Contribution of Trp63CreERT2-labeled cells to alveolar regeneration is independent of tuft cells
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This study addresses an interesting question in lung regeneration following viral injury. The authors describe the ectopic differentiation of tuft cells that were derived from lineage-tagged p63+ cells post influenza virus infection. These tuft cells do not appear to proliferate or give rise to other lineages. Importantly, they demonstrate that depletion of tuft cells caused by genetic deletion of Pou2f3 in p63+ cells has no effect on the expansion or resolution of Krt5+ pods after infection, implying that tuft cells play no functional role in this process.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
- Evaluated articles (ScreenIT)
Abstract
Viral infection often causes severe damage to the lungs, leading to the appearance of ectopic basal cells (EBCs) and tuft cells in the lung parenchyma. Thus far, the roles of these ectopic epithelial cells in alveolar regeneration remain controversial. Here, we confirm that the ectopic tuft cells are originated from EBCs in mouse models and COVID-19 lungs. The differentiation of tuft cells from EBCs is promoted by Wnt inhibition while suppressed by Notch inhibition. Although progenitor functions have been suggested in other organs, pulmonary tuft cells don’t proliferate or give rise to other cell lineages. Consistent with previous reports, Trp63 CreERT2 and KRT5-CreERT2 -labeled ectopic EBCs do not exhibit alveolar regeneration potential. Intriguingly, when tamoxifen was administrated post-viral infection, Trp63 CreERT2 but not KRT5-CreERT2 labels islands of alveolar epithelial cells that are negative for EBC biomarkers. Furthermore, germline deletion of Trpm5 significantly increases the contribution of Trp63 CreERT2 -labeled cells to the alveolar epithelium. Although Trpm5 is known to regulate tuft cell development, complete ablation of tuft cell production fails to improve alveolar regeneration in Pou2f3 -/- mice, implying that Trpm5 promotes alveolar epithelial regeneration through a mechanism independent of tuft cells.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
Huang et al. sought to study the cellular origin of Tuft cells and the molecular mechanisms that govern their specification in severe lung injury. First the authors show ectopic emergence of Tuft cells in airways and distal parenchyma following different injuries. The authors also used lineage tracing models and uncovered that p63-expressing cells and to some extent Scgb1a1-lineaged labeled cells contribute to tuft cells after injury. Further, the authors modulated multiple pathways and claim that Notch inhibition blocks tuft cells whereas Wnt inhibition enhances Tuft cell development in basal cell cultures. Finally, the authors used Trpm5 and Pou2f3 knock-out models to claim that tuft cells are indispensable for alveolar regeneration.
In summary, the findings described in this …
Author Response
Reviewer #1 (Public Review):
Huang et al. sought to study the cellular origin of Tuft cells and the molecular mechanisms that govern their specification in severe lung injury. First the authors show ectopic emergence of Tuft cells in airways and distal parenchyma following different injuries. The authors also used lineage tracing models and uncovered that p63-expressing cells and to some extent Scgb1a1-lineaged labeled cells contribute to tuft cells after injury. Further, the authors modulated multiple pathways and claim that Notch inhibition blocks tuft cells whereas Wnt inhibition enhances Tuft cell development in basal cell cultures. Finally, the authors used Trpm5 and Pou2f3 knock-out models to claim that tuft cells are indispensable for alveolar regeneration.
In summary, the findings described in this manuscript are somewhat preliminary. The claim that the cellular origin of Tuft cells in influenza infection was not determined is incorrect. Current data from pathway modulation is preliminary and this requires genetic modulation to support their claims.
We thank the reviewer for the comments and we have performed extensive experiments to address the reviewer’s comments. In the revised manuscript we provide additional data including genetic modulation findings to support our model.
Major comments:
- The abstract sounds incomplete and does not cover all key aspects of this manuscript. Currently, it is mainly focusing on the cellular origin of Tuft cells and the role of Wnt and notch signaling. However, it completely omits the findings from Trpm5 and Pou2f3 knock-out mice. In fact, the title of the manuscript highlights the indispensable nature of tuft cells in alveolar regeneration.
We have modified the abstract and title accordingly.
- In lines 93-94, the authors state that "It is also unknown what cells generate these tuft cells.....". This statement is incorrect. Rane et al., 2019 used the same p63-creER mouse line and demonstrated that all tuft cells that ectopically emerge following H1N1 infection originate from p63+ lineage labeled basal cells. Therefore, this claim is not new.
We thank the reviewer’s comment. Although Rane et al. reported the p63-expressing lineage-negative epithelial stem/progenitor cells (LNEPs) could contribute to the ectopic tuft cells after PR8 virus infection, it is still not clear whether the p63+ cells immediately give rise to tuft cells or though EBCs. Thus, we performed TMX injection after PR8 infection, different from Rane et al (Rane et al., 2019). who performed Tmx injection before viral infection to indicate the ectopic tuft cells are derived from EBCs, as shown in revised Figure 2.
- Lines 152-153 state that "21.0% +/- 2.0 % tuft cells within EBCs are labeled with tdT when examined at 30 dpi...". It is not clear what the authors meant here ("within EBC's")? And also, the same sentence states that "......suggesting that club cell-derived EBCs generate a portion of tuft cells....". In this experiment, the authors used club cell lineage tracing mouse lines. So, how do the authors know that the club cell lineage-derived tuft cells came through intermediate EBC population? Current data do not show evidence for this claim. Is it possible that club cells can directly generate tuft cells?
