Spatial modeling reveals nuclear phosphorylation and subcellular shuttling of YAP upon drug-induced liver injury
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The authors made an important extension of the canonical Hippo pathway by showing that nuclear phosphorylation of the pathway components YAP/TAZ contributes to the shuttling between different cellular compartments. The conclusions are well supported by the experimental evidence under both physiological and tissue-damaging conditions. Given the importance and developmental conserveness of the Hippo pathway, the work is of broad interest to the field of developmental and regenerative biology.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The Hippo signaling pathway controls cell proliferation and tissue regeneration via its transcriptional effectors yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ). The canonical pathway topology is characterized by sequential phosphorylation of kinases in the cytoplasm that defines the subcellular localization of YAP and TAZ. However, the molecular mechanisms controlling the nuclear/cytoplasmic shuttling dynamics of both factors under physiological and tissue-damaging conditions are poorly understood. By implementing experimental in vitro data, partial differential equation modeling, as well as automated image analysis, we demonstrate that nuclear phosphorylation contributes to differences between YAP and TAZ localization in the nucleus and cytoplasm. Treatment of hepatocyte-derived cells with hepatotoxic acetaminophen (APAP) induces a biphasic protein phosphorylation eventually leading to nuclear protein enrichment of YAP but not TAZ. APAP-dependent regulation of nuclear/cytoplasmic YAP shuttling is not an unspecific cellular response but relies on the sequential induction of reactive oxygen species (ROS), RAC-alpha serine/threonine-protein kinase (AKT, synonym: protein kinase B), as well as elevated nuclear interaction between YAP and AKT. Mouse experiments confirm this sequence of events illustrated by the expression of ROS-, AKT-, and YAP-specific gene signatures upon APAP administration. In summary, our data illustrate the importance of nuclear processes in the regulation of Hippo pathway activity. YAP and TAZ exhibit different shuttling dynamics, which explains distinct cellular responses of both factors under physiological and tissue-damaging conditions.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
The time-dependency of the model simulations was not analyzed, and the nature of the observed biphasic time-dependent APAP response remains elusive. It would be interesting to see how the model can explain the time course of the APAP stimulation experiment.
The alternative model at its current state can only describe steady state conditions. However, we understand that the reviewer is interested in the dynamic behavior of the model. However, our approach provides a proof of principle that the alternative model can phenomenologically explain the changes of YAP localization as a response to APAP treatment. The question of how to model Hippo pathway in a time-dependent manner as a response to APAP treatment is very challenging and would require further investigations and, most notably, …
Author Response
Reviewer #2 (Public Review):
The time-dependency of the model simulations was not analyzed, and the nature of the observed biphasic time-dependent APAP response remains elusive. It would be interesting to see how the model can explain the time course of the APAP stimulation experiment.
The alternative model at its current state can only describe steady state conditions. However, we understand that the reviewer is interested in the dynamic behavior of the model. However, our approach provides a proof of principle that the alternative model can phenomenologically explain the changes of YAP localization as a response to APAP treatment. The question of how to model Hippo pathway in a time-dependent manner as a response to APAP treatment is very challenging and would require further investigations and, most notably, further development of the PDE simulation algorithms and the SME software. Hence, a technical update of the software algorithms would be required, which cannot be in the scope of this manuscript.
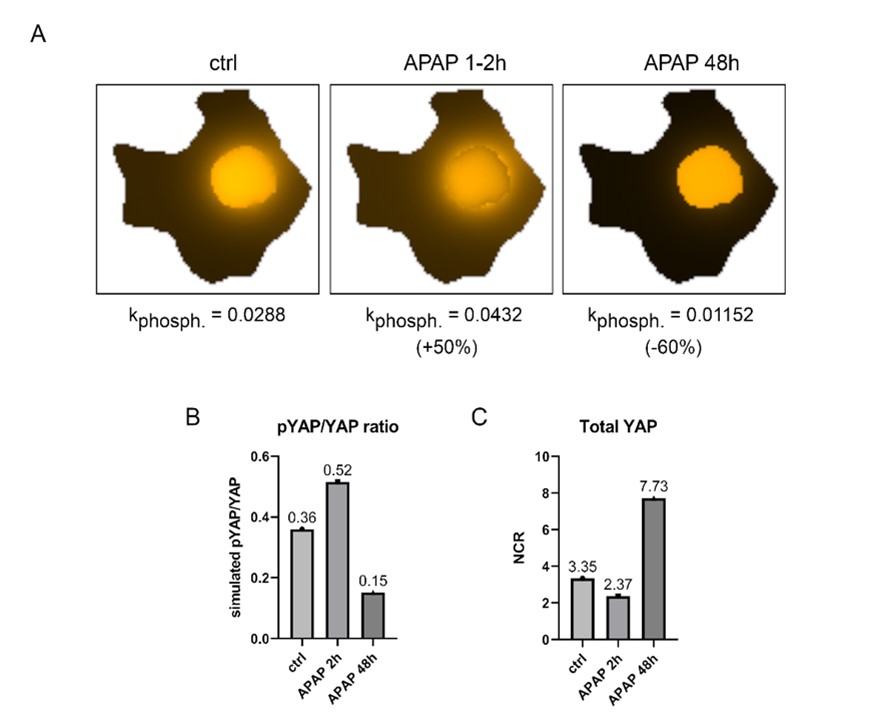
Nevertheless, we decided to share our first and preliminary analyses on dynamic processes caused by APAP with the reviewer. For this, we simulated the steady state model in an arbitrary manner, where APAP initiates (early time-point) and slows down (late time-points) YAP phosphorylation in the nucleus (see Figure below).
