Frequency-specific neural signatures of perceptual content and perceptual stability
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Bistable visual perception offers a unique window to study how perception arises and changes via an interaction between bottom-up and top-down processes. In three Magnetoencephalography (MEG) experiments with advanced neural state space analysis, this study demonstrates that two key aspects of bistable visual perception - perceptual content and perceptual stability - are mediated by slow cortical potential (SCP) and alpha-beta-band neural oscillations, respectively. The findings would be interesting for many fields, such as perception, consciousness, and attention.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
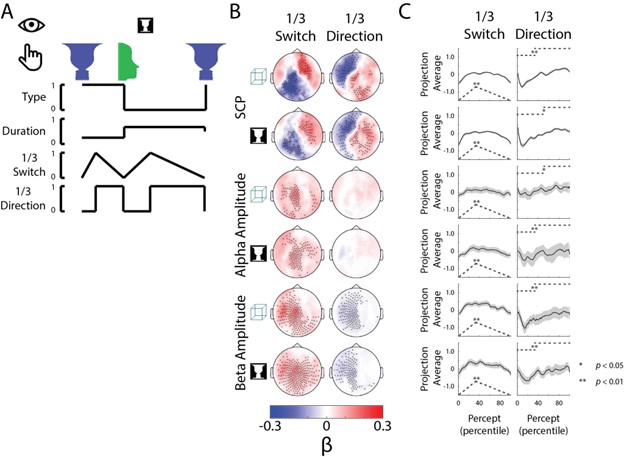
In the natural environment, we often form stable perceptual experiences from ambiguous and fleeting sensory inputs. Which neural activity underlies the content of perception and which neural activity supports perceptual stability remains an open question. We used a bistable perception paradigm involving ambiguous images to behaviorally dissociate perceptual content from perceptual stability, and magnetoencephalography to measure whole-brain neural dynamics in humans. Combining multivariate decoding and neural state-space analyses, we found frequency-band-specific neural signatures that underlie the content of perception and promote perceptual stability, respectively. Across different types of images, non-oscillatory neural activity in the slow cortical potential (<5 Hz) range supported the content of perception. Perceptual stability was additionally influenced by the amplitude of alpha and beta oscillations. In addition, neural activity underlying perceptual memory, which supports perceptual stability when sensory input is temporally removed from view, also encodes elapsed time. Together, these results reveal distinct neural mechanisms that support the content versus stability of visual perception.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
In this MEG work employing two types of bistable perception test and unique regression analyses, the authors identified different neural frequencies to different components of visual perception: its content and stability.
Strengths:
This study has a nice set of three different experiments to clarify neural differences between content, memory and stability of visual perception.
The state space analysis appears to be powerful to identify such different neural signatures for different cognitive components as well.
Weaknesses:
Despite such strengths, this work may have the somewhat critical weakness specified in the recommendations for the authors.
First, in the analysis to identify content-specific neural frequency, the authors concluded that the SCP is more relevant to the visual …
Author Response
Reviewer #2 (Public Review):
In this MEG work employing two types of bistable perception test and unique regression analyses, the authors identified different neural frequencies to different components of visual perception: its content and stability.
Strengths:
This study has a nice set of three different experiments to clarify neural differences between content, memory and stability of visual perception.
The state space analysis appears to be powerful to identify such different neural signatures for different cognitive components as well.
Weaknesses:
Despite such strengths, this work may have the somewhat critical weakness specified in the recommendations for the authors.
First, in the analysis to identify content-specific neural frequency, the authors concluded that the SCP is more relevant to the visual perceptual content compared to the neural activity in the alpha and beta-band frequencies. In my impression, to claim this, it would be necessary to show statistically significant differences in the prediction accuracy between the SCP and the other frequencies. Given the not-so-high prediction accuracy seen in the SCP-based analysis, such statistical supports appear essential.
We have now directly compared decoding accuracy for SCP and alpha/beta oscillations, which showed statistically significant differences in both the ambiguous and unambiguous conditions for both ambiguous images. We have added these results as a supplementary figure (new Figure 2—figure supplement 1).
Second, two behavioural metrics in the neural state space analysis-i.e., Switch and Direction-may be too arbitrary. As suggested by the power-law distribution of the percept duration, the neural dynamics during seemingly stable percept may not be able to be described in linear functions. Instead, the brain may go back and forth between several neural states even when we are thinking we're experiencing stable visual consciousness. If so, the current definition of the Switch metric and Direction index, which seems to be based on the behaviour of the Switch index, may be arbitrary. In other words, I feel the authors may have to elaborate the rationale for the definitions of such metrics.
First, we note it is generally accepted in the field that the distribution of percept durations follows a gamma distribution instead of a power-law distribution (e.g., Sterzer et al., TiCS 2009; Blake & Logothetis Nature Rev. Neurosci 2002; Kleinschmidt et al., 1998; Leopold et al., TiCS 1999), and microswitches have not been reported either using the more classic task as that employed here or the more recently developed ‘no-report’ task of using eye-tracking statistics to deduce perceptual switches without overt report (e.g., Frassle et al., J Neurosci 2014).
Second, while brain activity may fluctuate during these time periods, it never crosses the threshold of evoking a conscious report, and thus we would expect that such fluctuations, if they do occur, would be of a lower magnitude than those that do produce a conscious report.
Most importantly, our goal here is to define behavioral metrics in order to identify components of neural dynamics underpinning the relevant aspect of behavior. As such, our definition of the behavioral metric should not be directly informed by observed spontaneous dynamics of brain activity (especially those that may be observed in the data but are of unclear relevance to perceptual switching); otherwise the analysis would be prone to circularity and spurious correlations (i.e., using observed brain dynamics to inform construction of behavioral metrics might pick up aspect of brain dynamics not really relevant to behavior in the analysis results).
