Surface curvature and basal hydraulic stress induce spatial bias in cell extrusion
Curation statements for this article:-
Curated by eLife
eLife assessment
This paper presents important findings into the response of epithelial monolayers to the combined effects of surface curvature and hydraulic stress, offering insights into how these cues contribute to epithelial cell extrusion. Most of the evidence is convincing, relying mainly on a combination of imaging-based techniques. This paper is of interest to a broad and growing community of biologists, biophysicists, and engineers interested in cell-geometry interactions.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Abstract
Epithelial cell extrusion is employed in maintaining a healthy epithelium. It remains unclear how environmental conditions specific to various epithelial tissues, such as geometry and fluid osmolarity, affect cell extrusions. We found that, over curved surfaces, epithelial monolayers exhibited higher extrusion rates in concave regions than convex ones. This difference, and overall extrusions, decreased when osmotically induced basal hydraulic stress was reduced by increasing media osmolarity or by culturing monolayers on hydrogels. Mechanistically, basal fluid accumulation antagonized cell-substrate adhesions and the subsequent FAK-Akt survival pathway, leading to apoptotic cell death. Convex surfaces induced cellular forces that acted against osmosis, thereby promoting adhesions and lowering apoptosis. This effect was reversed in concave regions, and together, resulted in a curvature induced spatial bias in cell extrusions.
Article activity feed
-

-

-

Author Response
The following is the authors’ response to the original reviews.
Reviewer #1
Recommendation 1: The authors reasoned upon the presence of a differential basal hydraulic stress in waves' valleys vs hills at first from the observation of "domes" formation upon 48h cultivation. I suggest performing a quantification to support the statement as a good scientific practice. Furthermore, it would strengthen the concept when the formation of domes was compared between the waves' dimensions as a different grade of cell extrusion was quantified. i.e., 50, 100, and 200 µm.
Response 1: Upon seeing the phenomenon (Author response image 1 A), we performed a count for domes on the 100 µm and saw a significant effect. We refrained from including the results as it is the subject of ongoing research in our lab. In response to the reviewer’s …
Author Response
The following is the authors’ response to the original reviews.
Reviewer #1
Recommendation 1: The authors reasoned upon the presence of a differential basal hydraulic stress in waves' valleys vs hills at first from the observation of "domes" formation upon 48h cultivation. I suggest performing a quantification to support the statement as a good scientific practice. Furthermore, it would strengthen the concept when the formation of domes was compared between the waves' dimensions as a different grade of cell extrusion was quantified. i.e., 50, 100, and 200 µm.
Response 1: Upon seeing the phenomenon (Author response image 1 A), we performed a count for domes on the 100 µm and saw a significant effect. We refrained from including the results as it is the subject of ongoing research in our lab. In response to the reviewer’s suggestion, we have included a graph (Author response image 1 B) showing the increasing number of domes over 48 hours from three 100 µm wave samples.
We have updated Figure 2A and B in the manuscript to include the new graph.
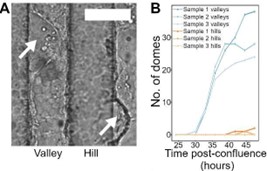
Author response image 1.
(A) shows dome (white arrows) over a 100 µm wave substrate. (B) is the number of accumulated domes in valley and hill regions, for 3 independent samples, over 48 hours.
Recommendation 2: Using RICM microscopy to quantify the cell basal separation with the substrate and hydraulic stress is very clever. Nevertheless, I am in doubt if the different intensity reported for the hills vs valley (Fig. 2G and H) is a result of the signal reduction at deeper Z levels. Since there is no difference in extrusion and forces between valleys and hills in the 200 µm waves but only in 50µm and 100µm, I would add this to the quantification. I would expect no intensity difference from RICM for the 200 µm sample if this is not an artefact of imaging.
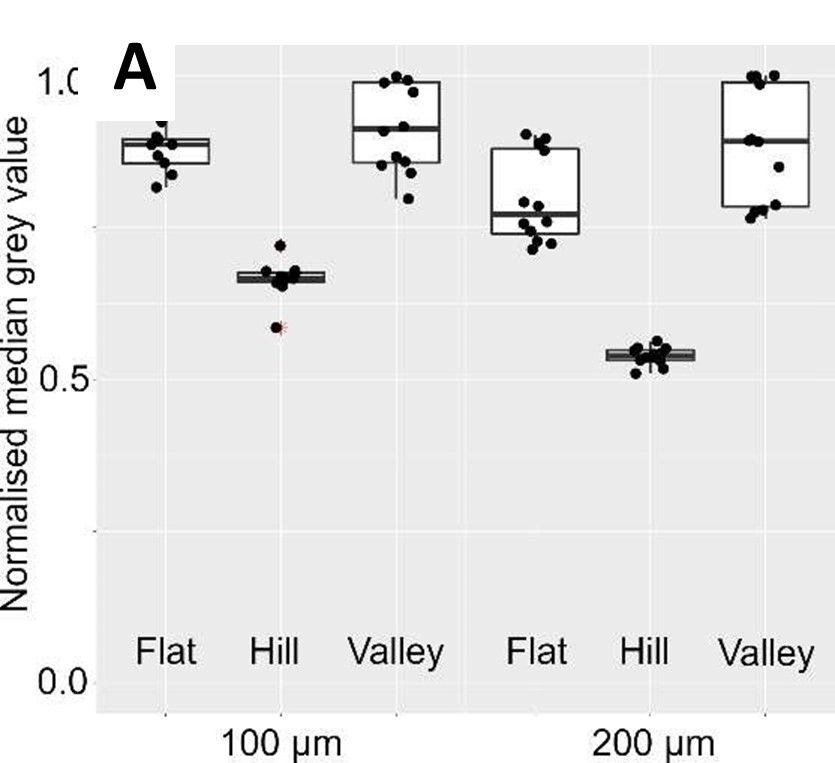
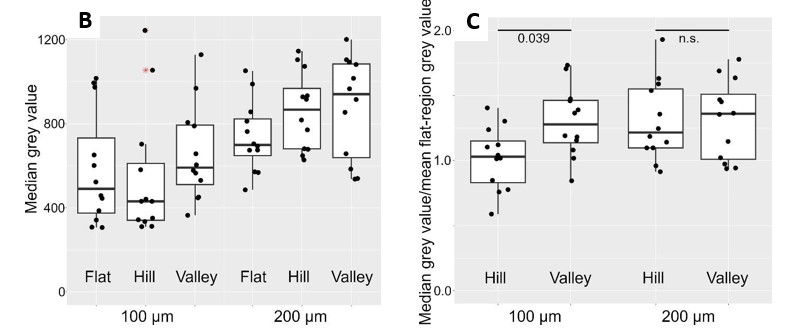
Response 2: We performed additional experiments on blank wave substrates (both 100 and 200 µm) to ascertain the extent of reflection intensity drop (Author response image 2A). And, as correctly pointed out by Reviewer #1, there was a drop in intensity even without cells. On the 100 µm waves, hill reflections are on average ~27 % dimmer than valley reflections. Whereas, on the 200 µm waves, hill reflections are on average ~39 % dimmer.