The simulated alternative model shows that increased YAP phosphorylation about 50% leads to the cytoplasmic localization of YAP (Rebuttal Figure R5A/B). However, this shuttling is not detectable in our protein fractionation and live-cell imaging experiments (see also Rebuttal Figure R7C/D). At late time points, decreasing YAP phosphorylation (about 60%) led to a clear nuclear enrichment and dephosphorylation of YAP was observed in our experiments. Thus, our mathematical model nicely describes cellular events of Hippo pathway dynamics observed at later stages after APAP treatment (nuclear enrichment). However, early events cannot be completely explained (suggested nuclear YAP exclusion is not detectable).
We suggest two explanations for this observation. First, other molecular mechanisms (not yet identified and therefore not part of the model topology) oppose the exclusion YAP enrichment that is expected at early time points. Second, detection methods used in this study (Western Blotting and life cell imaging) cannot capture minimal changes and cellular heterogeneity in the chosen experimental setup. We clarify this aspect/limitation of our study in the discussion chapter of the manuscript. Page 12, lines 436-440
Time-dependency of YAP (orange) localization based on the simulated APAP treatment. (A): Simulated control (ctrl) and APAP treatment for 2 and 48h. The treatment was simulated by changing the phosphorylation coefficient of YAP in the nucleus. (B): Simulated pYAP/YAP ratio during control and APAP treatment for 2 and 48 hours at the steady state of the model. (C): Simulated NCR of the total YAP during control and APAP treatment for 2 and 48 hours at the steady state.
-

Evaluation Summary:
The authors made an important extension of the canonical Hippo pathway by showing that nuclear phosphorylation of the pathway components YAP/TAZ contributes to the shuttling between different cellular compartments. The conclusions are well supported by the experimental evidence under both physiological and tissue-damaging conditions. Given the importance and developmental conserveness of the Hippo pathway, the work is of broad interest to the field of developmental and regenerative biology.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
The manuscript entitled "Spatial modeling reveals nuclear 1 phosphorylation and 2 subcellular shuttling of YAP upon drug-induced liver injury" by Wehling et al. sought to provide increased resolution for the dynamic regulation of the HIPPO/Yap signaling pathway in hepatocytes. The authors employed a multi-tier approach utilizing computational/machine learning as well as classical biochemical and molecular biology techniques both in vitro and in vivo. The authors demonstrate the nuclear phosphorylation of YAP as a critical regulator of subcellular localization. Furthermore, the authors provide a mechanism by which drug-induced liver injury activates a cascade of ROS/Akt to culminate on YAP phosphorylation to direct its activation/inactivation in vivo. This work extends our current understanding of the …
Reviewer #1 (Public Review):
The manuscript entitled "Spatial modeling reveals nuclear 1 phosphorylation and 2 subcellular shuttling of YAP upon drug-induced liver injury" by Wehling et al. sought to provide increased resolution for the dynamic regulation of the HIPPO/Yap signaling pathway in hepatocytes. The authors employed a multi-tier approach utilizing computational/machine learning as well as classical biochemical and molecular biology techniques both in vitro and in vivo. The authors demonstrate the nuclear phosphorylation of YAP as a critical regulator of subcellular localization. Furthermore, the authors provide a mechanism by which drug-induced liver injury activates a cascade of ROS/Akt to culminate on YAP phosphorylation to direct its activation/inactivation in vivo. This work extends our current understanding of the HIPPO/Yap pathway and provides novel signaling pathways that converge on Yap activation during pathophysiological conditions. The claims are well-supported by a variety of experimental conditions.