We apologize for the confusion and revised the text accordingly. Here, “within EBCs” means within the “pods” area where p63+ basal cells are ectopically present. The sentence is revised as “21.0% +/- 2.0 % tuft cells that are ectopically present in the parenchyma are labeled by tdT. Notably, these lineage labeled tuft cells were co-localized with EBCs.” We don’t know whether the club cell lineage-derived tuft cells transit through intermediate EBCs and that is why we use “suggest”. It is also possible that club cells can directly generate tuft cells. To avoid the confusion, we delete the sentence.
- Based on the data from Fig-3A, the authors claim that treatment with C59 significantly enhances tuft cell development in ALI cultures. Porcupine is known to facilitate Wnt secretion. So, which cells are producing Wnt in these cultures? It is important to determine which cells are producing Wnt and also which Wnt? Further, based on DBZ treatments, it appears that active Notch signaling is necessary to induce Tuft cell fate in basal cells. Where are Notch ligands expressed in these tissues? Is Notch active only in a small subset of basal cells (and hence generate rate tuft cells)? This is one of the key findings in this manuscript. Therefore, it is important to determine the expression pattern of Wnt and Notch pathway components.
We thank the reviewer’s interesting questions and agree the importance of identifying the specific ligands and receptors for relevant Wnt and Notch signaling during tuft cell derivation. That being said, we think the topic is beyond the scope of this study which is focused on the role of tuft cells in alveolar regeneration. The point is well taken and we will investigate the topic in our future study.
- How do the authors explain different phenotypes observed in Trpm5 knockout and Pou2f3 mutants? Is it possible that Trpm5 knockout mice have a subset of tuft cells and that they might be something to do with the phenotypic discrepancy between two mutant models?
Again we thank the reviewer for the interesting question. As discussed in the discussion section, Trpm5 is also reported to be expressed in B lymphocytes (Sakaguchi et al., 2020). It is possible that loss of Trpm5 modulates the inflammatory responses following viral infection, which may contribute to improved alveolar regeneration. However, it is also possible that Trpm5-/- mice keep a subset of tuft cells that facilitate lung regeneration as suggested by the reviewer.
- One of the key findings in this manuscript is that Wnt and Notch signaling play a role in Tuft cell specification. All current experiments are based on pharmacological modulation. These need to be substantiated using genetic gain loss of function models.
We have performed the genetic studies.
Reviewer #2 (Public Review):
In this manuscript, the authors describe the ectopic differentiation of tuft cells that were derived from lineage-tagged p63+ cells post influenza virus infection. These tuft cells do not appear to proliferate or give rise to other lineages. They then claim that Wnt inhibitors increase the number of tuft cells while inhibiting Notch signaling decreases the number of tuft cells within Krt5+ pods after infection in vitro and in vivo. The authors further show that genetic deletion of Trpm5 in p63+ cells post-infection results in an increase in AT2 and AT1 cells in p63 lineage-tagged cells compared to control. Lastly, they demonstrate that depletion of tuft cells caused by genetic deletion of Pou2f3 in p63+ cells has no effect on the expansion or resolution of Krt5+ pods after infection, implying that tuft cells play no functional role in this process.
Overall, in vivo and in vitro phenotypes of tuft cells and alveolar cells are clear, but the lack of detailed cellular characterization and molecular mechanisms underlying the cellular events limits the value of this study.
We thank the reviewer for the comments and acknowledging that our findings are clear. In the revised manuscript we provide more detailed characterization and genetic evidence to elucidate the role of tuft cells in lung regeneration.
- Origin of tuft cells: Although the authors showed the emergence of ectopic tuft cells derived from labelled p63+ cells after infection, it cannot be ruled out that pre-existing p63+Krt5- intrapulmonary progenitors, as previously reported, can also contribute to tuft cell expansion (Rane et al. 2019; by labelling p63+ cells prior to infection, they showed that the majority of ectopic tuft cells are derived from p63+ cells after viral infection). It would be more informative if the authors show the differentiation of tuft cells derived from p63+Krt5+ cells by tracing Krt5+ cells after infection, which will tell us whether ectopic tuft cells are differentiated from ectopic basal cells within Krt5+ pods induced by virus infection.
We thank the reviewer for the helpful suggestion. We have performed the experiment accordingly.
- Mechanisms of tuft cell differentiation: The authors tried to determine which signaling pathways regulate the differentiation of tuft cells from p63+ cells following infection. Although Wnt/Notch inhibitors affected the number of tuft cells derived from p63+ labelled cells, it remains unclear whether these signals directly modulate differentiation fate. The authors claimed that Wnt inhibition promotes tuft cell differentiation from ectopic basal cells. However, in Fig 3B, Wnt inhibition appears to trigger the expansion of p63+Krt5+ pod cells, resulting in increased tuft cell differentiation rather than directly enhancing tuft cell differentiation. Further, in Fig 3D, Notch inhibition appears to reduce p63+Krt5+ pod cells, resulting in decreased tuft cell differentiation. Importantly, a previous study has reported that Notch signalling is critical for Krt5+ pod expansion following influenza infection (Vaughan et al. 2015; Xi et al. 2017). Notch inhibition reduced Krt5+ pod expansion and induced their differentiation into Sftpc+ AT2 cells. In order to address the direct effect of Wnt/Notch signaling in the differentiation process of tuft cells from EBCs, the authors should provide a more detailed characterization of cellular composition (Krt5+ basal cells, club cells, ciliated cells, AT2 and AT1 cells, etc.) and activity (proliferation) within the pods with/without inhibitors/activators.