Using this information, we performed a calibration on the RICM results obtained from both the 100 and 200 µm waves (Author response image 3B). The calibrated 100 µm data showed residual signatures of difference, whereas the calibrated 200 µm distributions appeared very similar. We noticed large cross- sample variations in the registered intensities, which will negatively impact effect size if not accounted for. To do this, we subsequently normalized both hill and valley intensities against planar region intensities for each sample. As shown by the final output (Author response image 3C), we were able to remove the skewness in the distributions. Moreover, 1-way ANOVA followed by a post hoc analysis with BH correction revealed a significant reduction in 100 µm hill/flat intensity ratio compared to 100 µm valley/flat intensity ratios (Δ~-23 %). Conversely, no significance was observed for the same comparison on the 200 µm waves.
Author response image 2.
(A). RICM from blank wave samples reveal a reduction in reflection intensity in hill regions compared to flat and valley regions.
Author response image 3.
(B) shows the RICM intensities after adjusting for the inherent reflection intensity drop shown in (A). (C) show the RICM intensities after normalization against planar region signals; this removes cross-sample variations and improve effect size of differences.
We have updated the manuscript Figure 2I and text accordingly. The blank wave results are included in Figure 2-figure supplement 1 along with updated text and summary data table in Supplementary File 4.
Recommendation 3: To measure 3D forces on top of the hills and valleys, the use of PAA gels is necessary. Since in Fig 3B, the authors show a difference in cell extrusion number between substrates and stiffnesses, I think it is necessary to confirm the presence of more extrusion in valleys vs hills on PAA gels. This would ensure the conclusion between normal forces and extrusion.
Response 3: We do have time-lapse data with monolayers on the PAA waves. However, we felt results from the flat regions were sufficient in supporting the point being made in the text. Specifically, our original intention with PAA gels was to show that the extrusion reductions seen in osmotic perturbations were by virtue of removing basal stress and not some cryptic osmotic response. Hydrogels were chosen because they can effectively dilute basal solute concentration and thereby reduce the osmotically induced water transport. Moreover, as fluid could freely move within the gel, the fluid stress can quickly equilibrate across the basal surface. In contrast, poorly water/solute permeable substrates could lead to localized spikes in solute concentration and transient basal regions with high fluid stress.
To get a sense of the potential difference in basal solute concentration between the two materials, we can do a quick hand-waving estimation. For monolayers on non-water/solute permeable PDMS of 20x20 mm and using the laser wavelength (640 nm) for RICM as an extreme estimate of basal separation, we should expect ~0.25 µl of total basal water content. On the other hand, we typically produce our PAM gel slabs using ~150 µl of precursor solutions. This means that, given similar amounts of solute, PAM gels will lead to monolayer basal osmolarity that is around 3 orders of magnitude lower than monolayers on PDMS, producing significantly lower osmotic potential. This implies from the outset that we should expect high survivability of cells on these substrates irrespective of curvature domains. Indeed, later immunoblotting experiments showed MDCKs exhibiting hyper activated FAK and Akt on PAM gels.
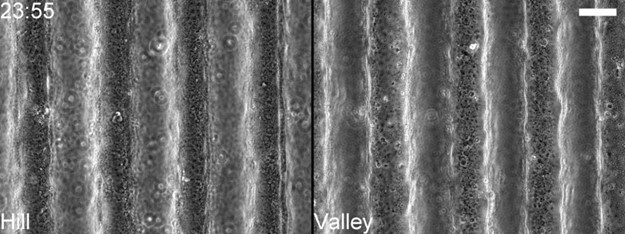
In response to Reviewer #1’s suggestion then, we have added another supporting time-lapse (Video 19) showing typical response of MDCK monolayers on 100 µm PAA waves (Author response image 4). Evident from the time-lapses, like the planar regions, cell extrusions were very rare. This supports the idea that on PAM gels the effects of basal hydraulic stress and asymmetric forces are marginal against the strong survival signals. And the response is similar to hyper-osmotic perturbations; there, we did not see a significant difference between valley and hill extrusions.
Author response image 4.
Time-lapse snapshot showing negligible MDCK extrusions 24 hours after confluency over PAM gel wave substrates.
Recommendation 4: Before proceeding with the FAK inhibitor experiment, the authors should better justify why the 4.1 wt % sucrose vs DMSO or NaCl is the most inert treatment. This can be done by citing relevant papers or showing time-lapses (as it is done for the higher FAKI14 dose).
Response 4: Although some cells have recently been shown to be able to transport and utilize sucrose, mammalian cells generally cannot directly take up polysaccharides for metabolism and this is frequently mentioned in literature: see (Ref. R1) for example. Without special enzymes to break sucrose down into monosaccharides, such as sucrase found in the gut, the sugars should remain spectators in the culture medium, contributing only to osmotic effects.
DMSO on the other hand, besides changing osmolarity, can also be integrated into cell membrane and pass through cells over time. It has been reported to chronically affect cell membrane properties and gene expressions (Ref. R2).
Finally, it is well known that both sodium and chloride ions are readily taken up and transported by cells (Ref R3). They help to regulate the transmembrane potential, which in turn can affect membrane bound proteins and biochemical reactions within a cell.
Hence, comparing the 3 hyper-osmotic perturbations, adding sucrose should have the least off- target effects on both the inhibitor study and the subsequent immunoblotting. And, in response to the reviewer’s recommendation, we have updated the text accordingly and included new references to support our statement.
Ref R1. H. Meyer, O. Vitavska, H. Wieczorek; Identification of an animal sucrose transporter. Journal of Cell Science 124, 1984–1991 (2011). Doi: 10.1242/jcs.082024
Ref R2. B. Gironi, Z. Kahveci, B. McGill, B.-D. Lechner, S. Pagliara, J. Metz, A. Morresi, F. Palombo, P. Sassi, P. G. Petrov; Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophysical Journal 119, 274-286 (2020). Doi: doi.org/10.1016/j.bpj.2020.05.037
Ref R3. X. Zhang, H. Li; Interplay between the electrostatic membrane potential and conformational changes in membrane proteins. Protein Science 28, 502-512 (2019). Doi: 10.1002/pro.3563
Recommendation 5: The data showing a FAK-dependent phosphorylation of AKT responsible for a higher cell survival rate in the hills is not yet completely convincing. Please show a reduced AKT phosphorylation level after FAK inhibition in high osmolarity levels. Furthermore, the levels of AKT activation seem to increase slightly upon substrate softening independently of FAK activation or osmotic pressure (i.e., Fig. 4E, Soft PDMS). The authors should comment on this in connection with the results shown for PAA gels.
