Single-cell profiling of lncRNAs in human germ cells and molecular analysis reveals transcriptional regulation of LNC1845 on LHX8
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This manuscript provides a comprehensive analysis of expression patterns and genomic features of long non-coding RNAs (lncRNAs) in the human developing gonad, using available single-cell RNA-seq datasets from both somatic and germ cells. Using multiple genetic strategies in an in vitro system of female germ cell differentiation, the study further shows a positive regulatory function of the LNC1845 lncRNA on its protein-coding neighbor LHX8, known to have a role in ovarian follicle development. This study has potential interest for reproductive biologists and for the non-coding RNA community.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Non-coding RNAs exert diverse functions in many cell types. In addition to transcription factors from coding genes, non-coding RNAs may also play essential roles in shaping and directing the fate of germ cells. The presence of many long non-coding RNAs (lncRNAs) which are specifically expressed in the germ cells during human gonadal development were reported and one divergent lncRNA, LNC1845 , was functionally characterized. Comprehensive bioinformatic analysis of these lncRNAs indicates that divergent lncRNAs occupied the majority of female and male germ cells. Integrating lncRNA expression into the bioinformatic analysis also enhances the cell-type classification of female germ cells. Functional dissection using in vitro differentiation of human pluripotent stem cells to germ cells revealed the regulatory role of LNC1845 on a transcription factor essential for ovarian follicle development, LHX8 , by modulating the levels of histone modifications, H3K4me3 and H3K27Ac. Hence, bioinformatical analysis and experimental verification provide a comprehensive analysis of lncRNAs in developing germ cells and elucidate how an lncRNA function as a cis regulator during human germ cell development.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
Wang et al. elegantly exploit single-cell RNA-seq datasets to question the putative involvement of lncRNAs in human germ cell development. In the first part of the study, the authors use computational approaches to identify and characterize, from existing data, lncRNAs expressed in the germline. Of note, the scRNA-seq data used were generated from polyA+ RNAs, and thus non-polyadenylated lncRNAs could not be retrieved. Most of the lncRNAs identified in the germ cells and in the somatic cells of the gonads were previously unannotated. While this increases the catalog of lncRNA genes in the human genome, further characterization is needed to determine which fraction of these newly identified lncRNAs represent bona fide transcripts or transcriptional noise.
Differential expression analysis …
Author Response
Reviewer #2 (Public Review):
Wang et al. elegantly exploit single-cell RNA-seq datasets to question the putative involvement of lncRNAs in human germ cell development. In the first part of the study, the authors use computational approaches to identify and characterize, from existing data, lncRNAs expressed in the germline. Of note, the scRNA-seq data used were generated from polyA+ RNAs, and thus non-polyadenylated lncRNAs could not be retrieved. Most of the lncRNAs identified in the germ cells and in the somatic cells of the gonads were previously unannotated. While this increases the catalog of lncRNA genes in the human genome, further characterization is needed to determine which fraction of these newly identified lncRNAs represent bona fide transcripts or transcriptional noise.
Differential expression analysis between developmental stages, sexes, or cell types led to several observations: (i) whatever the stage of development, the number of expressed lncRNAs is higher in fetal germ cells compared to gonadal somatic cells; (ii) there is a continuous increase in the number of expressed lncRNA during the development of the germline; of note, a similar, although the more subtle trend is observed for protein-coding genes; (iii) the developmental stage at which there is the highest number of lncRNA expressed differs between male and female germ cells. While convincing, the significance of these observations is difficult to assess. However, the authors remain prudent with their conclusion and are not over-interpreting their findings.
We appreciate Reviewer #2 precise summary of our analysis and highlighting the significances of these datasets for other researchers and future studies.
Interestingly, integrating lncRNA expression to classify cell types led to the identification of a novel population of cells in the female germline that had not been revealed by protein-coding gene only-based classification. The biological relevance of this population, which cluster with mitotic populations, remains to be demonstrated. Finally, by examining lncRNA biotype, the authors could demonstrate an enrichment, in the germ cells, of the antisense head-to-head organization (in relation to the nearby protein-coding gene) compared to other biotypes. Whether this is different from the general distribution of lncRNA should be discussed.