Again we thank the reviewer for the insightful suggestions. We agree that it will be interesting to further address the direct effect of Wnt/Notch signaling in the differentiation process of tuft cells from EBCs. In this revised manuscript we added new findings of EBC differentiation into tuft cells in mice with genetic deletion of Rbpjk.
- Impact of Trpm5 deletion in p63+ cells: It is interesting that Trpm5 deletion promotes the expansion of AT2 and AT1 cells derived from labelled p63+ cells following infection. It would be informative to check whether Trpm5 regulates Hif1a and/or Notch activity which has been reported to induce AT2 differentiation from ectopic basal cells (Xi et al. 2017). Although the authors stated that there was no discernible reduction in the size of Krt5+ pods in mutant mice, it would be interesting to investigate the relationship between AT2/AT1 cell retaining pods and the severity of injury (e.g. large Krt5+ pods retain more/less AT2/AT1 cells compared to small pods. What about other cell types, such as club and goblet cells, in Trpm5 mutant pods? Again, it cannot be ruled out that pre-existing p63+Krt5- intrapulmonary progenitor cells can directly convert into AT2/AT1 cells upon Trpm5 deletion rather than p63+Krt5+ cells induced by infection.
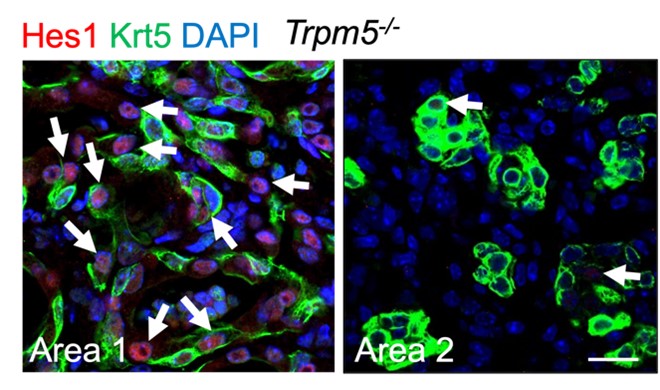
We thank the reviewer for the comments and suggestions. Our new data using KRT5-CreER mouse line confirmed that pod cells (Krt5+) do not contribute to AT2/AT1 cells, consistent with previous studies (Kanegai et al., 2016; Vaughan et al., 2015). Our data also show that p63-CreER lineage labeled AT2/AT1 cells are separated from pod cell area, suggesting pod cells and these AT2/AT1 cells are generated from different cell of origin. We also checked the Notch activity in pod cells in Trpm5-/- mice, and some pod cell-derived cells are Hes1 positive, whereas some are Hes1 negative (RLFigure 1). As indicated in discussion we think that AT2/AT1 cells are possibly derived from pre-existing AT2 cells that transiently express p63 after PR8 infection. It will be interesting to test whether Trpm5 regulates Hif1a in this population (p63+,Krt5-), and this will be our next plan.
RLFigure 1. Representative area staining in Trpm5-/- mice at 30 dpi. Area 1: Notch signaling is active (Hes1+, arrows) in pod cells following viral infection. Area 2: pod cells exhibit reduced Notch activities. Note few Hes1+ cells in pods (arrows). Scale bar: 50 µm.
- Ectopic tuft cells in COVID-19 lungs: The previous study by the authors' group revealed the presence of ectopic tuft cells in COVID-19 patient samples (Melms et al. 2021). There appears to be no additional information in this manuscript.
In Melms et al., Nature, 2021 (Melms et al., 2021), we showed tuft cell expansion in COVID-19 lungs but not the potential origin of tuft cells. In this manuscript we show some cells co-expressing POU2F3 and KRT5, suggesting a pod-to-tuft cell differentiation.
- Quantification information and method: Overall, the quantification method should be clarified throughout the manuscript. Further, in the method section, the authors stated that the production of various airway epithelial cell types was counted and quantified on at least 5 "random" fields of view. However, virus infection causes spatially heterogeneous injury, resulting in a difficult to measure "blind test". The authors should address how they dealt with this issue.
We clarified that quantification method as suggested. For the in vitro cell culture assays on the signaling pathways, we took pictures from at least five random fields of view for quantification. For lung sections, we tile-scanned the lung sections including at least three lung lobes and performed quantification.
Reviewer #3 (Public Review):
In this manuscript Huang et al. study how the lung regenerates after severe injury due to viral infection. They focus on how tuft cells may affect regeneration of the lung by ectopic basal cells and come to the conclusion that they are not required. The manuscript is intriguing but also very puzzling. The authors claim they are specifically targeting ectopic basal progenitor cells and show that they can regenerate the alveolar epithelium in the lung following severe injury. However, it is not clear that the p63-CreERT2 line the authors are using only labels ectopic basal cells. The question is what is a basal cell? Is an ectopic basal progenitor cell only defined by Trp63 expression?
