Murine blastocysts generated by in vitro fertilization show increased Warburg metabolism and altered lactate production
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Some children conceived by assisted reproductive technologies (ART) exhibit metabolic differences compared to those conceived naturally, and the causes are unknown. This work reveals possible explanations for the metabolic differences and provides opportunities to improve ART and prevent the differences. This is a valuable contribution and will be of special interest to practitioners of ART, as well as to developmental and reproductive biologists.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
In vitro fertilization (IVF) has resulted in the birth of over 8 million children. Although most IVF-conceived children are healthy, several studies suggest an increased risk of altered growth rate, cardiovascular dysfunction, and glucose intolerance in this population compared to naturally conceived children. However, a clear understanding of how embryonic metabolism is affected by culture condition and how embryos reprogram their metabolism is unknown. Here, we studied oxidative stress and metabolic alteration in blastocysts conceived by natural mating or by IVF and cultured in physiologic (5%) or atmospheric (20%) oxygen. We found that IVF-generated blastocysts manifest increased reactive oxygen species, oxidative damage to DNA/lipid/proteins, and reduction in glutathione. Metabolic analysis revealed IVF-generated blastocysts display decreased mitochondria respiration and increased glycolytic activity suggestive of enhanced Warburg metabolism. These findings were corroborated by altered intracellular and extracellular pH and increased intracellular lactate levels in IVF-generated embryos. Comprehensive proteomic analysis and targeted immunofluorescence showed reduction of lactate dehydrogenase-B and monocarboxylate transporter 1, enzymes involved in lactate metabolism. Importantly, these enzymes remained downregulated in the tissues of adult IVF-conceived mice, suggesting that metabolic alterations in IVF-generated embryos may result in alteration in lactate metabolism. These findings suggest that alterations in lactate metabolism are a likely mechanism involved in genomic reprogramming and could be involved in the developmental origin of health and disease.
Article activity feed
-

-

Author Response
Reviewer #3 (Public Review):
Q1) The manuscript reports that in vitro fertilization (including in vitro culture) of mouse embryos seemingly originates metabolic alterations probably caused by enhanced oxidative stress compared to in vivo development. Such alterations apparently increase anaerobic glycolysis, as evidenced by altered pH and lactate levels, and remain after birth, as evidenced by altered protein abundance of MCT1 and LDHB.
The manuscript concludes that IVF alters embryo metabolism, increasing oxidative damage and glycolytic activity. The topic is interesting but I consider that the conclusions are not well supported by the experiments:
- In vivo generated blastocysts are analyzed at a more advanced developmental stage than their in vitro counterparts as evidenced by their increased cell number (70 vs. …
Author Response
Reviewer #3 (Public Review):
Q1) The manuscript reports that in vitro fertilization (including in vitro culture) of mouse embryos seemingly originates metabolic alterations probably caused by enhanced oxidative stress compared to in vivo development. Such alterations apparently increase anaerobic glycolysis, as evidenced by altered pH and lactate levels, and remain after birth, as evidenced by altered protein abundance of MCT1 and LDHB.
The manuscript concludes that IVF alters embryo metabolism, increasing oxidative damage and glycolytic activity. The topic is interesting but I consider that the conclusions are not well supported by the experiments:
- In vivo generated blastocysts are analyzed at a more advanced developmental stage than their in vitro counterparts as evidenced by their increased cell number (70 vs. 50 cells). In this regard, the developmental timing when in vitro generated blastocysts are collected is undisclosed in the Materials and methods. This has an obvious effect on all experiments as the differences observed may be stage-specific rather than IVF vs. in vivo.