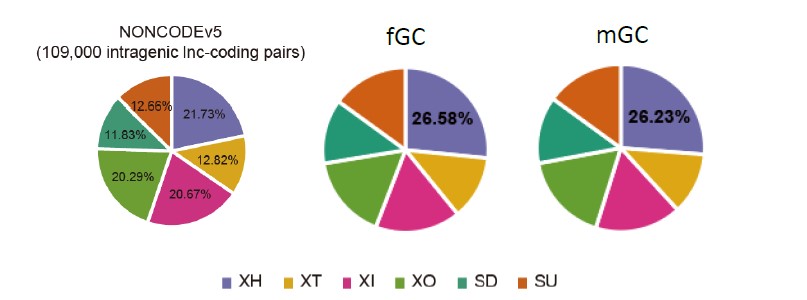
We analyzed the lncRNAs in NONCODEv5 database (human genome), and the result showed that XH type occupied 21.73% of the intragenic lncRNA-mRNA pairs in NONCODEv5 database (human genome), which is lower than 26.58% in fGC and 26.23% in mGC (Response Figure 1).
Response Figure 1. Genomic distribution and biotypes of the lncRNAs in NONCODEv5 database and lncRNAs expressed in human gonad.
In the second part of the manuscript, Wang et al focus on one pair of divergent lncRNA-protein coding genes (LNC1845-LHX8). To document the choice of this particular pair, it would be informative to have its correlation score indicated in Figure 3C. he existence of this transcript was validated using female fetal ovaries, and its function was addressed in late primordial germ cells like cells (PGCLC) derived from human embryonic stem cells (hESCs). The authors have used an admirable set of orthogonal approaches that led them to conclude as to a role for LNC1845 in regulating in cis the nearby gene LHX8. They further went on to identify the underlying mechanisms, which involve modification of the chromatin landscape through direct interaction of LNC1845 with a histone modifier. Among the different strategies used (KO, stop transcription, overexpression), the shRNA-mediated knock-down is the only one to specifically address the function of the transcript itself, as opposed to the active transcription. The result of this experiment led the authors to conclude that the LNC1845 RNA is functional, a conclusion that is reinforced by the demonstration of physical interaction between the LNC1845 RNA and WDR5, a component of MLL methyltransferase complexes. The result of the KD experiment is however puzzling as RNAi has been shown not to be the method of choice for targeting nuclear lncRNAs (Lennox et al. NAR 2016).
We thank the Reviewer #2’s suggestion to add the correlation score of LNC1845-LHX8 pair and the Pearson Correlation of this pair is 0.3268. We have added the number to Figure 4C because which the expression correlation of LNC1845 and LHX8 was first mentioned. We have compared many other similar studies, shRNA knockdown has been widely used to target nuclear lncRNAs (Guttman et al. Nature 2011; Luo et al. Cell Stem Cell 2016; Subhash et al. Nucleic Acids Res. 2018; Li et al. Genome Res 2021), and the knockdown efficiency seemed to be feasible and acceptable to be used. The knockdown results are consistent with the deletion mutation and stop transcription approaches, all three showed that LNC1845 transcriptional expression is required for proper LHX8 expression in late PGCLCs.
Overall, the functional investigation is convincing and strengthened by the inclusion of multiple clones for each approach, and by the convergence in the outcome of each individual approach. The depth of characterization is also remarkable. The analyses of the mechanisms at stake are somehow less solid, as there is less evidence demonstrating the involvement of the LNC1845 RNA and its interaction with WDR5.
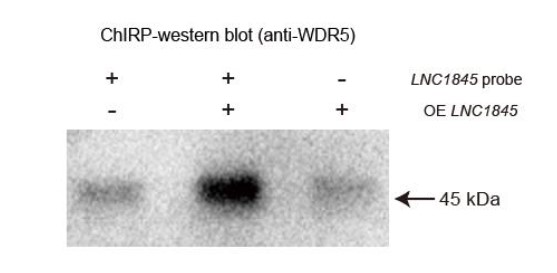
We have added more experimental evidence to strengthen the model especially the interaction of LNC1845 and WDR5. Apart from the RIP-qPCR results of WDR5 demonstrating the enrichment of LNC1845 by WDR5 pulldown (Figure S8D), we performed chromatin isolation by RNA purification (ChIRP) assay using antisense oligos along the entire LNC1845 transcript sequence. ChIRP results confirmed that WDR5 protein were enriched when anti-LNC1845 oligo probes were used to isolate the complex but not the controls without the probes or without overexpression of LNC1845 transcript (Response Figure 2). Taken together, the findings of both approaches support the model that LNC1845 directly interacts with WDR5 to modulate the H3K4me3 modification for LHX8 transcriptional activation. (Related to supplementary figure 8D and 8E.)