The accompanying manuscript by Barr et al. uses a Krt5-CreERT2 line to target ectopic basal cells and using that tool the authors do not see a signification contribution of ectopic basal cells towards alveolar epithelial regeneration. As such the claim that ectopic basal cell progenitors drive alveolar epithelial regeneration is not well-founded.
We appreciate the reviewer for the positive comments and agreeing that our findings are interesting.
The title itself is also not very informative and is a bit misleading. That being said I think the manuscript is still very interesting and can likely easily be improved through a better validation of which cells the p63-CreERT2 tool is targeting.
We have revised the title accordingly and performed extensive experiments to address the reviewer’s concerns.
I, therefore, suggest the following experiments.
- Please analyze which cells p63-CreERT2 labels immediately after PR8 and tamoxifen treatment. Are all the tdTomato labeled cells also Krt5 and p63 positive or are some alveolar epithelial cells or other airway cell types also labeled?
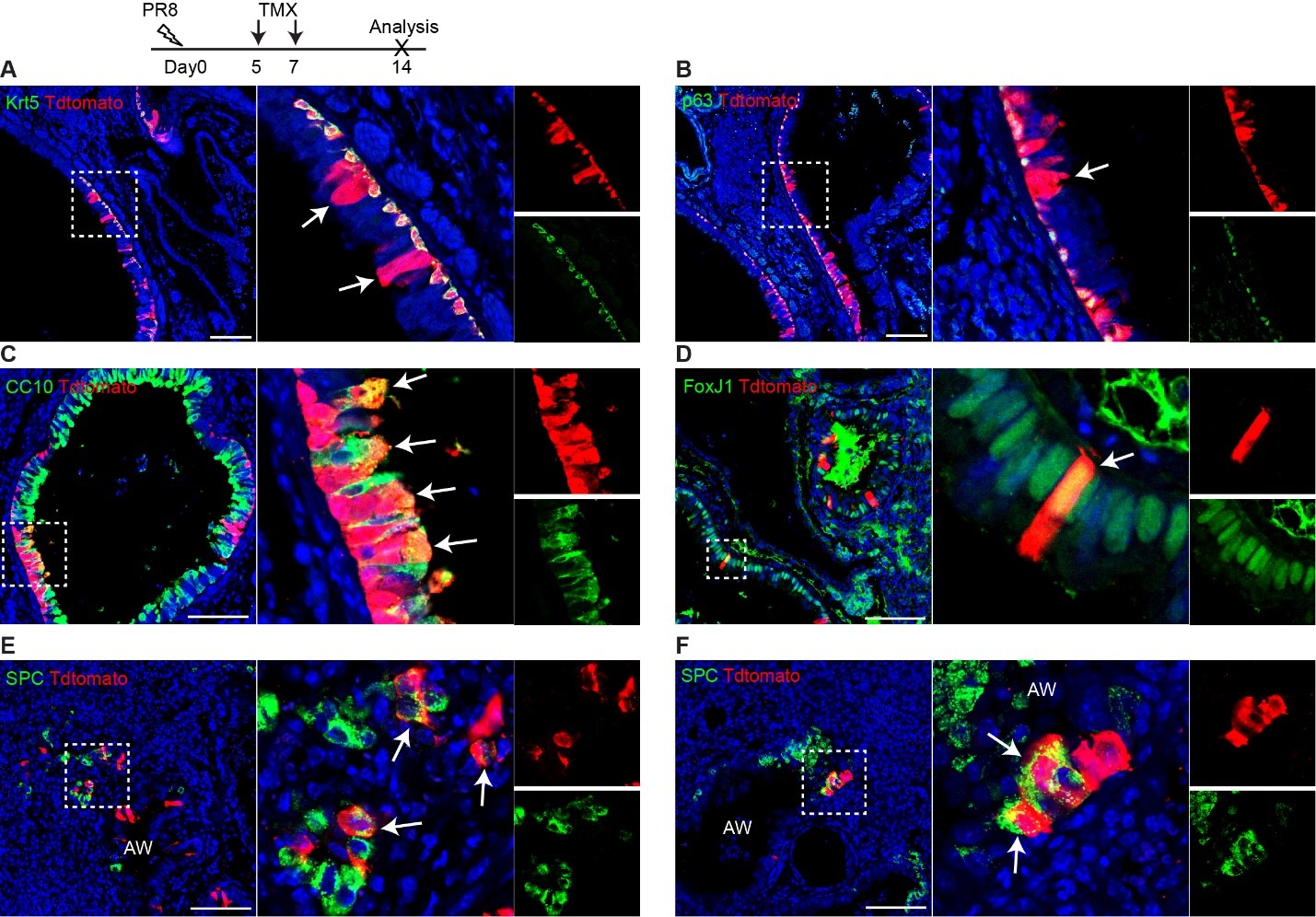
We thank the reviewer for the question. To answer the reviewer’s question, we performed PR8 infection (250 pfu) on three Trp63-CreERT2;R26tdT mice and TMX treatment at days 5 and 7 post viral infection. We didn't perform TMX injection immediately as the mice were sick at a few days post infection. The lung samples were collected at 14 dpi. We observed that tdT+ cells are present in the airways (rebuttal letter RLFigure 2A, B), and it appears that the lineage labeled cells (tdT+) include club cells (CC10+) that are underlined by tdT+Krt5+ basal cells (RLFigure 2C). We think that these labeled basal cells give rise to club cells. However, we also noticed that rare club cells and ciliated cells (FoxJ1+) are labeled by tdT in the areas absent of surrounding tdT+ basal cells (RLFigure 2D). Moreover, a minor population of tdT+ SPC+ cells are present in the terminal airways that were disrupted by viral infection (RLFigure 2E and D). We did not see any pods formed in this experiment and we did not observe any tdT+ cells in the intact alveoli (uninjured area).
