Neuroendocrinology of the lung revealed by single-cell RNA sequencing
Curation statements for this article:-
Curated by eLife
eLife assessment
This study delineates the transcriptomics of lung neuroendocrine cells and provides important new information on the nature of these cells in normal mouse lungs and in a sample of a human lung carcinoid. It will inform future studies investing the roles of PNECs in health and disease.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Pulmonary neuroendocrine cells (PNECs) are sensory epithelial cells that transmit airway status to the brain via sensory neurons and locally via calcitonin gene-related peptide (CGRP) and γ- aminobutyric acid (GABA). Several other neuropeptides and neurotransmitters have been detected in various species, but the number, targets, functions, and conservation of PNEC signals are largely unknown. We used scRNAseq to profile hundreds of the rare mouse and human PNECs. This revealed over 40 PNEC neuropeptide and peptide hormone genes, most cells expressing unique combinations of 5–18 genes. Peptides are packaged in separate vesicles, their release presumably regulated by the distinct, multimodal combinations of sensors we show are expressed by each PNEC. Expression of the peptide receptors predicts an array of local cell targets, and we show the new PNEC signal angiotensin directly activates one subtype of innervating sensory neuron. Many signals lack lung targets so may have endocrine activity like those of PNEC-derived carcinoid tumors. PNECs are an extraordinarily rich and diverse signaling hub rivaling the enteroendocrine system.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
In the article "Neuroendocrinology of the lung revealed by single cell RNA sequencing", Kuo et. al. described various aspects of pulmonary neuroendocrine cells (PNECs) including the scRNA-seq profile of one human lung carcinoid sample. Overall, although this manuscript does not have any specific storyline, it is informative and would be an asset for researchers exploring various new roles of PNECs.
Thank you for appreciating the significance of the data presented. Our storyline focuses on the newly uncovered molecular diversity of PNECs and the extraordinary repertoire of peptidergic signals they express and cell types these signals can directly target in (and outside) the lung, in mice and human, and in health and disease (human carcinoid tumor).
Major comments:
The major concern …
Author Response
Reviewer #1 (Public Review):
In the article "Neuroendocrinology of the lung revealed by single cell RNA sequencing", Kuo et. al. described various aspects of pulmonary neuroendocrine cells (PNECs) including the scRNA-seq profile of one human lung carcinoid sample. Overall, although this manuscript does not have any specific storyline, it is informative and would be an asset for researchers exploring various new roles of PNECs.
Thank you for appreciating the significance of the data presented. Our storyline focuses on the newly uncovered molecular diversity of PNECs and the extraordinary repertoire of peptidergic signals they express and cell types these signals can directly target in (and outside) the lung, in mice and human, and in health and disease (human carcinoid tumor).
Major comments:
The major concern about the work is most results are preliminary, and at a descriptive level, conclusions or sub-conclusions are derived from scRNA-seq analysis only, lacking in-depth functional analysis and validation in other methods or systems. There are many open-end results that have been predicted by the authors based on their scRNA-seq data analysis without functional validation. In order to give them a constructive roadmap, it would be better to investigate literature and put them in a potential or probable hypothesis by citing the available literature. This should be done in each section of the result part. The paper lacks a main theme or specific biology question to address. In addition, the description about the human lung carcinoid by scRNA-seq is somehow disconnected from the main study line. Also, these results are derived from the study on only one single patient, lacking statistical power.
We agree that much of the data and analysis presented in the paper is descriptive and hypothesis-generating for PNECs, however we do not consider it preliminary. We focused on validating two key conclusions from the scRNA-seq analysis: PNECs are extraordinarily diverse molecularly (as validated by multiplex in situ hybridization and immunostaining) and they express many different combinations of peptidergic signals (and appear to package them in separate vesicles). From the lung expression profiles of the cognate receptors, we also predicted the direct lung targets of the dozens of new PNEC peptidergic signals we uncovered, and validated the cell target (PSN4, a recently identified subtype of pulmonary sensory neuron) of one of the newly identified PNEC signals (the classic hormone angiotensin) by confirming expression of the cognate receptor gene in PSN4 neurons that innervate PNECs and showing that the hormone can directly activate PSN4 neurons. The characterized human carcinoid provided evidence that during tumorigenesis, the amplified PNECs retain a memory (albeit imperfect) of the molecular subtype of PNEC from which they originated. As suggested by the Reviewer, we have provided more background in Results by adding additional citations from the literature to clarify the rationale for each analysis and what was known prior to the analysis. We feel that our paper provides a broad foundation for exploring the diversity and signaling functions of PNECs, and although each molecular type of PNEC and new PNEC peptidergic signal we uncovered and potential target cell in (and outside) the lung warrants follow up (as do the sensory and other properties of PNECs we inferred from their expression profiles), such studies will require the effort of many individuals in many labs studying both normal and disease physiology in mouse and human, and exploiting the data, hypotheses, approaches, and framework we provide.
