviewers Figure 1’, we assume that the scarcity of docked SVs under control conditions is not due to depolarization induced by a solution containing 20 mM TEA and 1 mM Cs but is rather representative for the physiological resting state of IHC ribbon synapses. Upon 15 min high potassium depolarization, the number of docked SVs only slightly increased as shown in Chakrabarti et al., 2018 and Kroll et al. 2020, but it was not statistically significant. In the current study, we report a similar phenomenon, but here depolarization resulted in a more robust increase in the number of docked SVs.
To compare the data from the previous studies with the current study, we included an additional table 3 (line 676) now in the discussion with all total counts (and average per AZ) of docked SVs.
-

eLife assessment
This is an important and methodologically compelling paper that reports the first application of optogenetics to inner hair cell ribbon exocytosis mechanisms in the inner ear and rapid flash-and-freeze techniques to a ribbon synapse. The conclusions of the paper are mostly well supported by the data and it will capture the interests of a broad audience of neurobiologists and sensory physiologists. Paired recordings of inner hair cells and afferents validate the optogenetic protocols of stimulation. A surprising finding is the nearly complete absence of docked vesicles at rest and after stimulation, but upon stimulation vesicles rapidly associate with the ribbon. The reviewers agreed that this is a high-quality study, but that some additional work is needed to address certain pitfalls of the methods used and to rule out …
eLife assessment
This is an important and methodologically compelling paper that reports the first application of optogenetics to inner hair cell ribbon exocytosis mechanisms in the inner ear and rapid flash-and-freeze techniques to a ribbon synapse. The conclusions of the paper are mostly well supported by the data and it will capture the interests of a broad audience of neurobiologists and sensory physiologists. Paired recordings of inner hair cells and afferents validate the optogenetic protocols of stimulation. A surprising finding is the nearly complete absence of docked vesicles at rest and after stimulation, but upon stimulation vesicles rapidly associate with the ribbon. The reviewers agreed that this is a high-quality study, but that some additional work is needed to address certain pitfalls of the methods used and to rule out alternative explanations of the data.
-

Reviewer #1 (Public Review):
Chakrabarti et al study inner hair cell synapses using electron tomography of tissue rapidly frozen after optogenetic stimulation. Surprisingly, they find a nearly complete absence of docked vesicles at rest and after stimulation, but upon stimulation vesicles rapidly associate with the ribbon. Interestingly, no changes in vesicle size were found along or near the ribbon. This would have indicated a process of compound fusion prior to plasma membrane fusion, as proposed for retinal bipolar cell ribbons. This lack of compound fusion is used to argue against MVR at the IHC synapse. However, that is only one form of MVR. Another form, coordinated and rapid fusion of multiple docked vesicles at the bottom of the ribbon, is not ruled out. Therefore, I agree that the data set provides good evidence for rapid …
Reviewer #1 (Public Review):
Chakrabarti et al study inner hair cell synapses using electron tomography of tissue rapidly frozen after optogenetic stimulation. Surprisingly, they find a nearly complete absence of docked vesicles at rest and after stimulation, but upon stimulation vesicles rapidly associate with the ribbon. Interestingly, no changes in vesicle size were found along or near the ribbon. This would have indicated a process of compound fusion prior to plasma membrane fusion, as proposed for retinal bipolar cell ribbons. This lack of compound fusion is used to argue against MVR at the IHC synapse. However, that is only one form of MVR. Another form, coordinated and rapid fusion of multiple docked vesicles at the bottom of the ribbon, is not ruled out. Therefore, I agree that the data set provides good evidence for rapid replenishment of the ribbon-associated vesicles, but I do not find the evidence against MVR convincing. The work provides fundamental insight into the mechanisms of sensory synapses.
-