Response 5: For the additional immunoblotting experiments, work is currently underway. We could not, however, complete these experiments in time for this revision, as both Cheng-Kuang and Xianbin will shortly be taking on new jobs elsewhere. David will continue with the immunoblotting studies and should be able to include the results in an update in the coming months. As for the apparent elevated levels of AKT seen on soft silicones, we speculate that it is because we cannot immunoblot cells that have died and were inevitably washed out at the start of the procedure. Inferring from the higher extrusion rates on these soft substrates, we could be missing a significant portion of stats. Specifically, we are missing all the cells that would have lowered AKT activation but died, and had we been able to collect those statistics, perhaps both the FAK and AKT should have shown lower levels. We risk committing survival bias on the results if we read too much into the data as is.
Alternatively, another explanation could be that, by virtue of survival of the fittest, we might have effectively selected a subpopulation of cells that were able to survive on lower FAK signals, or completely irrespectively of it.
At any rate, to prove our foregoing hypothesis would require us to perform comprehensive immunoblotting and total transcriptome analysis over different duration conditions. Unfortunately, we do not have the time to do that for the current article, but it could be developed into a stand-alone molecular biology investigation in future. We have included similar discussion in the main text.
Recommendation 6: In the discussion, the authors suggest the reported findings be especially relevant for epithelia that significantly separate compartments and regulate water and soluble transport. These are for example kidney epithelia (i.e., MDCK is the best experimental choice), retinal epithelium or intestinal epithelium. I would suggest that some proof-of-concept experiments could be done to support this concept. For example, I would expect keratinocytes (i.e., HaCaT) not to show a strong difference in extrusion rate between valleys and hills since the monolayer is not so sealed as kidney epithelium. In general, this kind of experiment would significantly strengthen the finding of this work.
Response 6: As recommended, we tracked the behavior of retina pigment epithelial cells (hTERT RPE-1 from ATCC) which do not form tight monolayers like MDCKs (Ref. R4). We did not detect extrusion events occurring from monolayers of these cells (Author response image 5). This is true even for portions of monolayers over waved regions.
Author response image 5.
Time-lapse snapshot showing non-existent o cell extrusions from RPE monolayers confluent for over 21 hours.
We have updated these findings in the main text discussions and included a new supporting time- lapse (Video 15) in our article.
Ref R4 F. Liu, T. Xu, S. Peng, R. A. Adelman, L. I. Rizzolo; Claudins regulate gene and protein expression of the retinal pigment epithelium independent of their association with tight junctions. Experimental Eye Research 198, 108157 (2020). Doi: 10.1016/j.exer.2020.108157
Recommendation 7 (minor point): Figure S1 needs to have clear notes indicating in each step what is what. i.e., where is glass, PDMS, NOA73, etc? A more detailed caption will help the figure's comprehension. Also "Cy52" should be changed to "soft silicone" to be consistent with the text (or Cy52 should be mentioned in the text).
Response 7 (minor point): Changes were made to Figure 1-figure supplement 1 to improve comprehension accordingly. CY52 was added to the main-text, next to the first appearance of the word soft silicone, to be consistent with the figures.
Recommendation 8 (minor point): The authors often mentioned that epithelial monolayers are denser on PAA gels. Please add a reference(s) to this statement.
Response 8 (minor point): The statement is an inference from visually comparing monolayers on PAM gels and PDMS. The difference is quite evident (Author response image 6). The density difference is in spite of the fact that the substrates share similar starting cell numbers.
To address the reviewer’s comment, we have combined time-lapses of monolayers on silicones and PAM gels side-by-side in Video 17 to facilitate convenient comparisons.
Author response image 6.
Time-lapse snapshot at 24 hours after confluence, showing conspicuously higher density of MDCK monolayers on PAM gel compared to those on silicon elastomer.
Reviewer #2
Recommendation 1: The sinusoidal wavy substrate that the authors use in their investigation is interesting and relevant, but it is important to realize that this is a single-curved surface (also known as a developable surface). This means that the Gaussian curvature is zero and that monolayers need to undergo (almost) no stretching to conform to the curvature. The authors should at least discuss other curved surfaces as an option for future research, and highlight how the observations might change. Convex and concave hemispherical surfaces, for example, might induce stronger differences than observed on the sinusoidal substrates, due to potentially higher vertical resultant forces that the monolayer would experience. The authors could discuss this geometry aspect more in their manuscript and potentially link it to some other papers exploring cell-curvature interactions in more complex environments (e.g. non-zero Gaussian curvature).
Response 1: In response to reviewer #2’s recommendation we have highlighted in the discussion of our text that our waves constitute a developable surface and that cells will experience little stretching for the most part. Based on our knowledge of how curvature can modulate forces and thus osmotic effects, we included some rudimentary analysis of what one would expect on hemispherical surfaces of two types: one that is periodic and contiguous (Ref. R5), and another with delineating flat regions (Ref. R6).
For epithelial monolayers in the first scenario, and on poorly solute/water permeable substrates, we should also expect to see a relatively higher likelihood of extrusions from concave regions compared to convex ones. Moreover, as the surfaces are now curved in both principal directions (producing larger out-of-plane forces), we should see the onset of differential extrusions seen in this study, but at larger length scales. For example, the effects seen on 100 µm hemicylindrical waves might now happen at larger feature size for hemispherical waves. Furthermore, as this kind of surface would invariably contain hyperbolic regions (saddle points), we might expect an intermediate response from these locations. If the forces in both principal directions offset each other, the extrusion response may parallel planar regions. On the other hand, if one dominates over the other, we may see extrusion responses tending to the dominating curvature (concave of convex).
On the other hand, on curved landscapes with discrete convex or concave regions, we should expect, within the curved surface, extrusion behaviors paralleling findings in this study. What would be interesting would be to see what happens at the rims (or skirt regions) of the features. At these locations we effectively have hyperbolically curved surfaces, and like before, we should expect some sort of competing effect between the forces generated from the principal directions. So, for dome skirts, we should see fewer extrusions when the domes are small, and vice versa, when they are larger. Meanwhile, for pit rims, we should see a reversed behavior. It should also be noted that the transitioning curvature between convex/concave and planar regions would also modulate the effect.
