Population dynamics of immunological synapse formation induced by bispecific T cell engagers predict clinical pharmacodynamics and treatment resistance
Curation statements for this article:-
Curated by eLife
eLife assessment
The authors have developed a useful model for how proteins that mediate a connection between invariant components of the T cell antigen receptor and leukaemic cells antigens, called bispecific engagers (BiTEs), mediate immunological synapse formation and impact T cell search for tumour cells in vivo. The model was compared against the in vitro experiments and in vivo data following a solid approach. The developed framework could provide a direction for employing computational mechanistic models for evaluating various strategies for BiTE treatments.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Effector T cells need to form immunological synapses (IS) with recognized target cells to elicit cytolytic effects. Facilitating IS formation is the principal pharmacological action of most T cell-based cancer immunotherapies. However, the dynamics of IS formation at the cell population level, the primary driver of the pharmacodynamics of many cancer immunotherapies, remains poorly defined. Using classic immunotherapy CD3/CD19 bispecific T cell engager (BiTE) as our model system, we integrate experimental and theoretical approaches to investigate the population dynamics of IS formation and their relevance to clinical pharmacodynamics and treatment resistance. Our models produce experimentally consistent predictions when defining IS formation as a series of spatiotemporally coordinated events driven by molecular and cellular interactions. The models predict tumor-killing pharmacodynamics in patients and reveal trajectories of tumor evolution across anatomical sites under BiTE immunotherapy. Our models highlight the bone marrow as a potential sanctuary site permitting tumor evolution and antigen escape. The models also suggest that optimal dosing regimens are a function of tumor growth, CD19 expression, and patient T cell abundance, which confer adequate tumor control with reduced disease evolution. This work has implications for developing more effective T cell-based cancer immunotherapies.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
The authors set out to extend modeling of bispecific engager pharmacology through explicit modelling of the search of T cells for tumour cells, the formation of an immunological synapse and the dissociation of the immunological synapse to enable serial killing. These features have not been included in prior models and their incorporation may improve the predictive value of the model.
Thank you for the positive feedback.
The model provides a number of predictions that are of potential interest- that loss of CD19, the target antigen, to 1/20th of its initial expression will lead to escape and that the bone marrow is a site where the tumour cells may have the best opportunity to develop loss variants due to the limited pressure from T cells.
Thank you for the positive feedback.
A …
Author Response
Reviewer #1 (Public Review):
The authors set out to extend modeling of bispecific engager pharmacology through explicit modelling of the search of T cells for tumour cells, the formation of an immunological synapse and the dissociation of the immunological synapse to enable serial killing. These features have not been included in prior models and their incorporation may improve the predictive value of the model.
Thank you for the positive feedback.
The model provides a number of predictions that are of potential interest- that loss of CD19, the target antigen, to 1/20th of its initial expression will lead to escape and that the bone marrow is a site where the tumour cells may have the best opportunity to develop loss variants due to the limited pressure from T cells.
Thank you for the positive feedback.
A limitation of the model is that adhesion is only treated as a 2D implementation of the blinatumomab mediated bridge between T cell and B cells- there is no distinct parameter related to the distinct adhesion systems that are critical for immunological synapse formation. For example, CD58 loss from tumours is correlated with escape, but it is not related to the target, CD19. While they begin to consider the immunological synapse, they don't incorporate adhesion as distinct from the engager, which is almost certainly important.
We agree that adhesion molecules play critical roles in cell-cell interaction. In our model, we assumed these adhesion molecules are constant (or not showing difference across cell populations). This assumption made us to focus on the BiTE-mediated interactions.
Revision: To clarify this point, we added a couple of sentences in the manuscript.
“Adhesion molecules such as CD2-CD58, integrins and selectins, are critical for cell-cell interaction. The model did not consider specific roles played by these adhesion molecules, which were assumed constant across cell populations. The model performed well under this simplifying assumption”.
