Global chromatin mobility induced by a DSB is dictated by chromosomal conformation and defines the HR outcome
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This study is of relevance to the field of DNA repair. It uses a cleverly designed new recombination assay in yeast to address the impact of DNA break position on global genome mobility. A centromere-proximal DNA double-strand break (DSB) induces an H2A(X) phosphorylation-dependent global mobility that accelerates but is not essential for DSB repair, while a centromere-distal DSB triggers global mobility that is essential for repair and which depends on H2A(X) phosphorylation, Rad9 and Rad51. Together, these data support a model where global genome mobility promotes homologous recombination repair, particularly for centromere-distal DSBs, and help settle some recent controversy in the field.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Repair of DNA double-strand breaks (DSBs) is crucial for genome integrity. A conserved response to DSBs is an increase in chromatin mobility that can be local, at the site of the DSB, or global, at undamaged regions of the genome. Here, we address the function of global chromatin mobility during homologous recombination (HR) of a single, targeted, controlled DSB. We set up a system that tracks HR in vivo over time and show that two types of DSB-induced global chromatin mobility are involved in HR, depending on the position of the DSB. Close to the centromere, a DSB induces global mobility that depends solely on H2A(X) phosphorylation and accelerates repair kinetics, but is not essential. In contrast, the global mobility induced by a DSB away from the centromere becomes essential for HR repair and is triggered by homology search through a mechanism that depends on H2A(X) phosphorylation, checkpoint progression, and Rad51. Our data demonstrate that global mobility is governed by chromosomal conformation and differentially coordinates repair by HR.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
The data support the claims, and the manuscript does not have significant weaknesses in its present form. Key strengths of the paper include using a creative HR-based reporter system combining different inducible DSB positions along a chromosome arm and testing plasmid-based and chromosomal donor sequences. Combining that system with the visualization of specific chromosomal sites via microscopy is powerful. Overall, this work will constitute a timely and helpful contribution to the field of DSB/genome mobility in DNA repair, especially in yeast, and may inform similar mechanisms in other organisms. Importantly, this study also reconciles some of the apparent contradictions in the field.
We thank the reviewer for these positive comments on the quality of the THRIV system, in helping us to …
Author Response
Reviewer #1 (Public Review):
The data support the claims, and the manuscript does not have significant weaknesses in its present form. Key strengths of the paper include using a creative HR-based reporter system combining different inducible DSB positions along a chromosome arm and testing plasmid-based and chromosomal donor sequences. Combining that system with the visualization of specific chromosomal sites via microscopy is powerful. Overall, this work will constitute a timely and helpful contribution to the field of DSB/genome mobility in DNA repair, especially in yeast, and may inform similar mechanisms in other organisms. Importantly, this study also reconciles some of the apparent contradictions in the field.
We thank the reviewer for these positive comments on the quality of the THRIV system, in helping us to understand global mobility and to reconcile the different studies in the field. The possibility that these mobilities also exist in other organisms is attractive because they could be a way to anticipate the position of the damage in the genome and its possible outcome.
Reviewer #2 (Public Review):
The authors are clarifying the role of global mobility in homologous recombination (HR). Global mobility is positively correlated with recombinant product formation in some reports. However, some studies argue the contrary and report that global mobility is not essential for HR. To characterize the role of global chromatin mobility during HR, the authors set up a system in haploid yeast cells that allows simultaneously tracking of HR at the single-cell level and allows the analysis of different positions of the DSB induction. By moving the position of the DSB within their system, the authors postulate that the chromosomal conformation surrounding a DNA break affects the global mobility response. Finally, the authors assessed the contributions of H2A(X) phosphorylation, checkpoint progression and Rad51 in the mobility response.
One of the strengths of the manuscript is the development of "THRIV" as an efficient method for tracking homologous recombination in vivo. The authors take advantage of the power of yeast genetics and use gene deletions and as well as mutations to test the contribution of H2A(X) phosphorylation, checkpoint progression and Rad51 to the mobility response in their THRIV system.
A major weakness in the manuscript is the lack of a marker to indicate that DSB formation has occurred (or is occurring)? Although at 6 hours there is 80% I-SceI cutting, around 20% of the cells are uncut and cannot be distinguished from the ones that are cut (or have already been repaired). Thus, the MSD analysis is done in the blind with respect to cells actually undergoing DSB repair.
The authors clearly outlined their aims and have substantial evidence to support their conclusions. They discovered new features of global mobility that may clear up some of the controversies in the field. They overinterpreted some of their observations, but these criticisms can be easily addressed.