These effects might have interesting developmental implications. For instance, in developing pillar like tissues (e.g., villi) structures, the strong curvatures of nascent lumps would favor accumulation of cell numbers. However, once the size of the lumps reaches some critical value, epithelial cell extrusions might begin to appear at the roots of the developing structures, offsetting cell division, and eventually halting growth.
Ref R5. L. Pieuchot, J. Marteau, A. Guignandon, T. Dos Santos, I. Brigaud, P. Chauvy, T. Cloatre, A. Ponche, T. Petithory, P. Rougerie, M. Vassaux, J. Milan, N. T. Wakhloo, A. Spangenberg, M. Bigerelle, K. Anselme, Curvotaxis directs cell migration through cell-scale curvature landscapes. Nature Communications 9, 3995 (2018). Doi: 10.1038/s41467-018-06494-6
Ref R6. M. Werner, S. B.G. Blanquer, S. P. Haimi, G. Korus, J. W. C. Dunlop, G. N. Duda, D. W. Grijpma, A. Petersen, Surface curvature differentially regulates stem cell migration and differentiation via altered attachment morphology and nuclear deformation. Advanced Science 4, 1–11 (2017). Doi: 10.1002/advs.201600347
Recommendation 2: The discussion of the experiments on PAM gels is rather limited. The authors describe that cells on the PAM gels experience fewer extrusions than on the PDMS substrates, but this is not discussed in sufficient detail (e.g. why is this the case). Additionally, the description of the 3D traction force microscopy and its validation is quite limited and should be extended to provide more convincing evidence that the measured force differences are not an artefact of the undulations of the surface.
Response 2: We first saw a significant reduction in cell extrusions when we performed hyper-osmotic perturbations, and to eliminate possible off-target effects of the compounds used to increase osmolarity, we used three different compounds to be sure. In spite of this, we felt it would further support our argument, that basal accumulation of fluid stress was responsible for the extrusions, if we had some other independent means of removing fluid stress without directly tuning osmolarity through addition of extraneous solutes. We hence thought of culturing MDCK monolayers on hydrogels.
Hydrogels were chosen because they can effectively dilute basal solute concentration (for reference ions (Na+) are continuously pumped out basally by the monolayer) and thereby reduce the associated osmotically induced water transport. Moreover, as fluid could freely move within the gel, the fluid stress can quickly equilibrate across the basal surface. In contrast, poorly water/solute permeable substrates will lead to localized spikes in solute concentration and transient basal regions with high fluid stress.
To get a sense of the extent of difference in basal solute concentration between the two materials, we can do a quick hand-waving estimation. For monolayers on non-water-permeable PDMS of 20x20 mm, and using the laser wavelength (640 nm) for RICM as an extreme estimate of basal separation, we should expect ~0.25 µl of total basal water content. On the other hand, we typically produce our PAM gel slabs using ~150 µl of precursor solutions. This means that, given similar amounts of solute, PAM gels will lead to monolayer basal osmolarity that is around 3 orders of magnitude lower than monolayers on PDMS, producing significantly lower osmotic potential. This implies from the outset that we should expect high survivability of cells on these substrates. Indeed, later immunoblotting experiments showed MDCKs exhibiting hyper activated FAK and Akt on PAM gels.
As for the 3D TFM used in this study, it is actually implemented from a well-established finite element method to solve inverse problems in engineering and has been repeatedly validated in larger scale engineering contexts (Ref. R7). The novelty and contribution of our article is in its adaptation to reconstruct cellular forces at microscopic scales.
In brief, soft materials, such as hydrogels used in our case, are doped with fluorescent particles, coated with ECM, and then seeded with cells. The cells would exert forces that deform the soft substrate, thereby displacing the fluorescent particles from their equilibrium positions. This particle displacement can be extracted by producing an image pair with microscopy; first one with the cells, and subsequent one of relaxed gel after removal of cells with acutely cytotoxic reagents, such as SDS. There are several ways in which the displacement field can be extracted from the image pair. These include particle tracking velocimetry, particle image velocimetry, digital volume correlation, and optical flow.
We employed 3D Farneback optical flow in our study for its superior computational performance. The method was validated using synthetically generated images from Sample 14 of the Society for Experimental Mechanics DIC challenge. The accuracy of the calculated displacements using the 3D Farneback optical flow was then compared to the provided ground truth displacements. For the highest frequency displacement image pairs, an x-component root-mean-square-error (RMSE) value of 0.0113 was observed. This was lower than the 0.0141 RMSE value for the Augmented Lagrangian Digital Volume Correlation method. This suggested that the 3D Farneback optical flow is capable of accurately calculating the displacement between two bead images.
The displacement fields are then fed into a finite element suite (ANSYS in our case) along with the model and mesh of the underlying substrate structure to obtain node specific displacements. This is required because mech nodes do not typically align with voxel positions of displacements. With these node specific displacements, we subsequently solve the inverse problem for the forces using Tikhonov regularization (Ref. R8). The outcome is a vector of node specific forces.
In light of the above, to physically validate the method in our context would require the generation of a known ground truth force on the scale of pico- to nano-newtons and subsequently image the particle displacements from this force using confocal microscopy. The force must then be released in situ in order for the relaxed gel to be imaged again. This is not a straightforward feat at this scale, and a method that immediately springs to mind is magnetic tweezers. Unfortunately, this is a tool that we cannot develop within reasonable timeframes, as the method will have to be seamlessly integrated with our spinning-disk confocal. However, as a compromise, we have included an in-silico validation with our revised manuscript.
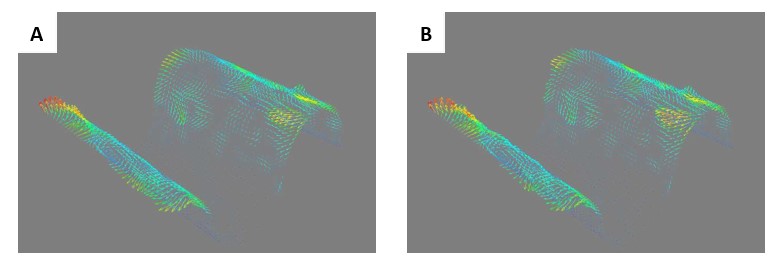
Specifically, given a finite element model with a predefined curvature, a known force was applied to the surface of the model (Author response image 7A). The resulting displacements were then calculated from the finite element solution. A 10% random noise is then added to the resulting displacement. The traction force recovery (Fig. R2-1 B) was then performed using the in-silico noisy displacements. To evaluate the accuracy of the recovery, the cosine similarity along with the mean norm of the force vectors were calculated. A value closer to 1 for both evaluation metrics indicates a more accurate reconstruction of the simulated traction force. The cosine similarity of the recovered traction forces to the original applied force was 0.977±0.056 while the norm of the recovered traction forces as a proportion of the original applied force was 1.016±0.165. As both values are close to 1 (i.e., identical), this suggested that the traction forces could be satisfactorily recovered using the finite-element based method.