Reviewer #2 (Public Review):
Chakrabarti et al. aimed to investigate exocytosis from ribbon synapses of cochlear inner hair cells with high-resolution electron microscopy with tomography. Current methods to capture the ultrastructure of the dynamics of synaptic vesicle release in IHCs rely on the application of potassium for stimulation, which constrains temporal resolution to minutes rather than the millisecond resolution required to analyse synaptic transmission. Here the authors implemented a high-pressure freezing method relying on optogenetics for stimulation (Opto-HPF), granting them both high spatial and temporal resolutions. They provide an extremely well-detailed and rigorously controlled description of the method, falling in line with previously use of such "Opto-HPF" studies. They successfully applied Opto-HPF to IHCs and had …
Reviewer #2 (Public Review):
Chakrabarti et al. aimed to investigate exocytosis from ribbon synapses of cochlear inner hair cells with high-resolution electron microscopy with tomography. Current methods to capture the ultrastructure of the dynamics of synaptic vesicle release in IHCs rely on the application of potassium for stimulation, which constrains temporal resolution to minutes rather than the millisecond resolution required to analyse synaptic transmission. Here the authors implemented a high-pressure freezing method relying on optogenetics for stimulation (Opto-HPF), granting them both high spatial and temporal resolutions. They provide an extremely well-detailed and rigorously controlled description of the method, falling in line with previously use of such "Opto-HPF" studies. They successfully applied Opto-HPF to IHCs and had several findings at this highly specialised ribbon synapse. They observed a stimulation-dependent accumulation of docked synaptic vesicles at IHC active-zones, and a stimulation-dependent reduction in the distance of non-docked vesicles to the active zone membrane; while the total number of ribbon-associated vesicles remained unchanged. Finally, they did not observe increases in diameter of synaptic vesicles proximal to the active zone, or other potential correlates to compound fusion - a potential mode of multivesicular release. The conclusions of the paper are mostly well supported by data, but some aspects of their findings and pitfalls of the methods should be better discussed.
Strengths:
While now a few different groups have used "Opto-HPF" methods (also referred to as "Flash and Freeze) in different ways and synapses, the current study implemented the method with rigorous controls in a novel way to specifically apply to cochlear IHCs - a different sample preparation than neuronal cultures, brain slices or C. elegans, the sample preparations used so far. The analysis of exocytosis dynamics of IHCs with electron microscopy with stimulation has been limited to being done with the application of potassium, which is not physiological. While much has been learned from these methods, they lacked time resolution. With Opto-HPF the authors were successfully able to investigate synaptic transmission with millisecond precision, with electron tomography analysis of active zones. I have no overall questions regarding the methodology as they were very thoroughly described. The authors also employed electrophysiology with optogenetics to characterise the optical simulation parameters and provided a well described analysis of the results with different pulse durations and irradiance - which is crucial for Opto-HPF.
Further, the authors did a superb job in providing several tables with data and information across all mouse lines used, experimental conditions, and statistical tests, including source code for the diverse analysis performed. The figures are overall clear and the manuscript was well written. Such a clear representation of data makes it easier to review the manuscript.
Weaknesses:
There are two main points that I think need to be better discussed by the authors.
The first refers to the pitfalls of using optogenetics to analyse synaptic transmission. While ChR2 provides better time resolution than potassium application, one cannot discard the possibility that calcium influx through ChR2 alters neurotransmitter release. This important limitation of the technique should be properly acknowledged by the authors and the consequences discussed, specifically in the context in which they applied it: a single sustained pulse of light of ~20ms (ShortStim) and of ~50ms (LongStim). While longer, sustained stimulation is characteristic for IHCs, these are quite long pulses as far as optogenetics and potential consequences to intrinsic or synaptic properties.
The second refers to the finding that the authors did not observe evidence of compound fusion (or homotypic fusion) in their data. This is an interesting finding in the context of multivesicular release in general, as well as specifically for IHCs. While the authors discussed the potential for "kiss-and-run" and/or "kiss-and-stay", it would be valuable if they could discuss their findings further in the context of the field for multivesicular release. For example, the evidence in support of the potential of multiple independent release events. Further, as far as such function-structure optical-quick-freezing methods, it is not unusual to not capture fusion events (so-called omega-shapes or vesicles with fusion pores); this is largely because these are very fast events (less than 10 ms), and not easily captured with optical stimulation.
-