Finally, the timing characteristics of ‘Switch’ and ‘Direction’ behavioral metrics are not arbitrary; instead they are the simplest behavioral functions that allow a comparison of pre- and post-switching periods (or when the percepts might be in the ‘stabilizing’ phase vs. the ‘destabilizing’ phase). Nevertheless, the regression analysis can pick up on other temporal patterns of changes not exactly the same as our defined behavioral metric. This can be seen for SCP and beta activity projected onto the Direction axis, where it has the lowest value at ~20th percentile of the trial (not 50th percentile as assumed by the behavioral metric). To confirm that the analysis is not highly dependent on the precise timing definition of the behavioral metrics, we ran a control analysis, where the switching point was set at 30%tile (rather than 50%tile as in the original analysis). This control analysis resulted in a similar pattern of neural results (Figure R1).
Figure R1: Changing temporal behavior definition (switching point moved from 50th percentile to 30th percentile of percept duration) does not significantly alter the neural results. Compare to Figure 4—figure supplement 1, ‘Switch’ and “Direction’ Columns.
-

Evaluation Summary:
Bistable visual perception offers a unique window to study how perception arises and changes via an interaction between bottom-up and top-down processes. In three Magnetoencephalography (MEG) experiments with advanced neural state space analysis, this study demonstrates that two key aspects of bistable visual perception - perceptual content and perceptual stability - are mediated by slow cortical potential (SCP) and alpha-beta-band neural oscillations, respectively. The findings would be interesting for many fields, such as perception, consciousness, and attention.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
Bistable visual perception, given its switching visual perception for constant inputs, offers a unique window to study how our perception arises and changes via an interaction between bottom-up and top-down processes. Previous studies have mainly focused on perceptual contents, while how perception is sustained over time, i.e., perceptual stability, as well as its potential dissociated neural mechanism from perceptual content, remain unclear. Hardstone et al., used magnetoencephalography (MEG) recordings in combination with advanced approaches and revealed dissociated neural implementations for perceptual content and perceptual stability, that is, slow cortical potential (SCP) and alpha-beta neural oscillations, respectively.
This is a very interesting study addressing important questions in bistable …
Reviewer #1 (Public Review):
Bistable visual perception, given its switching visual perception for constant inputs, offers a unique window to study how our perception arises and changes via an interaction between bottom-up and top-down processes. Previous studies have mainly focused on perceptual contents, while how perception is sustained over time, i.e., perceptual stability, as well as its potential dissociated neural mechanism from perceptual content, remain unclear. Hardstone et al., used magnetoencephalography (MEG) recordings in combination with advanced approaches and revealed dissociated neural implementations for perceptual content and perceptual stability, that is, slow cortical potential (SCP) and alpha-beta neural oscillations, respectively.
This is a very interesting study addressing important questions in bistable perception and the findings would be generally interesting for broad fields, including perception, consciousness, attention, and working memory. The authors have carefully designed several conditions for comparisons, e.g., Ambiguous, Unambiguous, Discontinuous. The last condition is particularly interesting given its close link to perceptual memory, a less touched field. Moreover, the neural state-space analysis is an innovative approach to complement the widely used multivariate decoding method, by projecting MEG data to the subspace that covaries with various behavioral matrices. The experiment was well-motivated based on previous findings, and the paper was well written.
Meanwhile, although the multivariate content decoding results support the separation of SCP and alpha-beta oscillation, it seems to be a mixed case for perceptual stability, i.e. SCP still shows representations of perceptual duration as well as the trend of tracking perceptual memory for the Discontinuous condition. These seem to somewhat weaken the perception/stability dissociation conclusion.
-

Reviewer #2 (Public Review):
In this MEG work employing two types of bistable perception test and unique regression analyses, the authors identified different neural frequencies to different components of visual perception: its content and stability.
Strengths:
This study has a nice set of three different experiments to clarify neural differences between content, memory and stability of visual perception.
The state space analysis appears to be powerful to identify such different neural signatures for different cognitive components as well.
Weaknesses:
Despite such strengths, this work may have the somewhat critical weakness specified in the recommendations for the authors.
First, in the analysis to identify content-specific neural frequency, the authors concluded that the SCP is more relevant to the visual perceptual content compared to …
Reviewer #2 (Public Review):
In this MEG work employing two types of bistable perception test and unique regression analyses, the authors identified different neural frequencies to different components of visual perception: its content and stability.
Strengths:
This study has a nice set of three different experiments to clarify neural differences between content, memory and stability of visual perception.
The state space analysis appears to be powerful to identify such different neural signatures for different cognitive components as well.
Weaknesses:
Despite such strengths, this work may have the somewhat critical weakness specified in the recommendations for the authors.
First, in the analysis to identify content-specific neural frequency, the authors concluded that the SCP is more relevant to the visual perceptual content compared to the neural activity in the alpha and beta-band frequencies. In my impression, to claim this, it would be necessary to show statistically significant differences in the prediction accuracy between the SCP and the other frequencies. Given the not-so-high prediction accuracy seen in the SCP-based analysis, such statistical supports appear essential.
Second, two behavioural metrics in the neural state space analysis-i.e., Switch and Direction-may be too arbitrary. As suggested by the power-law distribution of the percept duration, the neural dynamics during seemingly stable percept may not be able to be described in linear functions. Instead, the brain may go back and forth between several neural states even when we are thinking we're experiencing stable visual consciousness. If so, the current definition of the Switch metric and Direction index, which seems to be based on the behaviour of the Switch index, may be arbitrary. In other words, I feel the authors may have to elaborate the rationale for the definitions of such metrics.
-