In response to the reviewer’s recommendations then, additional content has been included in the main text to explain the use of PAM gels and the workings of our 3D TFM pipeline.
Ref R7. James F. Doyle, Modern Experimental Stress Analysis: Completing the Solution of Partially Specified Problems (John Wiley & Sons, Chichester, 2004).
Ref R8. Per Christian Hansen, Discrete Inverse Problems: Insight and Algorithms (siam, Philadelphia, 2010).
Author response image 7.
(A) shows simulated force field to generate simulated displacements. (B) shows force field reconstructed from simulated displacements with noise.
Recommendation 3: The authors show nuclear deformation on the hills and use this as evidence for a resultant downward-pointing force vector. This has, indeed, also been observed in other works referenced by the authors (e.g. Werner et al.), and could be interesting evidence to support the current observations, provided the authors also show a nuclear shape on the concave and flat regions. The authors could potentially also characterize this shape change better using higher-resolution data.
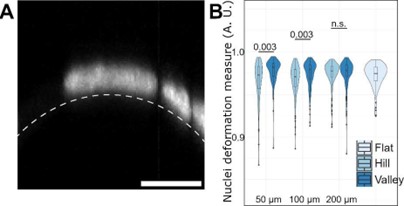
Response 3: We characterized nucleus deformation using Hoechst-stained samples as per recommendation. The deformation is estimated by dividing segmented nuclei volumes by best-fit ellipsoid volumes of same objects. In this way, objects exhibiting minimal bending will lead to values close to 1.0. The obtained graph is shown in figure Author response image 8B (and manuscript Figure 3D).
Author response image 8.
(A) an example of deformed nuclei on 50 µm wave hill region. (B) a Violin plot of calculated nuclear deformations across dimensions and features using segmented volume normalized against best-fit ellipsoid volume.
Our quantifications show a statistically significant difference in nuclei deformation measure medians between hill and valley cells on the 50 µm (0.973 vs 0.982) and 100 µm (0.971 vs 0.979) waves; this indicates that cells on the hills tend to have more deformed nuclei compared to cells in the valleys. Meanwhile, no significant difference was found for a similar comparison on 200 µm (0.978 vs 0.978) samples. For reference, the median found for cells pooled from planar regions was 0.975.
In response to the reviewer’s suggestions Figure 3 of our manuscript has been updated to include the new results on nuclei deformation. The text has also been updated to account for the new information to support our claims. The statistics are included in a new summary data table in Supplementary File 6.
Recommendation 4: The U-net for extrusion detection is a central tool used within this study, though the explanation and particularly validation of the tool are somewhat lacking. More clarity in the explanation and more examples of good (or bad) detections would help establish this tool as a more robust component of the data collection (on all geometries).
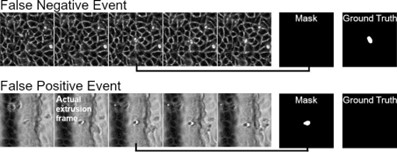
Response 4: The architecture of the neural network used in this study is outlined in supplementary figure S5a. To validate the performance of the model, a test dataset consisting of 200 positive examples and 100 negative examples were fed into the network and the resulting prediction was obtained from model. The confusion matrix of the model is shown in supplementary figure S5c. The weighted precision and recall of the model are 0.958 and 0.953 respectively.
Additionally, we have included examples of false positive and false negative detections in Figure 1-figure supplement 5 (Author response image 8). For false positive detections, these were typically observed to be extrusions that were labelled to have occurred the frame prior to the frame of interest (Author response image 9 bottom sequence). However, as the extrusion process is incomplete in the prior frame, there are still changes in the extruded cell body and the network falsely predicts this as a detection.
Author response image 9.
Examples of false negative and false positive extrusions registration.
Recommendation 5: The authors study the involvement of FAK in the observed curvature-dependent and hydraulic stress-dependent spatial regulation of cell extrusion. In one of the experiments, the authors supplement the cell medium with FAK inhibitors, though only in a hyper-osmotic medium. They show that FAK inhibition counteracts the extrusion-suppressing effect of a hyper-osmotic medium. However, no data is shown on the effect of FAK inhibitors within the control medium. Would the extrusion rates be even higher then?
Response 4: We proceeded, as suggested by the reviewer, to explore the effects of the FAK inhibitor on MDCK monolayers in our control medium. The results revealed that, at the 3 µM FAK concentration, where cells in sucrose media showed an elevated extrusion rate, monolayers in control medium quickly suffered massive cell death (Author response image 10) similar to what was seen when 6 µM FAK was introduced to sucrose medium.
This finding suggests that osmolarity protects against FAK inhibitors in a dose dependent manner. Moreover, as cell extrusions require an intact monolayer, its rates cannot increase indefinitely: a point will be reached where an intact monolayer can no longer be maintained.
We have updated the main text of our article to mention this observation, and also included a new time-lapse (Video 22) to demonstrate the effect.
Author response image 10.
Timelapse snapshot of MDCK monolayers over waves 4 hours after inclusion of focal adhesion kinase inhibitor.
Recommendation 6: The supplementary videos show two fields of view next to each other, which is not immediately clear to the viewer. I strongly advise the authors to add a clear border between the two panels, so that it is clear that the cells from one panel are not migrating into the next panel.
Response 6: A distinctive border has been added to the movies to separate panels showing different focal planes of the same stack.
Recommendation 7: The general quality and layout of the figures could be improved. Some figures would benefit from higher-resolution or larger cell images (e.g. Figure 2A, C, D), and the organisation of subpanels could be improved (e.g. especially in Figure 2). The box plots and bar graphs are also not consistent throughout the manuscript in terms of colouring and style, which should be improved.
Response 7: We have enlarged the figures in question accordingly, at the cost of reducing some information. However, the full scope of the sub-figures remains accessible in the supplementary movies. We have also tried to change the placement of the panels to improve readability. We have also adjusted the valley, hill, and flat coloring scheme for the extrusion boxplots in Figures 1 and 2 to make them consistent.
Recommendation 8: The graphs in Figures 3E and F are confusing and difficult to interpret. The x-axis states "Position along curve in radians" but it is unclear how to relate this to the position on the wavy substrate. The graphs also have a second vertical axis on the right ("valley-interface-hill"), which adds to the confusion. I would recommend the authors provide more explanation and consider a different approach of plotting this.