Response Figure 2. LNC1845 binding for WDR5 was verified by CHIRP-western blot.
Altogether, this study provides a convincing demonstration of the role of a lncRNA on the regulation of a nearby gene in the context of the germline. However, to have a better understanding of the functionality of lncRNA genes in general, it would be interesting to know whether other pairs of lncRNA-PC genes have been functionally investigated in this context,
where no function for the lncRNA gene could be demonstrated. Negative results are highly informative and if so, these could be included in the manuscript.
We appreciate Reviewer #2 suggestion to add other lncRNA-PC gene pairs results. In fact, we have analyzed and presented the results of another 2 pairs in figure 7D. LncRNAs LNC3346 and LNC15266 were also transcriptionally regulated by FOXP3, and they may regulate their neighbor genes TMCO1 and MPP5, as figure 7D showed. Our analysis showed that other lncRNA-PC gene pairs may also have the similar transcriptional regulation as LNC1845-LHX8 during germ cell development.
-

Evaluation Summary:
This manuscript provides a comprehensive analysis of expression patterns and genomic features of long non-coding RNAs (lncRNAs) in the human developing gonad, using available single-cell RNA-seq datasets from both somatic and germ cells. Using multiple genetic strategies in an in vitro system of female germ cell differentiation, the study further shows a positive regulatory function of the LNC1845 lncRNA on its protein-coding neighbor LHX8, known to have a role in ovarian follicle development. This study has potential interest for reproductive biologists and for the non-coding RNA community.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the …
Evaluation Summary:
This manuscript provides a comprehensive analysis of expression patterns and genomic features of long non-coding RNAs (lncRNAs) in the human developing gonad, using available single-cell RNA-seq datasets from both somatic and germ cells. Using multiple genetic strategies in an in vitro system of female germ cell differentiation, the study further shows a positive regulatory function of the LNC1845 lncRNA on its protein-coding neighbor LHX8, known to have a role in ovarian follicle development. This study has potential interest for reproductive biologists and for the non-coding RNA community.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
Using available single-cell transcriptomic data, Wang et al., annotated thousands of long noncoding RNAs (lncRNAs) in human germ cells. From their lncRNA data set, the authors focused on one particular lncRNA, lnc1845 located in the genomic proximity to the LHX8 gene that encodes a transcription factor essential for ovarian follicle development. Applying a set of different genetic approaches, the authors report that lnc1845 regulates LHX8 transcript in cis by modulating chromatin modifications.
The manuscript in its current form consists of three parts: (1) analyses of the available single-cell RNA seq data to identify and catalog lncRNAs expressed in human gonads; (2) dissection of the molecular mechanism of action of one of the lncRNAs named lnc1845 (3) identification of a transcription factor that …Reviewer #1 (Public Review):
Using available single-cell transcriptomic data, Wang et al., annotated thousands of long noncoding RNAs (lncRNAs) in human germ cells. From their lncRNA data set, the authors focused on one particular lncRNA, lnc1845 located in the genomic proximity to the LHX8 gene that encodes a transcription factor essential for ovarian follicle development. Applying a set of different genetic approaches, the authors report that lnc1845 regulates LHX8 transcript in cis by modulating chromatin modifications.
The manuscript in its current form consists of three parts: (1) analyses of the available single-cell RNA seq data to identify and catalog lncRNAs expressed in human gonads; (2) dissection of the molecular mechanism of action of one of the lncRNAs named lnc1845 (3) identification of a transcription factor that regulates expression of many of the identified gonad-specific lncRNAs including lnc1845.Strengths of the study:
- The study provides a useful and novel data set of lncRNAs expressed in human germ cells and can be a valuable resource for the community.
- To dissect the regulatory molecular interplay between lnc1845 and its protein-coding neighbor LHX8, the authors applied high-end genome editing strategies using multiple complementary approaches to inactivate or overexpress lnc1845.Weaknesses:
- In a current form, the three parts of the manuscript look like independent studies put together in one paper, with the last part being the least developed.