In addition, we acknowledged the fact that “synapse formation is a set of precisely orchestrated molecular and cellular interactions. Our model merely investigated the components relevant to BiTE pharmacologic action and can only serve as a simplified representation of this process”.
While the random search is a good first approximation, T cell behaviour is actually guided by stroma and extracellular matrix, which are non-isotropic. In a lymphoid tissue the stroma is optimised for a search that can be approximated as brownian, or more accurately, a correlated random walk, but in other tissues, particularly tumours, the Brownian search is not a good approximation and other models have been applied. It would be interesting to look at observations from bone marrow or other sites to determine the best approximating for the search related to BiTE targets.
We agree that the tissue stromal factors greatly influence the patterns of T cell searching strategy. Our current model considered Brownian motion as a good first approximation for two reasons: 1) we define tissues as homogeneous compartments to attain unbiased evaluations of factors that influence BiTE-mediated cell-cell interaction, such as T cell infiltration, T: B ratio, and target expression. The stromal factors were not considered in the model, as they require spatially resolved tissue compartments to represent the gradients of stromal factors; 2) our model was primarily calibrated against in vitro data obtained from a “well-mixed” system that does not recapitulate specific considerations of tissue stromal factors. We did not obtain tissue-specific data to support the prediction of T cell movement. This is under current investigation in our lab. Therefore, we are cautious about assuming different patterns of T cell movement in the model when translating into in vivo settings. We acknowledged the limitation of our model for not considering the more physiologically relevant T-cell searching strategies.
Revision: In the Discussion, we added a limitation of our model: “We assumed Brownian motion in the model as a good first approximation of T cell movement. However, T cells often take other more physiologically relevant searching strategies closely associated with many stromal factors. Because of these stromal factors, the cell-cell encounter probabilities would differ across anatomical sites.”
Reviewer #3 (Public Review):
Liu et al. combined mechanistic modeling with in vitro experiments and data from a clinical trial to develop an in silico model to describe response of T cells against tumor cells when bi-specific T cell engager (BiTE) antigens, a standard immunotherapeutic drug, are introduced into the system. The model predicted responses of T cell and target cell populations in vitro and in vivo in the presence of BiTEs where the model linked molecular level interactions between BiTE molecules, CD3 receptors, and CD19 receptors to the population kinetics of the tumor and the T- cells. Furthermore, the model predicted tumor killing kinetics in patients and offered suggestions for optimal dosing strategies in patients undergoing BiTE immunotherapy. The conclusions drawn from this combined approach are interesting and are supported by experiments and modeling reasonably well. However, the conclusions can be tightened further by making some moderate to minor changes in their approach. In addition, there are several limitations in the model which deserves some discussion.
Strengths
A major strength of this work is the ability of the model to integrate processes from the molecular scales to the populations of T cells, target cells, and the BiTE antibodies across different organs. A model of this scope has to contain many approximations and thus the model should be validated with experiments. The authors did an excellent job in comparing the basic and the in vitro aspects of their approach with in vitro data, where they compared the numbers of engaged target cells with T cells as the numbers of the BiTE molecules, the ratio of effector and target cells, and the expressions of the CD3 and CD19 receptors were varied. The agreement with the model with the data were excellent in most cases which led to several mechanistic conclusions. In particular, the study found that target cells with lower CD19 expressions escape the T cell killing.
The in vivo extension of the model showed reasonable agreements with the kinetics of B cell populations in patients where the data were obtained from a published clinical trial. The model explained differences in B cell population kinetics between responders and non-responders and found that the differences were driven by the differences in the T cell numbers between the groups. The ability of the model to describe the in vivo kinetics is promising. In addition, the model leads to some interesting conclusions, e.g., the model shows that the bone marrow harbors tumor growth during the BiTE treatment. The authors then used the model to propose an alternate dosage scheme for BiTEs that needed a smaller dose of the drug.
Thank you for the positive comments.