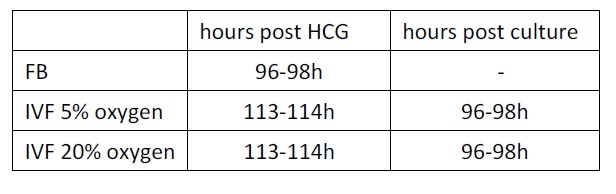
A1) Thank you for the comment. The reviewer is correct and it is indeed well known that in vitro fertilization and embryo culture results in profound changes to the embryo. Overall, embryos generated in vitro are delayed compared to embryos generated in vivo. To control for this, as done in our past publications (Belli 2019; Bloise 2014; Delle Piane 2010; Giritharan 2012; Giritharan 2010; Giritharan 2006; Giritharan 2007; Rinaudo 2006; Rinaudo 2004), or by others (Doherty 2000; Ecker 2004; Weinerman 2016), we limited the analysis to expanded blastocysts of similar morphology (under microscopic examination) in all of the groups. Therefore the embryos appeared morphological similar in all of the groups. As an alternative, we could have waited longer time in vitro, but this would have resulted in embryo hatching and being not morphological similar to in vivo embryos. In addition, the 2 IVF groups provide an internal control: embryos were at the same developmental stage, but showed significant changes in metabolism and cell numbers. (96 hours of culture +13-14hours for egg collection+ 4hours of fertilization= time post HCG administration)
We have added this information as follows: Line 377-382: To control for the known delay in development after culture in vitro, for all experiments, only expanded blastocysts of similar morphology were used, as done before (Doherty 2000; Rinaudo 2006; Rinaudo 2004). The in vivo-generated blastocysts were isolated by flushing 96-98 hours after hCG administration. IVF- 5% O2 and 20% O2 generated embryos reached the blastocyst stage after 96-98 hours following in vitro culture and 113-114 hours after hCG administration, respectively.
Q2) Several methods are not reliable to quantify the parameters analyzed. For instance, determining protein content by immunofluorescence has been largely shown to be misleading as immunofluorescence can be affected by multiple parameters. Intracellular pH was also analyzed by an assay also based on immunofluorescence, which can also be affected by embryo size (the blastocoel is a call-devoid cavity). These analyses are not reliable.
A2) Thank you for the comments.
We appreciate the comments and concerns. Any single method can result in error and possible bias. Immunofluorescence analysis is a robust method that has been used to analyze the distribution of proteins in cells or tissues. For instance, oxidative stress (Liu et al., 2022, Reprod Domest Anim), several signaling molecule (Spirkova et al., 2022, Biol Reprod) and DNA methylation level (Diaz et al, 2021, Fron Gent) have been measured by immunofluorescence in preimplantation embryos and oocytes. It our study, to minimize errors, we followed exactly the same protocol and we found immunofluorescence to be reliable. In addition, global proteomics analysis of blastocysts provide partial independent confirmation of our results. While LDH-A and MCT1 were not detected, LDH-B was detected and found to be lower in IVF blastocysts, exactly as show by IF studies. Finally, western blot analysis of adult tissues confirmed reduction in LDH-B and MCT-1 levels.
These comments have been added to the discussion as follows:
Line 299-302: Unsupervised global proteomics analysis revealed that LDH-B was downregulated in IVF embryos. We confirmed these results by performing immunofluorescence studies. In addition we found that IVF embryos showed downregulation of both LDHA and B and of the monocarboxylate transporter, MCT 1, providing an explanation for the increase in their lactate levels
Regarding pH measurement: to control for the possible variation in blastocoel size in different embryos, we compared immunofluorescence level of only the inner cell mass and trophoblast region of blastocysts and excluded the blastocoel region.
This clarification has been added to the method section as follow:
Line 488-491: To control for the possible variation in blastocoel size in different embryos, we compared immunofluorescence level of only the inner cell mass and trophoblast region of blastocysts and excluded the blastocoel region.
Q3) Identifying proteins and metabolites in such small samples is technically difficult and error-prone, requiring validation by alternative techniques.