- The data sets of the lnc1845 functional part sometimes lack consistency i.e. looking at one but not another mutant allele by given molecular approaches. -

Reviewer #2 (Public Review):
Wang et al. elegantly exploit single-cell RNA-seq datasets to question the putative involvement of lncRNAs in human germ cell development. In the first part of the study, the authors use computational approaches to identify and characterize, from existing data, lncRNAs expressed in the germline. Of note, the scRNA-seq data used were generated from polyA+ RNAs, and thus non-polyadenylated lncRNAs could not be retrieved. Most of the lncRNAs identified in the germ cells and in the somatic cells of the gonads were previously unannotated. While this increases the catalog of lncRNA genes in the human genome, further characterization is needed to determine which fraction of these newly identified lncRNAs represent bona fide transcripts or transcriptional noise.
Differential expression analysis between developmental …
Reviewer #2 (Public Review):
Wang et al. elegantly exploit single-cell RNA-seq datasets to question the putative involvement of lncRNAs in human germ cell development. In the first part of the study, the authors use computational approaches to identify and characterize, from existing data, lncRNAs expressed in the germline. Of note, the scRNA-seq data used were generated from polyA+ RNAs, and thus non-polyadenylated lncRNAs could not be retrieved. Most of the lncRNAs identified in the germ cells and in the somatic cells of the gonads were previously unannotated. While this increases the catalog of lncRNA genes in the human genome, further characterization is needed to determine which fraction of these newly identified lncRNAs represent bona fide transcripts or transcriptional noise.
Differential expression analysis between developmental stages, sexes, or cell types led to several observations: (i) whatever the stage of development, the number of expressed lncRNAs is higher in fetal germ cells compared to gonadal somatic cells; (ii) there is a continuous increase in the number of expressed lncRNA during the development of the germline; of note, a similar, although the more subtle trend is observed for protein-coding genes; (iii) the developmental stage at which there is the highest number of lncRNA expressed differs between male and female germ cells. While convincing, the significance of these observations is difficult to assess. However, the authors remain prudent with their conclusion and are not over-interpreting their findings.
Interestingly, integrating lncRNA expression to classify cell types led to the identification of a novel population of cells in the female germline that had not been revealed by protein-coding gene only-based classification. The biological relevance of this population, which cluster with mitotic populations, remains to be demonstrated. Finally, by examining lncRNA biotype, the authors could demonstrate an enrichment, in the germ cells, of the antisense head-to-head organization (in relation to the nearby protein-coding gene) compared to other biotypes. Whether this is different from the general distribution of lncRNA should be discussed.
In the second part of the manuscript, Wang et al focus on one pair of divergent lncRNA-protein coding genes (LNC1845-LHX8). To document the choice of this particular pair, it would be informative to have its correlation score indicated in Figure 3C. The existence of this transcript was validated using female fetal ovaries, and its function was addressed in late primordial germ cells like cells (PGCLC) derived from human embryonic stem cells (hESCs). The authors have used an admirable set of orthogonal approaches that led them to conclude as to a role for LNC1845 in regulating in cis the nearby gene LHX8. They further went on to identify the underlying mechanisms, which involve modification of the chromatin landscape through direct interaction of LNC1845 with a histone modifier. Among the different strategies used (KO, stop transcription, overexpression), the shRNA-mediated knock-down is the only one to specifically address the function of the transcript itself, as opposed to the active transcription. The result of this experiment led the authors to conclude that the LNC1845 RNA is functional, a conclusion that is reinforced by the demonstration of physical interaction between the LNC1845 RNA and WDR5, a component of MLL methyltransferase complexes. The result of the KD experiment is however puzzling as RNAi has been shown not to be the method of choice for targeting nuclear lncRNAs (Lennox et al. NAR 2016).
Overall the functional investigation is convincing and strengthened by the inclusion of multiple clones for each approach, and by the convergence in the outcome of each individual approach. The depth of characterization is also remarkable. The analyses of the mechanisms at stake are somehow less solid, as there is less evidence demonstrating the involvement of the LNC1845 RNA and its interaction with WDR5.
Altogether, this study provides a convincing demonstration of the role of a lncRNA on the regulation of a nearby gene in the context of the germline. However, to have a better understanding of the functionality of lncRNA genes in general, it would be interesting to know whether other pairs of lncRNA-PC genes have been functionally investigated in this context, where no function for the lncRNA gene could be demonstrated. Negative results are highly informative and if so, these could be included in the manuscript.
-