RLFigure 2. Trp63-CreERT2 lineage labeled cells in the airways but not alveoli when Tamoxifen was induced at day 5 and 7 after PR8 H1N1 viral infection. Trp63-CreERT2;R26-tdT mice were infected with PR8 at 250 pfu and Tmx were delivered at a dose of 0.25 mg/g bodyweight by oral gavage. Lung samples were collected and analyzed at 14 dpi. Stained antibodies are as indicated. Scale bar: 100 µm.
- Please also show if p63-CreERT2 labels any cells in the adult lung parenchyma in the absence of injury after tamoxifen treatment.
Dr. Wellington Cardoso’s group demonstrated that Trp63-CreERT2 only labels very few cells in the airways but not the lung parenchyma in the absence of injury after tamoxifen treatment (Yang et al., 2018). Dr. Ying Yang has revisited the data and she did not observe any labeling in the lung parenchyma (n = 2).
- Please analyze if p63-CreERT2 labels any cells with tdTomato in the absence of injury or after PR8 infection but without tamoxifen treatment.
We performed the experiment and didn't observe any labeled cells in the lung parenchyma without Tamoxifen treatment (n = 4).
- Please analyze when after PR8 infection do the first p63-CreERT2 labeled tdTomato positive alveolar epithelial cells appear.
We administered tamoxifen at day 5 and 7 after PR8 infection and harvested lung tissues at day 14. As shown in Figure 1, we observed a few tdT+ SPC+ cells in the terminal airways that are disrupted by viral infection. Notably, we did not observe any lineage labeled cells in the intact alveoli (uninjured) in this experiment..
- A clonal analysis of p63-CreERT2 labeled cells using a confetti reporter might also help interpret the origin of p63-CreERT2 labeled cells.
We thank the reviewer for the suggestion. Our new data demonstrate that a rare population of SPC+tdT+ cells are present in the disrupted terminal airways of Trp63-CreERT2;R26tdT mice. Our data in the original manuscript and the new data suggest that the initial SPC+;tdT+ cells are rare because we have to administrate multiple doses of Tamoxifen to label them. Given the less labeling efficiency of confetti than R26tdT mice, it is possible we will not be able to label these SPC+ cells. Moreover, our original manuscript clearly shows individual clones of SPC+tdT+ cells in the regenerated lung, and they do not seem to compose of multiple clones. Therefore we think that use of confetti mice may not add new information..
- Lastly could the authors compare the single-cell RNAseq transcription profile of p63-CREERT2 labeled cells immediately after PR8 and tamoxifen treatment and also at 60dpi. A pseudotime analysis and trajectory interference analysis could help elucidate the identity of p63-CreERT2 labeled cells that are actually not ectopic basal progenitor cells.
We appreciated the reviewer’s suggestion and agree that single cell RNA sequencing with pseudotime analysis can provide further information regarding the origin of the lineage labeled alveolar cells of Trp63-CreERT2;R26tdT mice. That said, our new data clearly show that KRT5-CreER lineage labeled cells do not give rise to AT1/2 cells as previously described (Kanegai et al., 2016; Vaughan et al., 2015), suggesting that the ectopic basal progenitor cells do not generate alveolar cells. By contrast, Trp63-CreERT2 lineage labeled cells do give rise to AECs, suggesting that this p63+ cell population capable of generating AECs are different from Krt5+ ectopic basal progenitor cells. Our single cell core has an extremely long waiting list due to the pandemic and we hope that our new findings are enough to address the reviewer’s concern without the need of single cell analysis..
-

Evaluation Summary:
This study addresses an interesting question in lung regeneration following viral injury. The authors describe the ectopic differentiation of tuft cells that were derived from lineage-tagged p63+ cells post influenza virus infection. These tuft cells do not appear to proliferate or give rise to other lineages. Importantly, they demonstrate that depletion of tuft cells caused by genetic deletion of Pou2f3 in p63+ cells has no effect on the expansion or resolution of Krt5+ pods after infection, implying that tuft cells play no functional role in this process.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
Huang et al. sought to study the cellular origin of Tuft cells and the molecular mechanisms that govern their specification in severe lung injury. First the authors show ectopic emergence of Tuft cells in airways and distal parenchyma following different injuries. The authors also used lineage tracing models and uncovered that p63-expressing cells and to some extent Scgb1a1-lineaged labeled cells contribute to tuft cells after injury. Further, the authors modulated multiple pathways and claim that Notch inhibition blocks tuft cells whereas Wnt inhibition enhances Tuft cell development in basal cell cultures. Finally, the authors used Trpm5 and Pou2f3 knock-out models to claim that tuft cells are indispensable for alveolar regeneration.