-

Reviewer #2 (Public Review):
The study used two different model versions to distinguish two competing hypotheses concerning the mechanisms of YAP and TAZ phosphorylation and their regularization of nuclear localization. In the first (canonical) model, YAP and TAZ are phosphorylated in the cytosol, whereas in the second (alternative) model YAP and TAZ are phosphorylated in the nucleus. By comparing the model predictions to spatially resolved data, the authors could convincingly reject the first model and show that the second model explains the observed data better. The authors conclude that YAP and TAZ cytoplasmic-nuclear shuttling is regulated by phosphorylation in the nucleus and this conclusion is well supported by their data. Albeit not modeled, the authors also show this phosphorylation depends on ROS and AKT signaling.
Strengths: …
Reviewer #2 (Public Review):
The study used two different model versions to distinguish two competing hypotheses concerning the mechanisms of YAP and TAZ phosphorylation and their regularization of nuclear localization. In the first (canonical) model, YAP and TAZ are phosphorylated in the cytosol, whereas in the second (alternative) model YAP and TAZ are phosphorylated in the nucleus. By comparing the model predictions to spatially resolved data, the authors could convincingly reject the first model and show that the second model explains the observed data better. The authors conclude that YAP and TAZ cytoplasmic-nuclear shuttling is regulated by phosphorylation in the nucleus and this conclusion is well supported by their data. Albeit not modeled, the authors also show this phosphorylation depends on ROS and AKT signaling.
Strengths: The study is a very nice example of systems biology and how modelling can be used to make different assumptions explicit to test different hypotheses. A key strength is the mathematical modelling with partial differential equations and the use of spatially resolved data. Spatial features of the data were compared to the models and used to distinguish two different, competing hypotheses. The results reveal a new mechanism of YAP and TAZ phosphorylation in the nucleus. Uncertain parameters in the model were estimated and predictions arising from the model were validated using multiple experimental techniques, increasing the confidence of the findings. In vivo significance was validated in a mouse model.
Weaknesses: As is to be expected, the parameters in the model were not identifiable leading to large variability in the estimates. However, this is typical for these types of systems biology models. Another potential weakness is that different diffusion rates were assumed for the canonical and alternative models, and the significance of having different diffusion rates for two different model versions remains unclear. To implement the different hypotheses, two extreme model versions were analyzed (the alternative model had no phosphorylation in the cytosol, only the unphosphorylated form of the YAP/TAZ is shuttled into the nucleus and only the phosphorylated YAP/TAZ is exported). The reality is most likely less discrete and somewhere in between with some phosphorylation/dephosphorylation occurring in both compartments and nuclear shuttling occurring for both the unphosphorylated and phosphorylated forms. The time-dependency of the model simulations was not analyzed, and the nature of the observed biphasic time-dependent APAP response remains elusive. It would be interesting to see how the model can explain the time course of the APAP stimulation experiment.
-

Reviewer #3 (Public Review):
In this manuscript, Wehling and colleagues discovered an important role of nuclear phosphorylation in the Hippo pathway that regulates the cellular localization of YAP and TAZ in the nucleus and cytoplasm. Using PDE modeling and imaging assays, they showed that the difference in shuttling dynamics between YAP and TAZ can be accounted for by a single parameter determining the nuclear phosphorylation capability. The authors further demonstrated that in a drug-induced liver injury model, YAP shuttling is also regulated by nuclear phosphorylation, which may be mediated by the induction of reactive oxygen species and subsequently AKT. The work has added the cell nucleus as a critical compartment in the regulation of the Hippo pathway, which challenged and extended the conventional model. The conclusions are …
Reviewer #3 (Public Review):
In this manuscript, Wehling and colleagues discovered an important role of nuclear phosphorylation in the Hippo pathway that regulates the cellular localization of YAP and TAZ in the nucleus and cytoplasm. Using PDE modeling and imaging assays, they showed that the difference in shuttling dynamics between YAP and TAZ can be accounted for by a single parameter determining the nuclear phosphorylation capability. The authors further demonstrated that in a drug-induced liver injury model, YAP shuttling is also regulated by nuclear phosphorylation, which may be mediated by the induction of reactive oxygen species and subsequently AKT. The work has added the cell nucleus as a critical compartment in the regulation of the Hippo pathway, which challenged and extended the conventional model. The conclusions are mostly well supported by the experimental evidence, although some improvements can be made in data presentation.
Overall, the work is of general interest to the field of developmental and regenerative biology. It will benefit a broader audience if the role of nuclear phosphorylation can be further linked to tissue damage and the regeneration process in future studies.
-