Reviewer #2 (Public Review):
Pulmonary neuroendocrine cells (PNECs) are known to monitor oxygen levels in the airway and can serve as stem cells that repair the lung epithelium after injury. Due to their rarity, however, their functions are still poorly understood. To identify potential sensory functions of PNECs, the authors have used single-cell RNA-sequencing (scRNA-seq) to profile hundreds of mouse and human PNECs. They report that PNECs express over 40 distinct peptidergic genes, and over 150 distinct combinations of these genes can be detected. Receptors for these neuropeptides and peptide hormones are expressed in a wide range of lung cell types, suggesting that PNECs may have mechanical, thermal, acid, and oxygen sensory roles, among others. However, since some of these cognate receptors are not expressed in the lung, PNECs may also have systemic endocrine functions. Although these data are largely descriptive, the results represent a significant resource for understanding the potential roles of PNECs in normal biology as well as in pulmonary diseases and cancer and are likely to be relevant for understanding neuroendocrine cells in other tissue contexts.
However, there are several aspects of the data analysis that are unclear and require clarification, most notably the definition of a neuroendocrine cell (points #1 and #2 below).
- Figure S1 shows the sorting strategy used for isolation of putative PNECs from Ascl1CreER/+; Rosa26ZsGreen/+ mice, and distinguishes neuroendocrine cells defined as ZsGreen+ EpCAM+ and "neural" cells defined as ZsGreen+ EpCAM-; the figure legend also refers to the ZsGreen+ EpCAM- cells as "control" cells. However, the table shown in panel D indicates that the NE population combines 112 ZsGreen+ EpCAM+ cells together with 64 ZsGreen+ EpCAM- cells to generate the 176 cells used for subsequent analyses. Why are these ZsGreen+ EpCAM- cells initially labeled as neural or control, but are then defined as neuroendocrine? If these do not express an epithelial marker, can they be rigorously considered as neuroendocrine?
As explained above in the response to Essential Revision point 1, we define pulmonary neuroendocrine cells (PNECs) throughout the paper by their transcriptomic clustering and signatures, which includes the dozens of newly identified PNEC markers as well as the few extant marker genes available before this study (listed in Table S2). The confusion here arises from the two previously known markers (Ascl1 lineage marker ZsGreen, EpCAM) we used for flow sorting to enrich for these rare cells for transcriptomic profiling (Fig. S1). Although most of the cells with PNEC transcriptomic profiles were from the ZsGreenhi EpCAMhi sorted population (as expected), some were from the ZsGreenhi EpCAMlo sorted population. The latter resulted from the high EpCAM gating threshold we used during flow sorting, which excluded some PNECs with intermediate levels of surface EpCAM. Indeed, nearly all PNECs (> 95%) expressed EpCAM by scRNAseq, and there was no difference in EpCAM transcript levels or transcriptomic clustering of PNECs that were from the ZsGreenhi EpCAMhi vs. ZsGreenhi EpCAMlo sorted populations, as we now show in the new panels (C', C'') added to Fig S1C. This point is now clarified in the legend to Fig. S1C, and it nicely demonstrates that transcriptomic profiling is a more robust method of identifying PNECs than flow sorting based on two classical markers.
- Similarly, in the human scRNA-seq analysis, how were PNECs defined? The methods description states that these cells were identified by their expression of CALCA and ASCL1, but does not indicate whether they also expressed epithelial markers.