Response 8: We have removed the confusing plot of cross-sectional profile from the force graphs. To indicate positions on the waves, we have augmented radian values with Hill, Interface, and Valley accordingly.
Recommendation 9: Specify which silicone was used for the low-stiffness silicone substrates in the methods and in the main text.
Response 9: CY52 has been added to the main-text, next to the first appearance of the word soft silicone, to be consistent with the figures.
Recommendation 10: The flow lines that are plotted over the RICM data make it difficult to see the underlying RICM images. I would advise to also show the RICM images without the flow lines.
Response 10: The original movie S15 (now Video 16) showing the RICM overlapped with optical flow paths has now been replaced by a movie showing the same, but with the flow paths and RICM in separate panels.
Recommendation 11: In the first paragraph of the discussion, the authors write: "And this difference was both dependent on the sense (positive or negative)...". This is superfluous since the authors already mentioned earlier in the paragraph that the convex and concave regions (i.e. different signs of curvature) show differences in extrusion rates.
Response 11: The sentence has been changed to “And this difference was also dependent on the degree of curvature.”
Recommendation 12: In the second paragraph of the discussion, the authors mention that "basal fluid spaces under monolayers in hill regions were found consistently smaller than those in valley regions". Is this data shown in the figures of the manuscript? If so, a reference should be made because it was unclear to me.
Response 12: This statement is an inference from the comparison of the hill and valley RICM grey values. Specifically, RICM intensities are direct surrogates for basal separations (i.e., fluid space (as there cannot be a vacuum)) by virtue of the physics underlying the effect. To be more precise then, “inferred from RICM intensity differences (Figure 2I)” has been added to support the statement.
Recommendation 13: On page 7 of the discussion, the authors talk about positively and negatively curved surfaces. This type of description should be avoided, as this depends on the definition of the surface normal (i.e. is positive convex or concave?). Rather use convex and concave in this context.
Response 13: The wording has been changed accordingly.
Recommendation 14: The label of Table 8 reads "Table 2".
Response 14: The error has been corrected.
Reviewer #3
Recommendation 1: The central finding seems to be opposite to an earlier report (J Cell Sci (2019) 132, jcs222372), where MDCK cells in curved alginate tubes exhibit increased extrusion on a convex surface. I suggest that you comment on possible explanations for the different behaviors.
Response 1: The article in question primarily reported the phenomenon of MDCK and J3B1A monolayers detaching from the concave alginate tube walls coated with Matrigel. The authors attributed this to the curvature induced out-of-plane forces towards the center of the tubes. Up to this point, the findings and interpretation are consistent with our current study where we also find a similar force trend in concave regions.
To further lend support to the importance of curvature in inducing detachment, the authors cleverly bent the tubes to introduce asymmetry in curvature between outer and inner surfaces. Specifically, the outside bend is concave in both principal directions, whereas the inside bend is convex in one of its principal directions. As expected, the authors found that detachment rates from the outer surface were much larger compared to the inner one. Again, the observations and interpretations are consistent with our own findings; the convex direction will generate out-of-plane forces pointing into the surface, serving to stabilize the monolayer against the substrate. It should be noted however, since the inner-side tube is characterized by both convex and concave curvatures in its two principal directions, the resulting behavior of overlaying monolayers will depend on which of the two resulting forces become dominant. So, for gradual bends, one should expect the monolayers to still be able to detach from the inner tube surface. This is what was reported in their findings.
For their extrusion observations, I am surprised. Because their whole material (hydrogels) is presumably both solute and water permeable, I would be more inclined to expect very few extrusions irrespective of curvature. This is indeed the case with our study of MDCKs on PAM hydrogels, where the hydrogel substrate effectively buffers against the quick build-up of solute concentration and basal hydraulic stress. Without the latter, concave monolayer forces alone are unlikely to be able to disrupt cell focal adhesions. Indeed, the detachments seen in their study are more likely by exfoliation of Matrigel rather than pulling cells off Matrigel matrix entirely.
My guess is that the extrusions seen in their study are solely of the canonical crowding effect. If this was the case, then the detached monolayer on the outside bend could buffer against crowding pressure by buckling. Meanwhile, the monolayer on the inside bend, being attached to the surface, can only regulate crowding pressure by removing cells through extrusions. This phenomenon should be particular to soft matrices such as Matrigel. Using stiffer and covalently bonded ECM should be sufficient to prevent monolayers from detaching, leading to similar extrusion behaviors. In response to the reviewer’s recommendation then, we have included a short paragraph to state the points discussed in this response.
Recommendation 2: Fig 3E, F: The quantities displayed on the panels are not forces, but have units of pressure (or stress).
Response 2: we have changed “force” to “stress” according to the reviewer’s suggestion. The reason we kept the use of force in the original text was due to the fact that we were reconstructing forces. Due to discretization, the resulting forces will inevitably be assigned to element nodes. In between the nodes, in the faces, there will be no information. So, in order to have some form of continuity to plot, the face forces are obtained by averaging the 4 nodes around the element face. Unfortunately, element face areas are not typically of the same size, therefore the average forces obtained needs to be further normalized against the face area, leading to a quantity that has units of stress.
Recommendation 3: Fig 2D: Asterisks are hard to see.
Response 3: the color of the asterisks has been changed to green for better clarity against a B&W background.
Recommendation 4: p 19, l 7: Word missing in "the of molding"
Response 4: the typo has been amended to “the molding of”.
-

eLife assessment
This paper presents important findings into the response of epithelial monolayers to the combined effects of surface curvature and hydraulic stress, offering insights into how these cues contribute to epithelial cell extrusion. Most of the evidence is convincing, relying mainly on a combination of imaging-based techniques. This paper is of interest to a broad and growing community of biologists, biophysicists, and engineers interested in cell-geometry interactions.
-

Reviewer #1 (Public Review):
Huang C-K. and colleagues in this work address the understudied role of environmental conditions and external forces in cell extrusion as a fundamental part of epithelial homeostasis. They suggest that hydrostatic stress plays a significant role in counteracting cell extrusion forces through the indirect regulation of the focal adhesion kinase (FAK) - protein kinase B (AKT) survival pathway. The team nicely exploits their expertise in fabricating cell culture substrates to control hydrostatic stress on a common epithelial cell model from the kidney (i.e., MDCK). This was done by creating waving surfaces with different lengths from 50µm to 200 µm, thus creating a heterogenous distribution of monolayer forces towards the substrate. Finally, using a specific inhibitor for FAK, they suggest that the survivor …
Reviewer #1 (Public Review):
Huang C-K. and colleagues in this work address the understudied role of environmental conditions and external forces in cell extrusion as a fundamental part of epithelial homeostasis. They suggest that hydrostatic stress plays a significant role in counteracting cell extrusion forces through the indirect regulation of the focal adhesion kinase (FAK) - protein kinase B (AKT) survival pathway. The team nicely exploits their expertise in fabricating cell culture substrates to control hydrostatic stress on a common epithelial cell model from the kidney (i.e., MDCK). This was done by creating waving surfaces with different lengths from 50µm to 200 µm, thus creating a heterogenous distribution of monolayer forces towards the substrate. Finally, using a specific inhibitor for FAK, they suggest that the survivor pathway FAK-AKT is involved in the observed phenomenon.