We appreciate the comments and concerns. Any single method can result in error and possible bias. Immunofluorescence analysis is a robust method that has been used to analyze the distribution of proteins in cells or tissues. For instance, oxidative stress (Liu 2022), several signaling molecule (Spirkova 2022) and DNA methylation level (Diaz 2021) have been measured by immunofluorescence in preimplantation embryos and oocytes. It our study, to minimize errors, we followed exactly the same protocol and we found immunofluorescence to be reliable. In addition, global proteomics analysis of blastocysts (triplicate for each group; n=100 blastocysts for each replicate; total 900 embryos). provide partial independent confirmation of our results. While LDH-A and MCT1 were not detected, LDH-B was detected and found to be lower in IVF blastocysts, exactly as show by IF studies. Finally, western blot analysis of adult tissues confirmed reduction in LDH-B and MCT-1 levels.
These comments have been added to the discussion as follows:
Line 299-302: Unsupervised global proteomics analysis revealed that LDH-B was downregulated in IVF embryos. We confirmed these results by performing immunofluorescence studies. In addition we found that IVF embryos showed downregulation of both LDHA and B and of the monocarboxylate transporter, MCT 1, providing an explanation for the increase in their lactate levels
Q4) Given the small size of these embryos (~80 µm diameter), it is unclear how they can alter significantly the composition of 500 µl of medium (106 their own volume).
To collect 300 blastocysts, we performed multiple IVF, each IVF resulting in 10-20 blastocysts cultured in 30 microliters of media. While intracellular lactate and pyruvate were performed on the embryos collected, the media from different experiments was pooled to a final 500 microliter volume. Lactate and pyruvate levels were measured in this final volume for each group of embryo (FB, IVF5% and IVF20%)
This has been clarified in the method section as follows:
Line 516-519: To collect 300 blastocysts, we performed multiple IVF, each IVF resulting in 10-20 blastocysts cultured in 30 microliters of media. While intracellular lactate and pyruvate were performed on the embryos collected, the media from different experiments was pooled to a final 500 microliter volume.
Q5) The metabolic changes observed in the offspring lack a mechanistic explanation.
Thank you for the comment. We can formulate a hypothesis in which (Figure 8) oxidative stress from in vitro condition increase ROS and induce oxidative damage resulting in a shift toward Warburg metabolism, given that lactate is a critical energy source (Brooks, 2018). The higher intracellular lactate levels will likely induce epigenetic changes, to favor Warburg metabolism during development, as an embryonic attempt to optimize growth based on the environment predicted to be experienced in the future. When the environment does not match the prediction, disease risk increases (Godfrey 2007). Low lactate would be beneficial in a setting of low food resources because it could favor lipolysis (Brooks, 2020). In fact, lactate activates the hydroxycarboxylic acid receptor 1 (HCAR1), a G protein-coupled receptor, which in turn inhibits lipolysis in fat cells via cAMP and CREB (Liu 2009). However, since there is an abundance of food in our society, this mismatch could predispose IVF concepti to develop chronic disease like glucose intolerance.
This hypothesis has been added to line 333-344:
In summary, we can formulate a hypothesis in which (Figure 8) oxidative stress from in vitro condition increase ROS and induce oxidative damage resulting in a shift toward Warburg metabolism, given that lactate is a critical energy source (Brooks, 2018). The higher intracellular lactate levels will likely induce epigenetic changes, to favor Warburg metabolism during development, as an embryonic attempt to optimize growth based on the environment predicted to be experienced in the future. When the environment does not match the prediction, disease risk increases (Godfrey 2007). Low lactate would be beneficial in a setting of low food resources because it could favor lipolysis (Brooks, 2020). In fact, lactate activates the hydroxycarboxylic acid receptor 1 (HCAR1), a G protein-coupled receptor in turn inhibits lipolysis in fat cells via cAMP and CREB (Liu 2009). However, since there is an abundance of food in our society, this mismatch could predispose IVF concepti to develop chronic disease like glucose intolerance.
-

Evaluation Summary:
Some children conceived by assisted reproductive technologies (ART) exhibit metabolic differences compared to those conceived naturally, and the causes are unknown. This work reveals possible explanations for the metabolic differences and provides opportunities to improve ART and prevent the differences. This is a valuable contribution and will be of special interest to practitioners of ART, as well as to developmental and reproductive biologists.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
There is evidence to suggest that a percentage of the 8 million children conceived by in vitro fertilization (IVF) exhibit an increased risk of various maladies (e.g., altered growth rate, cardiovascular dysfunction, and glucose intolerance) compared to naturally conceived children. However, it is unclear how embryonic metabolism is affected by conditions, especially oxygen tension, used to culture blastocysts derived by IVF.