The authors addressed conflicting results concerning the importance of global mobility to HR and their results aid in reconciling some of the controversies in the field. A key strength of this manuscript is the analysis of global mobility in response to breaks at different locations within chromosomes? They identified two types of DSB-induced global chromatin mobility involved in HR and postulate that they differ based on the position of the DSB. For example, DSBs close to the centromere exhibit increased global mobility that is not essential for repair and depends solely on H2A(X) phosphorylation. However, if the DSB is far away from the centromere, then global mobility is essential for HR and is dependent on H2A(X) phosphorylation, checkpoint progression as well as the Rad51 recombinase.
The Bloom lab had previously identified differences in mobility based on the position of the tracked site. However, in the study reported here, the mobility response is analyzed after inducing DSBs located at different positions along the chromosome.
They also addressed the question of the importance of the Rad51 protein in increased global mobility in haploid cells. Previous studies used DNA damaging agents that induce DSBs randomly throughout the genome, where it would have been rare to induce DSBs near the centromere. In the studies reported in this manuscript, they find no increase in global mobility in a rad51∆ background for breaks induced near the centromere (proximal), but find that breaks induced near the telomeres (distal), are dependent on both gamma-H2A(X) spreading and the Rad51 recombinase.
We thank the referee for his constructive comments on the strength of our system to accurately determine the impact of a DSB according to its position in the genome. Concerning the issue of damaged cells that were not detected, it is a very important and exciting issue because it confronts our data with the question of biological heterogeneity. We provide evidence on the consistency of our findings despite the lack of detection of undamaged cells.
Reviewer #3 (Public Review):
In this study, Garcia Fernandez et al. employ a variety of genetic constructs to define the mechanism underlying the global chromatin mobility elicited in response to a single DNA double-strand break (DSB). Such local and global chromatin mobility increases have been described a decade ago by the Gasser and Rothstein laboratories, and a number of determinants have been identified: one epistasis group results in H2A-S129 phosphorylation via Rad9 and Mec1 activation. The mechanism is thought to be due to chromatin rigidification (Herbert 2017; Miné-Hattab 2017) or general eviction of histones (Cheblal 2020). More enigmatic, global chromatin mobility increase also depends on Rad51, a central recombination protein downstream of checkpoint activation (Smith & Rothstein 2017), which is also required for local DSB mobility (Dion .. Gasser 2012). The authors set out to address this difficulty in the field.
A premise of their study is the convergence of two types of observations: First, the H2A phosphorylation ChIP profile matches that of Rad51, with both spreading in trans on other chromosomes at the level of centromeres when a DSB occurs in the vicinity of one of them (Renkawitz 2014). Second, global mobility depends on H2A phosphorylation and on Rad51 (their previous study Herbert 2017). They thus address whether the Rad51-ssDNA filament (and associated proteins) marks the chromatin engaged during the homology search. They found that the extent of the mobility depends on the residency time of the filament in a particular genomic and nuclear region, which can be induced at an initially distant trans site by providing a region of homology. Unfortunately, these findings are not clearly apparent from the title and the abstract, and in fact somewhat misrepresented in the manuscript, which would call for a rewrite (see points below).
The main goal of our study was to understand the role of global mobility in the repair by homologous recombination, depending on the location of the damage. We found distinct global mobility mechanisms, in particular in the involvement of the Rad51 nucleofilament, depending on whether the DSB was pericentromeric or not. It is thus likely that when the DSB is far from the pericentromere, the residence time of the Rad51 nucleofilament with the donor has an impact on global mobility. Thus, if our experiments were not designed to answer directly the question of the residence time of the nucleofilament, we now discuss in more detail the causes and consequences of the global mobility.
To this end, they induce the formation of a site-specific DSB in either of two regions: a centromere-proximal region and a telomere-proximal region, and measure the mobility of an undamaged site near the centromere on another chromosome (with a LacO-LacI-GFP system). This system reveals that only the centromere-proximal DSB induces the mobility of the centromere-proximal undamaged site, in a Rad9- and Rad51-independent manner. Providing a homologous donor in the vicinity of the LacO array (albeit in trans) restores its mobility when the DSB is located in a subtelomeric region, in a Rad9- and Rad51-dependent fashion. These genetic requirements are the same as those described for local DSB mobility (Dion & Gasser 2012), drawing a link between the two types of mobility, which to my knowledge was not described. The authors should focus their message (too scattered in the current manuscript), on these key findings and the diffusive "painting" model, in which the canvas is H2A, the moving paintbrush Mec1, and the hand the Rad51-ssDNA filament whose movement depends on Rad9. In the absence of Rad51-Rad9 the hand stays still, only decorating H2A in its immediate environment. The amount of paint deposited depends on the residency time of the Rad51-ssDNA-Mec1 filament in a given nuclear region. This synthesis is in agreement with the data presented and contrasts with their proposal that "two types of global mobility" exist.