Weaknesses
There are several weaknesses in the development of the model. Multiscale models of this nature contain parameters that need to be estimated by fitting the model with data. Some these parameters are associated with model approximations or not measured in experiments. Thus, a common practice is to estimate parameters with some 'training data' and then test model predictions using 'test data'. Though Supplementary file 1 provides values for some of the parameters that appeared to be estimated, it was not clear which dataset were used for training and which for test. The confidence intervals of the estimated parameters and the sensitivity of the proposed in vivo dosage schemes to parameter variations were unclear.
We agree with the reviewer on the model validation.
Revision: To ensure reproducibility, we summarized model assumptions and parameter values/sources in the supplementary file 1. To mimic tumor heterogeneity and evolution process, we applied stochastic agent-based models, which are challenging to be globally optimized against the data. The majority of key parameters was obtained or derived from the literature. Details have been provided in the response to Reviewer 3 - Question 1. In our modeling process, we manually optimized sensitive coefficient (β) for base model using pilot in-vitro data and sensitive coefficient (β) for in-vivo model by re-calibrating against the in-vitro data at a low BiTE concentration. BiTE concentrations in patients (mostly < 2 ng/ml) is only relevant to the low bound of the concentration range we investigated in vitro (0.65-2000 ng/ml). We have added some clarification/limitation of this approach in the text (details are provided in the following question). We understand the concerns, but the agent-based modeling nature prevent us to do global optimization.
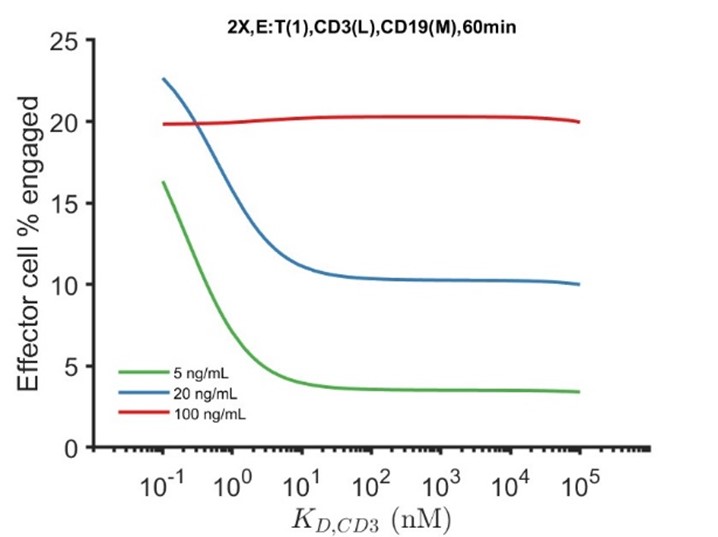
The model appears to show few unreasonable behaviors and does not agree with experiments in several cases which could point to missing mechanisms in the model. Here are some examples. The model shows a surprising decrease in the T cell-target cell synapse formation when the affinity of the BiTEs to CD3 was increased; the opposite should have been more intuitive. The authors suggest degradation of CD3 could be a reason for this behavior. However, this probably could be easily tested by removing CD3 degradation in the model. Another example is the increase in the % of engaged effector cells in the model with increasing CD3 expressions does not agree well with experiments (Fig. 3d), however, a similar fold increase in the % of engaged effector cells in the model agrees better with experiments for increasing CD19 expressions (Fig. 3e). It is unclear how this can be explained given CD3 and CD19 appears to be present in similar copy numbers per cell (~104 molecules/cell), and both receptors bind the BiTE with high affinities (e.g., koff < 10-4 s-1).