This manuscript describes a comprehensive investigation of the effects of IVF-embryo culture conditions on the metabolism of murine blastocysts and adult mice. The authors conclude that oxidative stress caused by culture conditions used for IVF-generated blastocysts results in various metabolic alterations and possibly epigenetic changes in embryos that lead to an increased risk of …
Reviewer #1 (Public Review):
There is evidence to suggest that a percentage of the 8 million children conceived by in vitro fertilization (IVF) exhibit an increased risk of various maladies (e.g., altered growth rate, cardiovascular dysfunction, and glucose intolerance) compared to naturally conceived children. However, it is unclear how embryonic metabolism is affected by conditions, especially oxygen tension, used to culture blastocysts derived by IVF.
This manuscript describes a comprehensive investigation of the effects of IVF-embryo culture conditions on the metabolism of murine blastocysts and adult mice. The authors conclude that oxidative stress caused by culture conditions used for IVF-generated blastocysts results in various metabolic alterations and possibly epigenetic changes in embryos that lead to an increased risk of various maladies in children and adults.
In this investigation, the authors studied oxidative stress and metabolic alterations in murine blastocysts conceived by natural mating or by IVF and cultured in either 5% (physiological) or 20% (atmospheric) oxygen. The authors found that compared to blastocysts conceived by natural mating (flushed blastocysts, FB), IVF-generated blastocysts exhibited: (1) increased reactive oxygen species (ROS), (2) oxidative damage to DNA, lipids, and proteins, (3) reduction in glutathione and NAD, (4) decreased mitochondria respiration, (5) increased glycolytic activity (enhanced Warburg metabolism), (6) altered intracellular and extracellular pH, (7) decreased intracellular pyruvate levels and increased intracellular lactate levels, and (8) a reduction in lactate dehydrogenase B (LDH-B) and monocarboxylate transporter (MCT1) involved in lactate transport.
This is a valuable contribution to the field of assisted reproductive technologies (ART) and will be of special interest to practitioners of IVF, as well as to developmental and reproductive biologists in general.
-

Reviewer #2 (Public Review):
This study discovers significant metabolic differences between naturally occurring murine blastocysts and those created by in-vitro fertilization. Although both groups of embryos appear similar morphologically, the IVF embryos experience increased oxidative damage, decreased mitochondrial activity, and increased glycolytic function, all indicative of increased Warburg metabolism, a mechanism commonly used by cancer cells to generate increased ATP.
These authors show that IVF-generated embryos show alterations in intracellular/extracellular pH, NAD, lactate, and pyruvate levels and this is accompanied by alterations in the expression of enzymes involved in lactate metabolism. All these aberrations are seen in the presence of both 5% and 20% oxygen, confirming that these embryos prefer glucose fermentation …
Reviewer #2 (Public Review):
This study discovers significant metabolic differences between naturally occurring murine blastocysts and those created by in-vitro fertilization. Although both groups of embryos appear similar morphologically, the IVF embryos experience increased oxidative damage, decreased mitochondrial activity, and increased glycolytic function, all indicative of increased Warburg metabolism, a mechanism commonly used by cancer cells to generate increased ATP.
These authors show that IVF-generated embryos show alterations in intracellular/extracellular pH, NAD, lactate, and pyruvate levels and this is accompanied by alterations in the expression of enzymes involved in lactate metabolism. All these aberrations are seen in the presence of both 5% and 20% oxygen, confirming that these embryos prefer glucose fermentation even when enough oxygen is present to undergo respiration. The authors note that this is possibly due to the impaired mitochondrial function that has been reported in IVF-obtained oocytes prior to fertilization. Finally, they report that metabolic tissues of IVF-generated adult mice show down-regulation of LDH-B and MCT1 protein, the same enzyme, and transporter found to be down-regulated in early embryos.