In summary, the findings described in this manuscript are somewhat …
Reviewer #1 (Public Review):
Huang et al. sought to study the cellular origin of Tuft cells and the molecular mechanisms that govern their specification in severe lung injury. First the authors show ectopic emergence of Tuft cells in airways and distal parenchyma following different injuries. The authors also used lineage tracing models and uncovered that p63-expressing cells and to some extent Scgb1a1-lineaged labeled cells contribute to tuft cells after injury. Further, the authors modulated multiple pathways and claim that Notch inhibition blocks tuft cells whereas Wnt inhibition enhances Tuft cell development in basal cell cultures. Finally, the authors used Trpm5 and Pou2f3 knock-out models to claim that tuft cells are indispensable for alveolar regeneration.
In summary, the findings described in this manuscript are somewhat preliminary. The claim that the cellular origin of Tuft cells in influenza infection was not determined is incorrect. Current data from pathway modulation is preliminary and this requires genetic modulation to support their claims.
Major comments:
1. The abstract sounds incomplete and does not cover all key aspects of this manuscript. Currently, it is mainly focusing on the cellular origin of Tuft cells and the role of Wnt and notch signaling. However, it completely omits the findings from Trpm5 and Pou2f3 knock-out mice. In fact, the title of the manuscript highlights the indispensable nature of tuft cells in alveolar regeneration.
2. In lines 93-94, the authors state that "It is also unknown what cells generate these tuft cells.....". This statement is incorrect. Rane et al., 2019 used the same p63-creER mouse line and demonstrated that all tuft cells that ectopically emerge following H1N1 infection originate from p63+ lineage labeled basal cells. Therefore, this claim is not new.
3. Lines 152-153 state that "21.0% +/- 2.0 % tuft cells within EBCs are labeled with tdT when examined at 30 dpi...". It is not clear what the authors meant here ("within EBC's")? And also, the same sentence states that "......suggesting that club cell-derived EBCs generate a portion of tuft cells....". In this experiment, the authors used club cell lineage tracing mouse lines. So, how do the authors know that the club cell lineage-derived tuft cells came through intermediate EBC population? Current data do not show evidence for this claim. Is it possible that club cells can directly generate tuft cells?
4. Based on the data from Fig-3A, the authors claim that treatment with C59 significantly enhances tuft cell development in ALI cultures. Porcupine is known to facilitate Wnt secretion. So, which cells are producing Wnt in these cultures? It is important to determine which cells are producing Wnt and also which Wnt? Further, based on DBZ treatments, it appears that active Notch signaling is necessary to induce Tuft cell fate in basal cells. Where are Notch ligands expressed in these tissues? Is Notch active only in a small subset of basal cells (and hence generate rate tuft cells)? This is one of the key findings in this manuscript. Therefore, it is important to determine the expression pattern of Wnt and Notch pathway components.
5. How do the authors explain different phenotypes observed in Trpm5 knockout and Pou2f3 mutants? Is it possible that Trpm5 knockout mice have a subset of tuft cells and that they might be something to do with the phenotypic discrepancy between two mutant models?
6. One of the key findings in this manuscript is that Wnt and Notch signaling play a role in Tuft cell specification. All current experiments are based on pharmacological modulation. These need to be substantiated using genetic gain loss of function models.
-

Reviewer #2 (Public Review):
In this manuscript, the authors describe the ectopic differentiation of tuft cells that were derived from lineage-tagged p63+ cells post influenza virus infection. These tuft cells do not appear to proliferate or give rise to other lineages. They then claim that Wnt inhibitors increase the number of tuft cells while inhibiting Notch signalling decreases the number of tuft cells within Krt5+ pods after infection in vitro and in vivo. The authors further show that genetic deletion of Trpm5 in p63+ cells post-infection results in an increase in AT2 and AT1 cells in p63 lineage-tagged cells compared to control. Lastly, they demonstrate that depletion of tuft cells caused by genetic deletion of Pou2f3 in p63+ cells has no effect on the expansion or resolution of Krt5+ pods after infection, implying that tuft …
Reviewer #2 (Public Review):
In this manuscript, the authors describe the ectopic differentiation of tuft cells that were derived from lineage-tagged p63+ cells post influenza virus infection. These tuft cells do not appear to proliferate or give rise to other lineages. They then claim that Wnt inhibitors increase the number of tuft cells while inhibiting Notch signalling decreases the number of tuft cells within Krt5+ pods after infection in vitro and in vivo. The authors further show that genetic deletion of Trpm5 in p63+ cells post-infection results in an increase in AT2 and AT1 cells in p63 lineage-tagged cells compared to control. Lastly, they demonstrate that depletion of tuft cells caused by genetic deletion of Pou2f3 in p63+ cells has no effect on the expansion or resolution of Krt5+ pods after infection, implying that tuft cells play no functional role in this process.
Overall, in vivo and in vitro phenotypes of tuft cells and alveolar cells are clear, but the lack of detailed cellular characterization and molecular mechanisms underlying the cellular events limits the value of this study.