Thank you for pointing this out. The bidirectional effect of CD3 affinity on IS formation is counterintuitive. In a hypothetical situation when there is no CD3 downregulation, the bidirectional effect disappears (as shown below), consistent with our view that CD3 downregulation accounts for the counterintuitive behavior. We have included the simulation to support our point. From a conceptual standpoint, the inclusion of CD3 degradation means the way to maximize synapse formation is for the BiTE to first bind tumor antigen, after which the tumor-BiTE complex “recruits” a T cell through the CD3 arm.
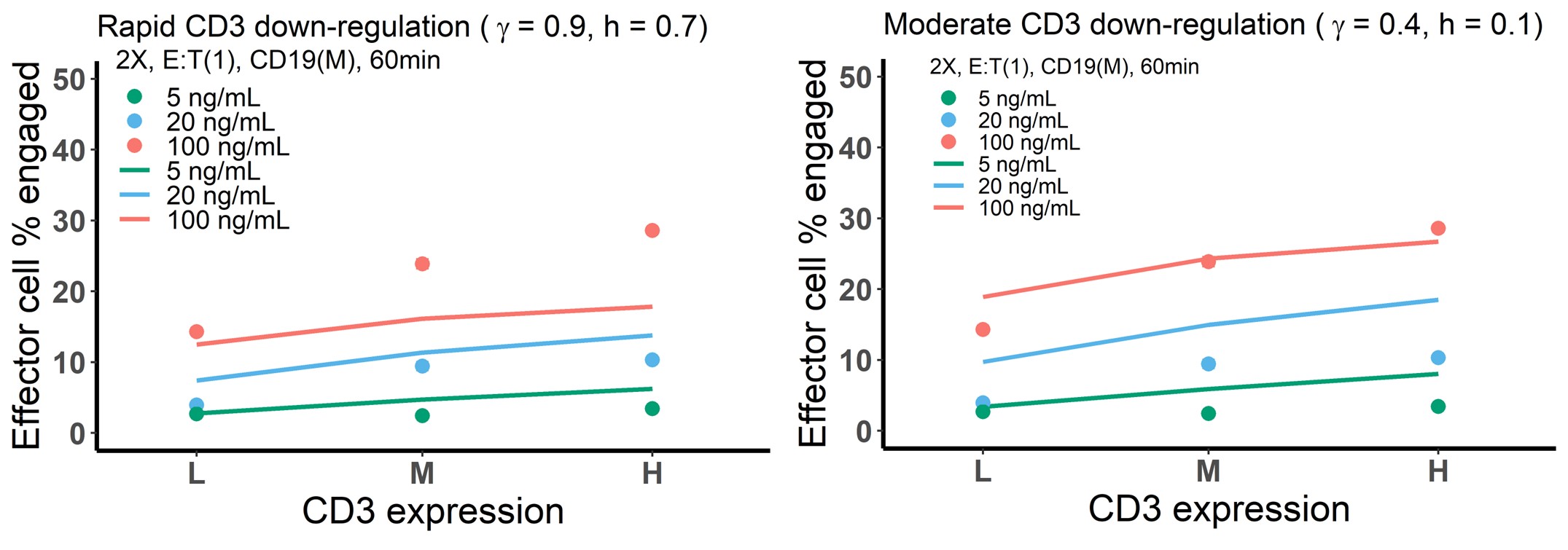
We agree that the model did not adequately capture the effect of CD3 expression at the highest BiTE concentration 100 ng/ml, while the effects at other BiTE concentrations were well captured (as shown below, left). The model predicted a much moderate effect of CD3 expression on IS formation at the highest concentration. This is partly because the model assumed rapid CD3 downregulation upon antibody engagement. We did a similar simulation as above, with moderate CD3 downregulation (as shown below, right). This increases the effect of CD3 expression at the highest BiTE concentration, consistent with experiments. Interestingly, a rapid CD3 downregulation rate, as we concluded, is required to capture data profiles at all other conditions. Considering BiTE concentration at 100 ng/ml is much higher than therapeutically relevant level in circulation (< 2 ng/ml), we did not investigate the mechanism underlying this inconsistent model prediction but we acknowledged the fact that the model under-predicted IS formation in Figure 3d. Notably, this discrepancy may rarely appear in our clinical predictions as the CD3 expression is low level and blood BiTE concentration is very low (< 2 ng/ml).