In conclusion, the presented data underline the importance of considering external forces and tissue geometry in regulating epithelial homeostasis and the selective transport of water and solutes. These results may have a significant impact on understanding the basic mechanisms of epithelial physiology and pathology, such as in the kidney, intestine, or retina.
Comments on the revised version:
Overall, most of my comments were reasonably addressed. Nevertheless, one comment was not convincingly addressed ("Recommendation 5" - reviewer #1).
The authors did not show that the FAK inhibitor directly induced the reduction of AKT phosphorylation but used this experiment to conclude that FAK - AKT survivor pathway is involved in the observed phenomenon (Fig. 4). The authors mentioned that additional immunoblotting experiments are currently underway. This is a minor control for the manuscript message, but I feel it is necessary. The connection between the levels of FAK and p-AKT shown in Fig. 4E is purely correlative and can be caused by ECM adhesion-independent reasons.
Alternatively, the authors could reduce the stress on the FAK - AKT survivor pathway's involvement and conclude only on the involvement of FAK.
-

Reviewer #2 (Public Review):
The paper by Huan, Yong, et al. studies epithelial cell extrusion in MDCK monolayers grown on sinusoidally wavy surfaces in varying media osmolarities, finding that both curvature and osmolarity-mediated basal hydraulic stress spatially regulate extrusion events. The authors fabricated wavy substrates of varying periods and amplitude out of PDMS (and PA hydrogels) and monitored monolayer evolution and cell extrusion over time, by combining live-cell imaging with a convolutional network-based algorithm for automatic detection of extrusions.
In general, the study has been elegantly designed, starting with convincing evidence for enhanced extrusion rates in concave valleys with respect to convex hills. Next, the authors showed that hyper-osmotic medium reduced cell extrusion rate, which was demonstrated in a …
Reviewer #2 (Public Review):
The paper by Huan, Yong, et al. studies epithelial cell extrusion in MDCK monolayers grown on sinusoidally wavy surfaces in varying media osmolarities, finding that both curvature and osmolarity-mediated basal hydraulic stress spatially regulate extrusion events. The authors fabricated wavy substrates of varying periods and amplitude out of PDMS (and PA hydrogels) and monitored monolayer evolution and cell extrusion over time, by combining live-cell imaging with a convolutional network-based algorithm for automatic detection of extrusions.
In general, the study has been elegantly designed, starting with convincing evidence for enhanced extrusion rates in concave valleys with respect to convex hills. Next, the authors showed that hyper-osmotic medium reduced cell extrusion rate, which was demonstrated in a variety of different media compositions (e.g. with sucrose, DMSO, or NaCl), while hypo-osmotic medium increased cell extrusion rate. Additionally, the authors applied reflection interference contrast microscopy to reveal fluid spaces between the substrate and the basal side of the monolayer, which were found to grow when media composition was altered from hyper-osmotic to normal osmotic conditions. Using a 3D traction force microscopy approach, the authors demonstrated that cells on convex regions apply a downward pointing force on the substrate, opposite to cells on the concave regions. This was linked to a larger basal separation on the concave valleys as opposed to the convex hills. Finally, the authors focussed on the FAK-Akt pathway to explore the hypothesis that basal hydraulic stress interferes with focal adhesions, leading to differences in cell extrusion rates in media of different osmolarity and on convex or concave surfaces.
Comments on the revised version:
My previous comments were reasonably answered. In response to the comment that "experiments that are currently underway" for "Recommendation 5 - reviewer #1", I would also suggest the authors to either add the additional data or alter the emphasis on the FAK-AKT pathway in the manuscript accordingly if additional data is not presented.
-

eLife assessment
This paper presents important findings into the response of epithelial monolayers to the combined effects of surface curvature and hydraulic stress, offering insights into how these cues contribute to epithelial cell extrusion. Most of the evidence is convincing, relying mainly on a combination of imaging-based techniques, though some parts would benefit from a more rigorous analysis and some claims require more evidence to be justified. This paper is of interest to a broad and growing community of biologists, biophysicists, and engineers interested in cell-geometry interactions.
-

Reviewer #1 (Public Review):
Huang C-K. and colleagues in this work address the understudied role of environmental conditions and external forces in cell extrusion as a fundamental part of epithelial homeostasis. They suggest that hydrostatic stress plays a significant role in counteracting cell extrusion forces through the indirect regulation of the focal adhesion kinase (FAK) - protein kinase B (AKT) survival pathway. The team nicely exploits their expertise in fabricating cell culture substrates to control hydrostatic stress on a common epithelial cell model from the kidney (i.e., MDCK). This was done by creating waving surfaces with different lengths from 50µm to 200 µm, thus creating a heterogenous distribution of monolayer forces towards the substrate. Finally, using a specific inhibitor for FAK, they suggest that the survivor …
Reviewer #1 (Public Review):
Huang C-K. and colleagues in this work address the understudied role of environmental conditions and external forces in cell extrusion as a fundamental part of epithelial homeostasis. They suggest that hydrostatic stress plays a significant role in counteracting cell extrusion forces through the indirect regulation of the focal adhesion kinase (FAK) - protein kinase B (AKT) survival pathway. The team nicely exploits their expertise in fabricating cell culture substrates to control hydrostatic stress on a common epithelial cell model from the kidney (i.e., MDCK). This was done by creating waving surfaces with different lengths from 50µm to 200 µm, thus creating a heterogenous distribution of monolayer forces towards the substrate. Finally, using a specific inhibitor for FAK, they suggest that the survivor pathway FAK-AKT is involved in the observed phenomenon.
In conclusion, the presented data underline the importance of considering external forces and tissue geometry in regulating epithelial homeostasis and the selective transport of water and solutes. These results may have a significant impact on understanding the basic mechanisms of epithelial physiology and pathology, such as in the kidney, intestine, or retina.
-

Reviewer #2 (Public Review):
The paper by Huan, Yong, et al. studies epithelial cell extrusion in MDCK monolayers grown on sinusoidally wavy surfaces in varying media osmolarities, finding that both curvature and osmolarity-mediated basal hydraulic stress spatially regulate extrusion events. The authors fabricated wavy substrates of varying periods and amplitude out of PDMS (and PA hydrogels) and monitored monolayer evolution and cell extrusion over time, by combining live-cell imaging with a convolutional network-based algorithm for automatic detection of extrusions.