1. Origin of tuft cells: Although the authors showed the emergence of ectopic tuft cells derived from labelled p63+ cells after infection, it cannot be ruled out that pre-existing p63+Krt5- intrapulmonary progenitors, as previously reported, can also contribute to tuft cell expansion (Rane et al. 2019; by labelling p63+ cells prior to infection, they showed that the majority of ectopic tuft cells are derived from p63+ cells after viral infection). It would be more informative if the authors show the differentiation of tuft cells derived from p63+Krt5+ cells by tracing Krt5+ cells after infection, which will tell us whether ectopic tuft cells are differentiated from ectopic basal cells within Krt5+ pods induced by virus infection.
2. Mechanisms of tuft cell differentiation: The authors tried to determine which signalling pathways regulate the differentiation of tuft cells from p63+ cells following infection. Although Wnt/Notch inhibitors affected the number of tuft cells derived from p63+ labelled cells, it remains unclear whether these signals directly modulate differentiation fate. The authors claimed that Wnt inhibition promotes tuft cell differentiation from ectopic basal cells. However, in Fig 3B, Wnt inhibition appears to trigger the expansion of p63+Krt5+ pod cells, resulting in increased tuft cell differentiation rather than directly enhancing tuft cell differentiation. Further, in Fig 3D, Notch inhibition appears to reduce p63+Krt5+ pod cells, resulting in decreased tuft cell differentiation. Importantly, a previous study has reported that Notch signalling is critical for Krt5+ pod expansion following influenza infection (Vaughan et al. 2015; Xi et al. 2017). Notch inhibition reduced Krt5+ pod expansion and induced their differentiation into Sftpc+ AT2 cells. In order to address the direct effect of Wnt/Notch signalling in the differentiation process of tuft cells from EBCs, the authors should provide a more detailed characterization of cellular composition (Krt5+ basal cells, club cells, ciliated cells, AT2 and AT1 cells, etc.) and activity (proliferation) within the pods with/without inhibitors/activators.
3. Impact of Trpm5 deletion in p63+ cells: It is interesting that Trpm5 deletion promotes the expansion of AT2 and AT1 cells derived from labelled p63+ cells following infection. It would be informative to check whether Trpm5 regulates Hif1a and/or Notch activity which has been reported to induce AT2 differentiation from ectopic basal cells (Xi et al. 2017). Although the authors stated that there was no discernible reduction in the size of Krt5+ pods in mutant mice, it would be interesting to investigate the relationship between AT2/AT1 cell retaining pods and the severity of injury (e.g. large Krt5+ pods retain more/less AT2/AT1 cells compared to small pods. What about other cell types, such as club and goblet cells, in Trpm5 mutant pods? Again, it cannot be ruled out that pre-existing p63+Krt5- intrapulmonary progenitor cells can directly convert into AT2/AT1 cells upon Trpm5 deletion rather than p63+Krt5+ cells induced by infection.
4. Ectopic tuft cells in COVID-19 lungs: The previous study by the authors' group revealed the presence of ectopic tuft cells in COVID-19 patient samples (Melms et al. 2021). There appears to be no additional information in this manuscript.
5. Quantification information and method: Overall, the quantification method should be clarified throughout the manuscript. Further, in the method section, the authors stated that the production of various airway epithelial cell types was counted and quantified on at least 5 "random" fields of view. However, virus infection causes spatially heterogeneous injury, resulting in a difficult to measure "blind test". The authors should address how they dealt with this issue.
-

Reviewer #3 (Public Review):
In this manuscript Huang et al. study how the lung regenerates after severe injury due to viral infection. They focus on how tuft cells may affect regeneration of the lung by ectopic basal cells and come to the conclusion that they are not required. The manuscript is intriguing but also very puzzling. The authors claim they are specifically targeting ectopic basal progenitor cells and show that they can regenerate the alveolar epithelium in the lung following severe injury. However, it is not clear that the p63-CreERT2 line the authors are using only labels ectopic basal cells. The question is what is a basal cell? Is an ectopic basal progenitor cell only defined by Trp63 expression?
The accompanying manuscript by Barr et al. uses a Krt5-CreERT2 line to target ectopic basal cells and using that tool the …
Reviewer #3 (Public Review):
In this manuscript Huang et al. study how the lung regenerates after severe injury due to viral infection. They focus on how tuft cells may affect regeneration of the lung by ectopic basal cells and come to the conclusion that they are not required. The manuscript is intriguing but also very puzzling. The authors claim they are specifically targeting ectopic basal progenitor cells and show that they can regenerate the alveolar epithelium in the lung following severe injury. However, it is not clear that the p63-CreERT2 line the authors are using only labels ectopic basal cells. The question is what is a basal cell? Is an ectopic basal progenitor cell only defined by Trp63 expression?
The accompanying manuscript by Barr et al. uses a Krt5-CreERT2 line to target ectopic basal cells and using that tool the authors do not see a signification contribution of ectopic basal cells towards alveolar epithelial regeneration. As such the claim that ectopic basal cell progenitors drive alveolar epithelial regeneration is not well-founded.
The title itself is also not very informative and is a bit misleading. That being said I think the manuscript is still very interesting and can likely easily be improved through a better validation of which cells the p63-CreERT2 tool is targeting.
I, therefore, suggest the following experiments.
Please analyze which cells p63-CreERT2 labels immediately after PR8 and tamoxifen treatment. Are all the tdTomato labeled cells also Krt5 and p63 positive or are some alveolar epithelial cells or other airway cell types also labeled?