Revision: we have made text adjustment to increase clarity on these points. In addition, we added: “The base model underpredicted the effect of CD3 expression on IS formation at 100 ng/ml BiTE concentration, which is partially because of the rapid CD3 downregulation upon BiTE engagement and assay variation across experimental conditions.”
The model does not include signaling and activation of T cells as they form the immunological synapse (IS) with target cells. The formation IS leads to aggregation of different receptors, adhesion molecules, and kinases which modulate signaling and activation. Thus, it is likely the variations of the copy numbers of CD3, and the CD19-BiTE-CD3 will lead to variations in the cytotoxic responses and presumably to CD3 degradation as well. Perhaps some of these missing processes are responsible for the disagreements between the model and the data shown in Fig. 3. In addition, the in vivo model does not contain any development of the T cells as they are stimulated by the BiTEs. The differences in development of T cells, such as generation of dysfunctional/exhausted T cells could lead to the differences in responses to BiTEs in patients. In particular, the in vivo model does not agree with the kinetics of B cells after day 29 in non-responders (Fig. 6d); could the kinetics of T cell development play a role in this?
We agree that intracellular signaling is critical to T cell activation and cytotoxic effects. IS formation, T cell activation, and cytotoxicity are a cascade of events with highly coordinated molecular and cellular interactions. Compared to the events of T cell activation and cytotoxicity, IS formation occurs at a relatively earlier time. As shown in our study, IS formation can occur at 2-5 min, while the other events often need hours to be observed. We found that IS formation is primarily driven by two intercellular processes: cell-cell encounter and cell-cell adhesion. The intracellular signaling would be initiated in the process of cell-cell adhesion or at the late stage of IS formation. We think these intracellular events are relevant but may not be the reason why our model did not adequately capture the profiles in Figure 3d at the highest BiTE concentrations. Therefore, we did not include intracellular signaling in the models. Another reason was that we simulated our models at an agent level to mimic the process of tumor evolution, which is computationally demanding. Intracellular events for each cell may make it more challenging computationally.
T cell activation and exhaustion throughout the BiTE treatment is very complicated, time-variant and impacted by multiple factors like T cell status, tumor burden, BiTE concentration, immune checkpoints, and tumor environment. T cell proliferation and death rates are challenging to estimate, as the quantitative relationship with those factors is unknown. Therefore, T cell abundance (expansion) was considered as an independent variable in our model. T cell counts are measured in BiTE clinical trials. We included these data in our model to reveal expanded T cell population. Patients with high T cell expansion are often those with better clinical response. Notably, the T cell decline due to rapid redistribution after administration was excluded in the model. T cell abundance was included in the simulations in Figure 6 but not proof of concept simulations in Figure 7.
In Figure 6d, kinetics of T cell abundance had been included in the simulations for responders and non-responders in MT103-211 study. Thus, the kinetics of T cell development can’t be used to explain the disagreement between model prediction and observation after day 29 in non-responders. The observed data is actually median values of B-cell kinetics in non-responders (N = 27) with very large inter-subject variation (baseline from 10-10000/μL), which makes it very challenging to be perfectly captured by the model. A lot of non-responders with severe progression dropped out of the treatment at the end of cycle 1, which resulted in a “more potent” efficacy in the 2nd cycle. This might be main reason for the disagreement.