In general, the study has been elegantly designed, starting with convincing evidence for enhanced extrusion rates in concave valleys with respect to convex hills. Next, the authors showed that hyper-osmotic medium reduced cell extrusion rate, which was demonstrated in a …
Reviewer #2 (Public Review):
The paper by Huan, Yong, et al. studies epithelial cell extrusion in MDCK monolayers grown on sinusoidally wavy surfaces in varying media osmolarities, finding that both curvature and osmolarity-mediated basal hydraulic stress spatially regulate extrusion events. The authors fabricated wavy substrates of varying periods and amplitude out of PDMS (and PA hydrogels) and monitored monolayer evolution and cell extrusion over time, by combining live-cell imaging with a convolutional network-based algorithm for automatic detection of extrusions.
In general, the study has been elegantly designed, starting with convincing evidence for enhanced extrusion rates in concave valleys with respect to convex hills. Next, the authors showed that hyper-osmotic medium reduced cell extrusion rate, which was demonstrated in a variety of different media compositions (e.g. with sucrose, DMSO, or NaCl), while hypo-osmotic medium increased cell extrusion rate. Additionally, the authors applied reflection interference contrast microscopy to reveal fluid spaces between the substrate and the basal side of the monolayer, which were found to grow when media composition was altered from hyper-osmotic to normal osmotic conditions. Using a 3D traction force microscopy approach, the authors demonstrated that cells on convex regions apply a downward pointing force on the substrate, opposite to cells on the concave regions. This was linked to a larger basal separation on the concave valleys as opposed to the convex hills. Finally, the authors focussed on the FAK-Akt pathway to explore the hypothesis that basal hydraulic stress interferes with focal adhesions, leading to differences in cell extrusion rates in media of different osmolarity and on convex or concave surfaces.
Despite the host of relevant experiments and the interesting data acquired with a variety of techniques, some aspects of the manuscript would need to be strengthened or explained in more detail to better support the claims and to provide more convincing evidence.
The sinusoidal wavy substrate that the authors use in their investigation is interesting and relevant, but it is important to realise that this is a single-curved surface (also known as a developable surface). This means that the Gaussian curvature is zero and that monolayers need to undergo (almost) no stretching to conform to the curvature. The authors should at least discuss other curved surfaces as an option for future research, and highlight how the observations might change. Convex and concave hemispherical surfaces, for example, might induce stronger differences than observed on the sinusoidal substrates, due to potentially higher vertical resultant forces that the monolayer would experience. The authors could discuss this geometry aspect more in their manuscript and potentially link it to some other papers exploring cell-curvature interactions in more complex environments (e.g. non-zero Gaussian curvature).
The discussion of the experiments on PAM gels is rather limited. The authors describe that cells on the PAM gels experience fewer extrusions than on the PDMS substrates, but this is not discussed in sufficient detail (e.g. why is this the case). Additionally, the description of the 3D traction force microscopy and its validation is quite limited and should be extended to provide more convincing evidence that the measured force differences are not an artefact of the undulations of the surface.
The authors show nuclear deformation on the hills and use this as evidence for a resultant downward-pointing force vector. This has, indeed, also been observed in other works referenced by the authors (e.g. Werner et al.), and could be interesting evidence to support the current observations, provided the authors also show a nuclear shape on the concave and flat regions. The authors could potentially also characterise this shape change better using higher-resolution data.
The U-net for extrusion detection is a central tool used within this study, though the explanation and particularly validation of the tool are somewhat lacking. More clarity in the explanation and more examples of good (or bad) detections would help establish this tool as a more robust component of the data collection (on all geometries).
The authors study the involvement of FAK in the observed curvature-dependent and hydraulic stress-dependent spatial regulation of cell extrusion. In one of the experiments, the authors supplement the cell medium with FAK inhibitors, though only in a hyper-osmotic medium. They show that FAK inhibition counteracts the extrusion-suppressing effect of a hyper-osmotic medium. However, no data is shown on the effect of FAK inhibitors within the control medium. Would the extrusion rates be even higher then?
-

Reviewer #3 (Public Review):
The authors study monolayers of MDCK cells on curved surfaces. These surfaces consist of hemicylindrical valleys and hills obtained through microfabrication involving glass rods and repeated molding steps. They find higher apoptotic extrusion rates in valleys compared to hills for patterns with 25 and 50 µm curvature radii, but not in valleys of 100 µm curvature radius. By using osmotic shocks and reflection interference contrast microscopy, they identify hydraulic stress to drive cell extrusion. 3D force microscopy reveals that cytoskeletal forces point towards the substrate on hills and away from the substrate in valleys. From these observations, the authors conclude that hydraulic stress-induced cell extrusion is assisted by cytoskeletal forces in the valleys and opposed on the hills. Finally, they link …
Reviewer #3 (Public Review):
The authors study monolayers of MDCK cells on curved surfaces. These surfaces consist of hemicylindrical valleys and hills obtained through microfabrication involving glass rods and repeated molding steps. They find higher apoptotic extrusion rates in valleys compared to hills for patterns with 25 and 50 µm curvature radii, but not in valleys of 100 µm curvature radius. By using osmotic shocks and reflection interference contrast microscopy, they identify hydraulic stress to drive cell extrusion. 3D force microscopy reveals that cytoskeletal forces point towards the substrate on hills and away from the substrate in valleys. From these observations, the authors conclude that hydraulic stress-induced cell extrusion is assisted by cytoskeletal forces in the valleys and opposed on the hills. Finally, they link the hydraulic stress to the activity of focal adhesion kinase, which in turn affects cell survival through Akt signaling.
Strengths:
This work combines a new microfabrication method with state of the art 3d force microscopy that allows the authors to study curvature-dependent cell extrusion. The application of various osmotic shocks to the system clearly identifies the role of hydraulic stress in cell extrusion. The decoupling of the main driver of cell extrusion (hydraulic stress) from its curvature-dependent modulation through cytoskeletal forces, together with the mechanical activation of apoptosis is an important new finding that significantly advances our understanding of epithelial cell extrusion and could be important during developmental processes and for maintaining intact epithelia in adult organisms.
Weaknesses:
The main weakness of this work is a lack of quantification of the hydraulic stress. Furthermore, the authors do not present data on other cell types such that the phenomenon studied in this work might be specific to MDCK cells. Finally, The authors do not modify cytoskeleton contractility to check how this parameter affects the threshold curvature below which cell extrusion is no longer curvature dependent.
-

-