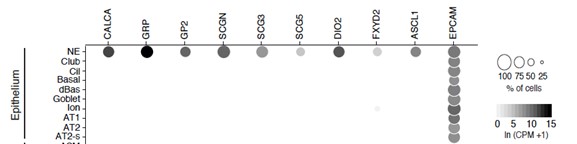
Human PNECs were identified in the single cell transcriptomic analysis by the same strategy described above for mouse PNECs: by their transcriptomic clustering and signatures, which includes the dozens of newly identified PNEC markers as well as the few extant marker genes available before this study (listed in Table S2). In addition to expression of classic and new markers, the human PNEC cluster defined by scRNA-seq indeed showed the expected expressed of epithelial markers (e.g, EPCAM, see dotplot below), like other epithelial cells.
- The presentation of sensitivity and specificity in Figure 1 is confusing and potentially misleading. According to Figure 1B, Psck1 and Nov are two of the top-ranked differentially expressed genes in PNECs with respect to both sensitivity and specificity. However, the specificity of these two genes appears to be lower than that of Scg5, Chgb, and several other genes, as suggested in Figure 1C and Figure S1E. In contrast, Chgb appears to have higher specificity and sensitivity than Psck1 in Figures 1C and E but is not shown in the list of markers in Figure 1B.
As explained above in the response to Essential Revision point 2, because different marker features are important for different applications, we have provided several different graphical formats (Figs. 1B,C, Fig. S1E) and a table (Table S1) to aid in selection of the optimal markers for each application. Fig. 1B shows the most sensitive and specific PNEC markers identified by ratio of the natural logs of the average expression of the marker in PNECs vs. non-PNEC epithelial cells (Table S1), and we have added a two-dimensional plot of this sensitivity and specificity for a large set of PNEC markers (new panel E of Fig. S1). The violin plots in Fig. 1C allow visual comparison of expression of selected markers across PNECs and 40 other lung cell types including non-epithelial cells (from our extensive mouse lung atlas in Travaglini, Nabhan et al, Nature 2020). Pcsk1 and Nov score high in the analysis of Fig. 1B because they are highly sensitive and specific markers within the pulmonary epithelium, and they are also valuable markers because they are highly expressed in PNECs. However, they appear slightly less specific in the violon plots of Fig. 1C (Pcsk1) and Fig. S1F (Nov) because of expression (though at much lower levels) in individual lung cell types outside the epithelium: Pcsk1 is expressed also at low levels in some Alox5+ lymphocytes, and Nov is expressed at low levels in some smooth muscle cells. Chgb is a new PNEC marker that did not make the cutoff for the list in Fig. 1B because it is expressed in a slightly higher percentage of non-PNEC epithelial cells than the markers shown, which ranked slightly above it by this metric (see Table S1).
- The expression of serotonin biosynthetic genes in mouse versus human PNECs deserves some comment. The authors fail to detect the expression of Tph1 and Tph2 in any of the mouse PNECs analyzed, but TPH1 is expressed in 76% of the human PNECs (Table S8). Is it possible that Tph1 and Tph2 are not detected in the mouse scRNA-seq data due to gene drop-out? If serotonin signaling by mouse PNECs is due to protein reuptake, as implied on p. 5, is there a discrepancy between serotonin expression as detected by smFISH versus immunostaining?
It is always possible that the failure to detect expression of Tph1 and Tph2 in the mouse scRNA-seq dataset is due to technical dropout, however when we analyzed this in our other mouse PNEC scRNA-seq dataset obtained using a microfluidic platform and also deeply-sequenced (Ouadah et al, Cell 2019), we found similar values as in the previously analyzed dataset: no Tph2 expression was detected and only 3% (3 of 92) of PNECs had detected Tph1 expression, whereas 24% (22 of 92) had detected expression of serotonin re-uptake transporter Slc6a4. Because our mouse and human scRNA-seq datasets were prepared similarly and sequenced to a similar depth (105 to 106 reads/cell), the difference observed in Tph1/TPH1 expression between mouse (0-3% PNECs) and human (76% PNECs) is more likely a true biological difference. We also analyzed serotonin levels in mouse PNECs by immunohistochemistry (not shown) and detected serotonin in nearly all (~90%) embryonic PNECs but only ~10% of adult PNECs. Systematic follow up studies will be necessary to resolve the mechanism of serotonin biogenesis and uptake in PNECs, and the potential stage and species-specific differences in these processes suggested by this initial data.