Variation in cytotoxic response was not included in our models. Tumor cells were assumed to be eradicated after the engagement with effecter cells, no killing rate or killing probability was implemented. This assumption reduced the model complexity and aligned well with our in-vitro and clinical data. Cytotoxic response in vivo is impacted by multiple factors like copy number of CD3, cytokine/chemokine release, tumor microenvironment and T cell activation/exhaustion. For example, the cytotoxic response and killing rate mediated by 1:1 synapse (ET) and other variants (ETE, TET, ETEE, etc.) are supposed to be different as well. Our model did not differentiate the killing rate of these synapse variants, but the model has quantified these synapse variants, providing a framework for us to address these questions in the future. We agree that differentiate the cytotoxic responses under different scenarios cell may improve model prediction and more explorations need to be done in the future.
Revision: We added a discussion of the limitations which we believe is informative to future studies.
“Our models did not include intracellular signaling processes, which are critical for T activation and cytotoxicity. However, our data suggests that encounter and adhesion are more relevant to initial IS formation. To make more clinically relevant predictions, the models should consider these intracellular signaling events that drive T cell activation and cytotoxic effects. Of note, we did consider the T cell expansion dynamics in organs as independent variable during treatment for the simulations in Figure 6. T cell expansion in our model is case-specific and time-varying.”
References:
Chen W, Yang F, Wang C, Narula J, Pascua E, Ni I, Ding S, Deng X, Chu ML, Pham A, Jiang X, Lindquist KC, Doonan PJ, Blarcom TV, Yeung YA, Chaparro-Riggers J. 2021. One size does not fit all: navigating the multi-dimensional space to optimize T-cell engaging protein therapeutics. MAbs 13:1871171. DOI: 10.1080/19420862.2020.1871171, PMID: 33557687
Dang K, Castello G, Clarke SC, Li Y, AartiBalasubramani A, Boudreau A, Davison L, Harris KE, Pham D, Sankaran P, Ugamraj HS, Deng R, Kwek S, Starzinski A, Iyer S, Schooten WV, Schellenberger U, Sun W, Trinklein ND, Buelow R, Buelow B, Fong L, Dalvi P. 2021. Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release. Journal for ImmunoTherapy of Cancer 9:e002488. DOI: 10.1136/jitc-2021-002488, PMID: 34088740
Gong C, Anders RA, Zhu Q, Taube JM, Green B, Cheng W, Bartelink IH, Vicini P, Wang BPopel AS. 2019. Quantitative Characterization of CD8+ T Cell Clustering and Spatial Heterogeneity in Solid Tumors. Frontiers in Oncology 8:649. DOI: 10.3389/fonc.2018.00649, PMID: 30666298
Mejstríková E, Hrusak O, Borowitz MJ, Whitlock JA, Brethon B, Trippett TM, Zugmaier G, Gore L, Stackelberg AV, Locatelli F. 2017. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer Journal 7: 659. DOI: 10.1038/s41408-017-0023-x, PMID: 29259173
Samur MK, Fulciniti M, Samur AA, Bazarbachi AH, Tai YT, Prabhala R, Alonso A, Sperling AS, Campbell T, Petrocca F, Hege K, Kaiser S, Loiseau HA, Anderson KC, Munshi NC. 2021. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nature Communications 12:868. DOI: 10.1038/s41467-021-21177-5, PMID: 33558511
Xu X, Sun Q, Liang X, Chen Z, Zhang X, Zhou X, Li M, Tu H, Liu Y, Tu S, Li Y. 2019. Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Frontiers in Immunology 10:2664. DOI: 10.3389/fimmu.2019.02664, PMID: 31798590
Yoneyama T, Kim MS, Piatkov K, Wang H, Zhu AZX. 2022. Leveraging a physiologically-based quantitative translational modeling platform for designing B cell maturation antigen-targeting bispecific T cell engagers for treatment of multiple myeloma. PLOS Computational Biology 18: e1009715. DOI: 10.1371/journal.pcbi.1009715, PMID: 35839267
-

eLife assessment
The authors have developed a useful model for how proteins that mediate a connection between invariant components of the T cell antigen receptor and leukaemic cells antigens, called bispecific engagers (BiTEs), mediate immunological synapse formation and impact T cell search for tumour cells in vivo. The model was compared against the in vitro experiments and in vivo data following a solid approach. The developed framework could provide a direction for employing computational mechanistic models for evaluating various strategies for BiTE treatments.