Reviewer #3 (Public Review):
Precise methods were developed to validate the expression of channelrhodopsin in inner hair cells of the Organ of Corti, to quantify the relationship between blue light irradiance and auditory nerve fiber depolarization, to control light stimulation within the chamber of a high-pressure freezing device, and to measure with good precision the delay between stimulation and freezing of the specimen. These methods represent a clear advance over previous experimental designs used to study this synaptic system and are an initial application of rapid high-pressure freezing with freeze substitution, followed by high-resolution electron tomography (ET), to sensory cells that operate via graded potentials.
Short-duration stimuli were used to assess the redistribution of vesicles among pools at hair cell ribbon …
Reviewer #3 (Public Review):
Precise methods were developed to validate the expression of channelrhodopsin in inner hair cells of the Organ of Corti, to quantify the relationship between blue light irradiance and auditory nerve fiber depolarization, to control light stimulation within the chamber of a high-pressure freezing device, and to measure with good precision the delay between stimulation and freezing of the specimen. These methods represent a clear advance over previous experimental designs used to study this synaptic system and are an initial application of rapid high-pressure freezing with freeze substitution, followed by high-resolution electron tomography (ET), to sensory cells that operate via graded potentials.
Short-duration stimuli were used to assess the redistribution of vesicles among pools at hair cell ribbon synapses. The number of vesicles linked to the synaptic ribbon did not change, but vesicles redistributed within the membrane-proximal pool to docked locations. No evidence was found for vesicle-to-vesicle fusion prior to vesicle fusion to the membrane, which is an important, ongoing question for this synapse type. The data for quantifying numbers of vesicles in membrane-tethered, non-tethered, and docked vesicle pools are compelling and important. These quantifications would benefit from additional presentation of raw images so that the reader can better assess their generality and variability across synaptic sites.
The images shown for each of the two control and two experimental (stimulated) preparation classes should be more representative. Variation in synaptic cleft dimensions and numbers of ribbon-associated and membrane-proximal vesicles do not track the averaged data. Since the preparation has novel stimulus features, additional images (as the authors employed in previous publications) exhibiting tethered vesicles, non-tethered vesicles, docked vesicles, several sections through individual ribbons, and the segmentation of these structures, will provide greater confidence that the data reflect the images.
The introduction raises questions about the length of membrane tethers in relation to vesicle movement toward the active zone, but this topic was not addressed in the manuscript. Seemingly quantification of this metric, and the number of tethers especially for vesicles near the membrane, is straightforward. The topic of EPSC amplitude as representing unitary events due to variation in vesicle volume, size of the fusion pore, or vesicle-vesicle fusion was partially addressed. Membrane fusion events were not evident in the few images shown, but these presumably occurred and could be quantified. Likewise, sites of membrane retrieval could also be marked. These analyses will broaden the scope of the presentation, but also contribute to a more complete story.
Overall, the methodology forms the basis for future studies by this group and others to investigate rapid changes in synaptic vesicle distribution at this synapse.
-

Reviewer #4 (Public Review):
This manuscript investigates the process of neurotransmitter release from hair cell synapses using electron microscopy of tissue rapidly frozen after optogenetic stimulation. The primary finding is that in the absence of a stimulus very few vesicles appear docked at the membrane, but upon stimulation vesicles rapidly associate with the membrane. In contrast, the number of vesicles associated with the ribbon and within 50 nm of the membrane remains unchanged. Additionally, the authors find no changes in vesicle size that might be predicted if vesicles fuse to one-another prior to fusing with the membrane. The paper claims that these findings argue for rapid replenishment and against a mechanism of multi-vesicular release, but neither argument is that convincing. Nonetheless, the work is of high quality, the …
Reviewer #4 (Public Review):
This manuscript investigates the process of neurotransmitter release from hair cell synapses using electron microscopy of tissue rapidly frozen after optogenetic stimulation. The primary finding is that in the absence of a stimulus very few vesicles appear docked at the membrane, but upon stimulation vesicles rapidly associate with the membrane. In contrast, the number of vesicles associated with the ribbon and within 50 nm of the membrane remains unchanged. Additionally, the authors find no changes in vesicle size that might be predicted if vesicles fuse to one-another prior to fusing with the membrane. The paper claims that these findings argue for rapid replenishment and against a mechanism of multi-vesicular release, but neither argument is that convincing. Nonetheless, the work is of high quality, the results are intriguing, and will be of interest to the field.
The abstract states that their results "argue against synchronized multiquantal release". While I might agree that the lack of larger structures is suggestive that homotypic fusion may not be common, this is far from an argument against any mechanisms of multi-quantal release. At least one definition of synchronized multiquantal release posits that multiple vesicles are fusing at the same time through some coordinated mechanism. Given that they do not report evidence of fusion itself, I fail to see how these results inform us one way or the other.
The complete lack of docked vesicles in the absence of a stimulus followed by their appearance with a stimulus is a fascinating result. However, since there are no docked vesicles prior to a stimulus, it is really unclear what these docked vesicles represent - clearly not the RRP. Are these vesicles that are fusing or recently fused or are they ones preparing to fuse? It is fine that it is unknown, but it complicates their interpretation that the vesicles are "rapidly replenished". How does one replenish a pool of docked vesicles that didn't exist prior to the stimulus?
-