Please also show if p63-CreERT2 labels any cells in the adult lung parenchyma in the absence of injury after tamoxifen treatment.
Please analyze if p63-CreERT2 labels any cells with tdTomato in the absence of injury or after PR8 infection but without tamoxifen treatment.
Please analyze when after PR8 infection do the first p63-CreERT2 labeled tdTomato positive alveolar epithelial cells appear.
A clonal analysis of p63-CreERT2 labeled cells using a confetti reporter might also help interpret the origin of p63-CreERT2 labeled cells.
Lastly could the authors compare the single-cell RNAseq transcription profile of p63-CREERT2 labeled cells immediately after PR8 and tamoxifen treatment and also at 60dpi. A pseudotime analysis and trajectory interference analysis could help elucidate the identity of p63-CreERT2 labeled cells that are actually not ectopic basal progenitor cells.
-

SciScore for 10.1101/2022.03.11.483948: (What is this?)
Please note, not all rigor criteria are appropriate for all manuscripts.
Table 1: Rigor
Ethics IACUC: Mouse studies were approved by Columbia University Medical Center (CUMC) Institutional Animal Care and Use Committees (IACUC).
Euthanasia Agents: Pulmonary function assessment: Trpm5+/- and Trpm5-/- mice (21 days after viral injury) were anesthetized with pentobarbital (50 mg/kg, i.p.).
IRB: All experiments involving human samples were performed in accordance with the protocols approved by the Institutional Review Boards at Columbia University Irving Medical CenterSex as a biological variable 8-12 weeks old animals of both sexes were used in equal proportions. Randomization The production of various airway epithelial cell types was counted and quantified on at least 5 random fields of view … SciScore for 10.1101/2022.03.11.483948: (What is this?)
Please note, not all rigor criteria are appropriate for all manuscripts.
Table 1: Rigor
Ethics IACUC: Mouse studies were approved by Columbia University Medical Center (CUMC) Institutional Animal Care and Use Committees (IACUC).
Euthanasia Agents: Pulmonary function assessment: Trpm5+/- and Trpm5-/- mice (21 days after viral injury) were anesthetized with pentobarbital (50 mg/kg, i.p.).
IRB: All experiments involving human samples were performed in accordance with the protocols approved by the Institutional Review Boards at Columbia University Irving Medical CenterSex as a biological variable 8-12 weeks old animals of both sexes were used in equal proportions. Randomization The production of various airway epithelial cell types was counted and quantified on at least 5 random fields of view with a 10X or a 20X objective, and the average and standard deviation was calculated. Blinding not detected. Power Analysis not detected. Table 2: Resources
Experimental Models: Organisms/Strains Sentences Resources Mouse Models: p63-CreER (Lee et al., 2014a), Scgb1a1-CreER (Rawlins et al., 2009), Pou2f3-CreER (McGinty et al., 2020a), Trpm5-GFP (Clapp et al., 2006), Trpm5-/- (Damak et al., 2006), and Pou2f3-/- (Matsumoto et al., 2011) mouse strains were previously described. Pou2f3-/-suggested: NoneB6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J(R26-tdT) mouse strain was purchased from The Jackson Laboratory (Stock #007914). B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J(R26-tdT)suggested: NoneAll mice were maintained on a C57BL/6 and 129SvEv mixed background and housed in the mouse facility at Columbia University according to institutional guidelines. C57BL/6suggested: None129SvEvsuggested: NoneFor lineage analysis and genetic targeting, Pou2f3-CreER mouse strain were administered 2 mg tamoxifen by oral gavage at days 0, 2, and 4 for control or 1 mg tamoxifen by oral gavage at days 14, 17, 20, 23, 25, 27 post viral infection. Pou2f3-CreERsuggested: Nonep63-CreER mice were administered with tamoxifen at a dose of 0.25 mg/g bodyweight by daily oral gavage for a total five doses as indicated. p63-CreERsuggested: NoneFor Scgb1a1-CreER mice, a period of ten or twenty-one days as indicated was used to wash out the residual tamoxifen before any further treatments. Scgb1a1-CreERsuggested: NoneMoreover, tracheal basal cells isolated from Trpm5-GFP mice were cultured and tested for drug effects in ALI. Trpm5-GFPsuggested: NonePulmonary function assessment: Trpm5+/- and Trpm5-/- mice (21 days after viral injury) were anesthetized with pentobarbital (50 mg/kg, i.p.). Trpm5-/-suggested: NoneResults from OddPub: We did not detect open data. We also did not detect open code. Researchers are encouraged to share open data when possible (see Nature blog).
Results from LimitationRecognizer: An explicit section about the limitations of the techniques employed in this study was not found. We encourage authors to address study limitations.Results from TrialIdentifier: No clinical trial numbers were referenced.
Results from Barzooka: We did not find any issues relating to the usage of bar graphs.
Results from JetFighter: We did not find any issues relating to colormaps.
Results from rtransparent:- Thank you for including a conflict of interest statement. Authors are encouraged to include this statement when submitting to a journal.
- Thank you for including a funding statement. Authors are encouraged to include this statement when submitting to a journal.
- No protocol registration statement was detected.
Results from scite Reference Check: We found no unreliable references.
-