-

Reviewer #1 (Public Review):
The authors set out to extend modeling of bispecific engager pharmacology through explicit modelling of the search of T cells for tumour cells, the formation of an immunological synapse and the dissociation of the immunological synapse to enable serial killing. These features have not been included in prior models and their incorporation may improve the predictive value of the model.
The model provides a number of predictions that are of potential interest- that loss of CD19, the target antigen, to 1/20th of its initial expression will lead to escape and that the bone marrow is a site where the tumour cells may have the best opportunity to develop loss variants due to the limited pressure from T cells.
A limitation of the model is that adhesion is only treated as a 2D implementation of the blinatumomab …
Reviewer #1 (Public Review):
The authors set out to extend modeling of bispecific engager pharmacology through explicit modelling of the search of T cells for tumour cells, the formation of an immunological synapse and the dissociation of the immunological synapse to enable serial killing. These features have not been included in prior models and their incorporation may improve the predictive value of the model.
The model provides a number of predictions that are of potential interest- that loss of CD19, the target antigen, to 1/20th of its initial expression will lead to escape and that the bone marrow is a site where the tumour cells may have the best opportunity to develop loss variants due to the limited pressure from T cells.
A limitation of the model is that adhesion is only treated as a 2D implementation of the blinatumomab mediated bridge between T cell and B cells- there is no distinct parameter related to the distinct adhesion systems that are critical for immunological synapse formation. For example, CD58 loss from tumours is correlated with escape, but it is not related to the target, CD19. While they begin to consider the immunological synapse, they don't incorporate adhesion as distinct from the engager, which is almost certainly important.
While the random search is a good first approximation, T cell behaviour is actually guided by stroma and extracellular matrix, which are non-isotropic. In a lymphoid tissue the stroma is optimised for a search that can be approximated as brownian, or more accurately, a correlated random walk, but in other tissues, particularly tumours, the Brownian search is not a good approximation and other models have been applied. It would be interesting to look at observations from bone marrow or other sites to determine the best approximating for the search related to BiTE targets.
-

Reviewer #2 (Public Review):
This mechanistic PK/PD model simultaneous characterized several important factors, including formation of immunological synapses synapse variants, target/tumor cell densities, target CD3/tumor antigen expression levels, tumor antigen escape and the associated cancer relapse, in a unified model structure.
This model has the potential to be used in optimization dosage of T cell redirecting bispecific treatment towards best clinical outcome.
-

Reviewer #3 (Public Review):
Liu et al. combined mechanistic modeling with in vitro experiments and data from a clinical trial to develop an in silico model to describe response of T cells against tumor cells when bi-specific T cell engager (BiTE) antigens, a standard immunotherapeutic drug, are introduced into the system. The model predicted responses of T cell and target cell populations in vitro and in vivo in the presence of BiTEs where the model linked molecular level interactions between BiTE molecules, CD3 receptors, and CD19 receptors to the population kinetics of the tumor and the T- cells. Furthermore, the model predicted tumor killing kinetics in patients and offered suggestions for optimal dosing strategies in patients undergoing BiTE immunotherapy. The conclusions drawn from this combined approach are interesting and are …
Reviewer #3 (Public Review):
Liu et al. combined mechanistic modeling with in vitro experiments and data from a clinical trial to develop an in silico model to describe response of T cells against tumor cells when bi-specific T cell engager (BiTE) antigens, a standard immunotherapeutic drug, are introduced into the system. The model predicted responses of T cell and target cell populations in vitro and in vivo in the presence of BiTEs where the model linked molecular level interactions between BiTE molecules, CD3 receptors, and CD19 receptors to the population kinetics of the tumor and the T- cells. Furthermore, the model predicted tumor killing kinetics in patients and offered suggestions for optimal dosing strategies in patients undergoing BiTE immunotherapy. The conclusions drawn from this combined approach are interesting and are supported by experiments and modeling reasonably well. However, the conclusions can be tightened further by making some moderate to minor changes in their approach. In addition, there are several limitations in the model which deserves some discussion.