- The smFISH and immunostaining analyses are often presented without any indication of the number of independent replicate samples analyzed (e.g., Figure 2B, Figure 3F, G).
The number of samples analyzed have been added (the values for Fig. 2B are given in legend to Fig. 2C, the quantification of Fig. 2B).
- It would be helpful to provide a statistical analysis of the similarities and differences shown in the graphs in Figures 1E and G.
We added a statistical analysis (Fisher's exact test, two-sided) of Fig. 1E comparing expression of each examined gene in the two scRNA-seq datasets (Table S4). We added a similar statistical analysis of Fig. 1G comparing the expression values of each examined gene by scRNA-seq vs smFISH (see Fig. 1G legend).
-

eLife assessment
This study delineates the transcriptomics of lung neuroendocrine cells and provides important new information on the nature of these cells in normal mouse lungs and in a sample of a human lung carcinoid. It will inform future studies investing the roles of PNECs in health and disease.
-

Reviewer #1 (Public Review):
In the article "Neuroendocrinology of the lung revealed by single cell RNA sequencing", Kuo et. al. described various aspects of pulmonary neuroendocrine cells (PNECs) including the scRNA-seq profile of one human lung carcinoid sample. Overall, although this manuscript does not have any specific storyline, it is informative and would be an asset for researchers exploring various new roles of PNECs.
Major comments:
The major concern about the work is most results are preliminary, and at a descriptive level, conclusions or sub-conclusions are derived from scRNA-seq analysis only, lacking in-depth functional analysis and validation in other methods or systems. There are many open-end results that have been predicted by the authors based on their scRNA-seq data analysis without functional validation. In order to …Reviewer #1 (Public Review):
In the article "Neuroendocrinology of the lung revealed by single cell RNA sequencing", Kuo et. al. described various aspects of pulmonary neuroendocrine cells (PNECs) including the scRNA-seq profile of one human lung carcinoid sample. Overall, although this manuscript does not have any specific storyline, it is informative and would be an asset for researchers exploring various new roles of PNECs.
Major comments:
The major concern about the work is most results are preliminary, and at a descriptive level, conclusions or sub-conclusions are derived from scRNA-seq analysis only, lacking in-depth functional analysis and validation in other methods or systems. There are many open-end results that have been predicted by the authors based on their scRNA-seq data analysis without functional validation. In order to give them a constructive roadmap, it would be better to investigate literature and put them in a potential or probable hypothesis by citing the available literature. This should be done in each section of the result part.
The paper lacks a main theme or specific biology question to address. In addition, the description about the human lung carcinoid by scRNA-seq is somehow disconnected from the main study line. Also, these results are derived from the study on only one single patient, lacking statistical power. -

Reviewer #2 (Public Review):
Pulmonary neuroendocrine cells (PNECs) are known to monitor oxygen levels in the airway and can serve as stem cells that repair the lung epithelium after injury. Due to their rarity, however, their functions are still poorly understood. To identify potential sensory functions of PNECs, the authors have used single-cell RNA-sequencing (scRNA-seq) to profile hundreds of mouse and human PNECs. They report that PNECs express over 40 distinct peptidergic genes, and over 150 distinct combinations of these genes can be detected. Receptors for these neuropeptides and peptide hormones are expressed in a wide range of lung cell types, suggesting that PNECs may have mechanical, thermal, acid, and oxygen sensory roles, among others. However, since some of these cognate receptors are not expressed in the lung, PNECs may …
Reviewer #2 (Public Review):
Pulmonary neuroendocrine cells (PNECs) are known to monitor oxygen levels in the airway and can serve as stem cells that repair the lung epithelium after injury. Due to their rarity, however, their functions are still poorly understood. To identify potential sensory functions of PNECs, the authors have used single-cell RNA-sequencing (scRNA-seq) to profile hundreds of mouse and human PNECs. They report that PNECs express over 40 distinct peptidergic genes, and over 150 distinct combinations of these genes can be detected. Receptors for these neuropeptides and peptide hormones are expressed in a wide range of lung cell types, suggesting that PNECs may have mechanical, thermal, acid, and oxygen sensory roles, among others. However, since some of these cognate receptors are not expressed in the lung, PNECs may also have systemic endocrine functions. Although these data are largely descriptive, the results represent a significant resource for understanding the potential roles of PNECs in normal biology as well as in pulmonary diseases and cancer and are likely to be relevant for understanding neuroendocrine cells in other tissue contexts.