Strengths: It is imperative to note the difficulty of these experiments due to the small number of cells per embryo, 70-80 in FB and 40-50 in the IVF embryos. Most assays and technologies for these types of measurements are not designed for these few cells and the authors have been able to adapt novel modern technologies, typically used for experiments with larger cell numbers, for this ultramicroanalysis. The measurements of ROS, glutathione, 8-OHdG, PGF2 alpha, and DNPH need to be measured fluorometrically and have been adapted for this cell system. The use of the Seahorse methodology has not been used successfully for this purpose but was adjusted for this cell system in this study to acquire the measurements of metabolic changes and this is a major strength. For the measurement of lactate and pyruvate, they used 900 blastocysts, since modern methods for the analysis of metabolites in this cell system have yet to be developed. The choice of analytic tools and the application to this unique embryo study to address a clinical phenomenon is a distinctive strength of this study. Another strength of this study is the finding of the same decrease in LDH-B and MCT1 protein (seen in blastocysts) in adult tissues of IVF-derived mice, suggesting a persistent effect manifested by a developmental alteration.
Weaknesses: The performance of this work in a mouse model is a limitation, however, due to the lack of adequate cell lines or access to human embryos, mouse embryos recapitulate the timing of the early developmental period in humans and are experimentally preferred due to the large number of embryos obtained with superovulation.
In summary, the authors have achieved their aims and the results support their conclusions. These findings strongly suggest that impaired mitochondrial function in the oocyte may be the cause of the Warburg effect in these in-vitro-derived embryos. This is a highly significant finding, implying that potential changes to ovulation stimulation methodologies that would improve oocyte metabolism may have the potential to prevent these metabolic differences in the offspring.
-

Reviewer #3 (Public Review):
The manuscript reports that in vitro fertilization (including in vitro culture) of mouse embryos seemingly originates metabolic alterations probably caused by enhanced oxidative stress compared to in vivo development. Such alterations apparently increase anaerobic glycolysis, as evidenced by altered pH and lactate levels, and remain after birth, as evidenced by altered protein abundance of MCT1 and LDHB.
The manuscript concludes that IVF alters embryo metabolism, increasing oxidative damage and glycolytic activity. The topic is interesting but I consider that the conclusions are not well supported by the experiments:
In vivo generated blastocysts are analyzed at a more advanced developmental stage than their in vitro counterparts as evidenced by their increased cell number (70 vs. 50 cells). In this regard, …
Reviewer #3 (Public Review):
The manuscript reports that in vitro fertilization (including in vitro culture) of mouse embryos seemingly originates metabolic alterations probably caused by enhanced oxidative stress compared to in vivo development. Such alterations apparently increase anaerobic glycolysis, as evidenced by altered pH and lactate levels, and remain after birth, as evidenced by altered protein abundance of MCT1 and LDHB.
The manuscript concludes that IVF alters embryo metabolism, increasing oxidative damage and glycolytic activity. The topic is interesting but I consider that the conclusions are not well supported by the experiments:
In vivo generated blastocysts are analyzed at a more advanced developmental stage than their in vitro counterparts as evidenced by their increased cell number (70 vs. 50 cells). In this regard, the developmental timing when in vitro generated blastocysts are collected is undisclosed in the Materials and methods. This has an obvious effect on all experiments as the differences observed may be stage-specific rather than IVF vs. in vivo.
Several methods are not reliable to quantify the parameters analyzed. For instance, determining protein content by immunofluorescence has been largely shown to be misleading as immunofluorescence can be affected by multiple parameters. Intracellular pH was also analyzed by an assay also based on immunofluorescence, which can also be affected by embryo size (the blastocoel is a call-devoid cavity). These analyses are not reliable.
Identifying proteins and metabolites in such small samples is technically difficult and error-prone, requiring validation by alternative techniques.
Given the small size of these embryos (~80 µm diameter), it is unclear how they can alter significantly the composition of 500 µl of medium (106 their own volume).
The metabolic changes observed in the offspring lack a mechanistic explanation.
-