Strengths
A major strength of this work is the ability of the model to integrate processes from the molecular scales to the populations of T cells, target cells, and the BiTE antibodies across different organs. A model of this scope has to contain many approximations and thus the model should be validated with experiments. The authors did an excellent job in comparing the basic and the in vitro aspects of their approach with in vitro data, where they compared the numbers of engaged target cells with T cells as the numbers of the BiTE molecules, the ratio of effector and target cells, and the expressions of the CD3 and CD19 receptors were varied. The agreement with the model with the data were excellent in most cases which led to several mechanistic conclusions. In particular, the study found that target cells with lower CD19 expressions escape the T cell killing.
The in vivo extension of the model showed reasonable agreements with the kinetics of B cell populations in patients where the data were obtained from a published clinical trial. The model explained differences in B cell population kinetics between responders and non-responders and found that the differences were driven by the differences in the T cell numbers between the groups. The ability of the model to describe the in vivo kinetics is promising. In addition, the model leads to some interesting conclusions, e.g., the model shows that the bone marrow harbors tumor growth during the BiTE treatment. The authors then used the model to propose an alternate dosage scheme for BiTEs that needed a smaller dose of the drug.
Weaknesses
There are several weaknesses in the development of the model. Multiscale models of this nature contain parameters that need to be estimated by fitting the model with data. Some these parameters are associated with model approximations or not measured in experiments. Thus, a common practice is to estimate parameters with some 'training data' and then test model predictions using 'test data'. Though Supplementary file 1 provides values for some of the parameters that appeared to be estimated, it was not clear which dataset were used for training and which for test. The confidence intervals of the estimated parameters and the sensitivity of the proposed in vivo dosage schemes to parameter variations were unclear.
The model appears to show few unreasonable behaviors and does not agree with experiments in several cases which could point to missing mechanisms in the model. Here are some examples. The model shows a surprising decrease in the T cell-target cell synapse formation when the affinity of the BiTEs to CD3 was increased; the opposite should have been more intuitive. The authors suggest degradation of CD3 could be a reason for this behavior. However, this probably could be easily tested by removing CD3 degradation in the model. Another example is the increase in the % of engaged effector cells in the model with increasing CD3 expressions does not agree well with experiments (Fig. 3d), however, a similar fold increase in the % of engaged effector cells in the model agrees better with experiments for increasing CD19 expressions (Fig. 3e). It is unclear how this can be explained given CD3 and CD19 appears to be present in similar copy numbers per cell (~104 molecules/cell), and both receptors bind the BiTE with high affinities (e.g., koff < 10-4 s-1).
The model does not include signaling and activation of T cells as they form the immunological synapse (IS) with target cells. The formation IS leads to aggregation of different receptors, adhesion molecules, and kinases which modulate signaling and activation. Thus, it is likely the variations of the copy numbers of CD3, and the CD19-BiTE-CD3 will lead to variations in the cytotoxic responses and presumably to CD3 degradation as well. Perhaps some of these missing processes are responsible for the disagreements between the model and the data shown in Fig. 3. In addition, the in vivo model does not contain any development of the T cells as they are stimulated by the BiTEs. The differences in development of T cells, such as generation of dysfunctional/exhausted T cells could lead to the differences in responses to BiTEs in patients. In particular, the in vivo model does not agree with the kinetics of B cells after day 29 in non-responders (Fig. 6d); could the kinetics of T cell development play a role in this?
Addressing these concerns and a discussion of the limitations will make the conclusions of the study stronger and will provide cues for extending the approach for future studies.
-