However, there are several aspects of the data analysis that are unclear and require clarification, most notably the definition of a neuroendocrine cell (points #1 and #2 below).
1. Figure S1 shows the sorting strategy used for isolation of putative PNECs from Ascl1CreER/+; Rosa26ZsGreen/+ mice, and distinguishes neuroendocrine cells defined as ZsGreen+ EpCAM+ and "neural" cells defined as ZsGreen+ EpCAM-; the figure legend also refers to the ZsGreen+ EpCAM- cells as "control" cells. However, the table shown in panel D indicates that the NE population combines 112 ZsGreen+ EpCAM+ cells together with 64 ZsGreen+ EpCAM- cells to generate the 176 cells used for subsequent analyses. Why are these ZsGreen+ EpCAM- cells initially labeled as neural or control, but are then defined as neuroendocrine? If these do not express an epithelial marker, can they be rigorously considered as neuroendocrine?
2. Similarly, in the human scRNA-seq analysis, how were PNECs defined? The methods description states that these cells were identified by their expression of CALCA and ASCL1, but does not indicate whether they also expressed epithelial markers.
3. The presentation of sensitivity and specificity in Figure 1 is confusing and potentially misleading. According to Figure 1B, Psck1 and Nov are two of the top-ranked differentially expressed genes in PNECs with respect to both sensitivity and specificity. However, the specificity of these two genes appears to be lower than that of Scg5, Chgb, and several other genes, as suggested in Figure 1C and Figure S1E. In contrast, Chgb appears to have higher specificity and sensitivity than Psck1 in Figures 1C and E but is not shown in the list of markers in Figure 1B.
4. The expression of serotonin biosynthetic genes in mouse versus human PNECs deserves some comment. The authors fail to detect the expression of Tph1 and Tph2 in any of the mouse PNECs analyzed, but TPH1 is expressed in 76% of the human PNECs (Table S8). Is it possible that Tph1 and Tph2 are not detected in the mouse scRNA-seq data due to gene drop-out? If serotonin signaling by mouse PNECs is due to protein reuptake, as implied on p. 5, is there a discrepancy between serotonin expression as detected by smFISH versus immunostaining?
5. The smFISH and immunostaining analyses are often presented without any indication of the number of independent replicate samples analyzed (e.g., Figure 2B, Figure 3F, G).
6. It would be helpful to provide a statistical analysis of the similarities and differences shown in the graphs in Figures 1E and G.
-

Reviewer #3 (Public Review):
The authors present a comprehensive profile of signals and sensors expressed in mouse and human PNECs by single-cell RNA sequencing. Analyses revealed a myriad combination of neuropeptide, neurotransmitter, receptor, and channel genes in PNECs. A diverse transcript combination is further enriched by alternative posttranscriptional and posttranslational processing. The authors also surveyed cognate receptors expressed in epithelial cells, endothelial cells, stromal cells, immune cells, and pulmonary sensory neurons, identifying potential local targets for the PNECs signals. The scRNA-seq profile from lung carcinoid tumors suggests that selected PNECs are susceptible to carcinoid transformation. Together, these data indicate that PNECs serve as sentinels to perceive multiple airway stimuli and express a …
Reviewer #3 (Public Review):
The authors present a comprehensive profile of signals and sensors expressed in mouse and human PNECs by single-cell RNA sequencing. Analyses revealed a myriad combination of neuropeptide, neurotransmitter, receptor, and channel genes in PNECs. A diverse transcript combination is further enriched by alternative posttranscriptional and posttranslational processing. The authors also surveyed cognate receptors expressed in epithelial cells, endothelial cells, stromal cells, immune cells, and pulmonary sensory neurons, identifying potential local targets for the PNECs signals. The scRNA-seq profile from lung carcinoid tumors suggests that selected PNECs are susceptible to carcinoid transformation. Together, these data indicate that PNECs serve as sentinels to perceive multiple airway stimuli and express a variety of signals that either act locally or potentially through circulation to regulate homeostasis.
-