The brush model is very useful in explaining the distal mobility, which indeed is linked to local mobility genetic requirements, but it is also helpful to think of different model than the brush model when pericentromeric damage occurs. To stay in the terms of painting technique, this model would be similar to the pouring technique, when oil paint is deposited on water and spreads in a multidirectional manner. It is likely that Mec1 or Tel1 are the factors responsible for this spreading pattern. We therefore propose to maintain the notion of two distinct types of mobilities. Without going into pictorial techniques in the text, we have attempted to clarify these two models in the manuscript.
The rest of the manuscript attempts to define a role in DSB repair of this phosphor-H2A-dependent mobility, using a fluorescence recovery assay upon DSB repair. They correlate a defect in the centromere-proximal mobility (in the rad9 or h2a-s129a mutant) when a DSB is distantly induced in the subtelomere with a defect in repairing the DSB. Repair efficiency is not affected by these mutations when the donor is located initially close to the DSB site. This part is less convincing, as repair failure specifically at a distant donor in the rad9 and H2A-S129A mutants may result from other defects relating to chromatin than its mobility (i.e. affecting homology sampling, DNA strand invasion, D-loop extension, D-loop disruption, etc), which could be partially alleviated by repeated DSB-donor encounters when the two are spatially close. In fact, suggesting that undamaged site mobility is required for the early step of the homology search directly contradicts the fact that the centromere-proximal mobility induced by a subtelomeric DSB depends on the presence of a donor near the centromere: mobility is thus a product of homology identification and increased Rad51-ssDNA filament residency in the vicinity of the centromere, and so downstream of homology search. This is a major pitfall in their interpretation and model.
We thank the referee for helping to clarify the question of the cause and consequence of global mobility. As he pointed out, the fact that a donor is required to observe both H2A phosphorylation and distal mobility implicates the recombination process itself, as well as the residence time of the Rad51 nucleofilament, in the ƴ--‐H2A(X) spreading and indicates that recombination would be the cause of distal mobility. In contrast, the fact that proximal mobility can exist independently of homologous recombination suggests that in this particular configuration, HR would then be a consequence of proximal mobility.
In conclusion, I think the data presented are of importance, as they identify a link between local and global chromatin mobility. The authors should rewrite their manuscript and reorganize the figures to focus on the painter model that their data support. I propose experiments that will help bolster the manuscript conclusions.
- Attempt dual-color tracking of the DSB (i.e. Rad52-mCherry or Ddc1-mCherry) and the donor site, and track MSD as a function of proximity between the DSB and the Lac array (with DSB +/-dCen). The expectation is that only upon contact (or after getting in close range) should the MSD at the centromere-proximal LacO array increase with a DSB at a subtelomere. Furthermore, this approach will help distinguish MSDs in cells bearing a DSB (Rad52 foci) from undamaged ones (no Rad52 foci)(see Mine-Hattab & Rothstein 2012). This would help overcome the inefficient DSB induction of their system (less than 50% at 1 hr post-galactose addition, and reaching 80% at 6 hr). For the reader to have a better appreciation of the data distribution, replace the whisker plots of MSD at 10 seconds with either scatter dot plot or violin plots, whichever conveys most clearly the distribution of the data: indeed, a bimodal distribution is expected in the current data, with undamaged cells having lower, and damaged cells having higher MSDs.
The reviewer raises two points here.
The first point concerns the residence time of the Rad51 filament with the donor when a subtelomeric DSB happens. Measuring the DSBs as a function of the distance between donor and Rad52mCherry (or Ddc1--‐mCherry) would allow deciding on the cause or the consequence of the global mobility. Thus, if mobility is the consequence of (stochastic) contact, leading to a better efficiency of homologous recombination, we would see an increase in MSDs only when the distance between donor and filament would be small. Conversely, if global mobility is the cause of contact, the increase in mobility would be visible even when the distance between donor and filament is large. It would be necessary to have a labelling system with 3 different fluorophores — the one for the global mobility, the one for the donor and the one allowing following the filament. This triple labelling is still to be developed.
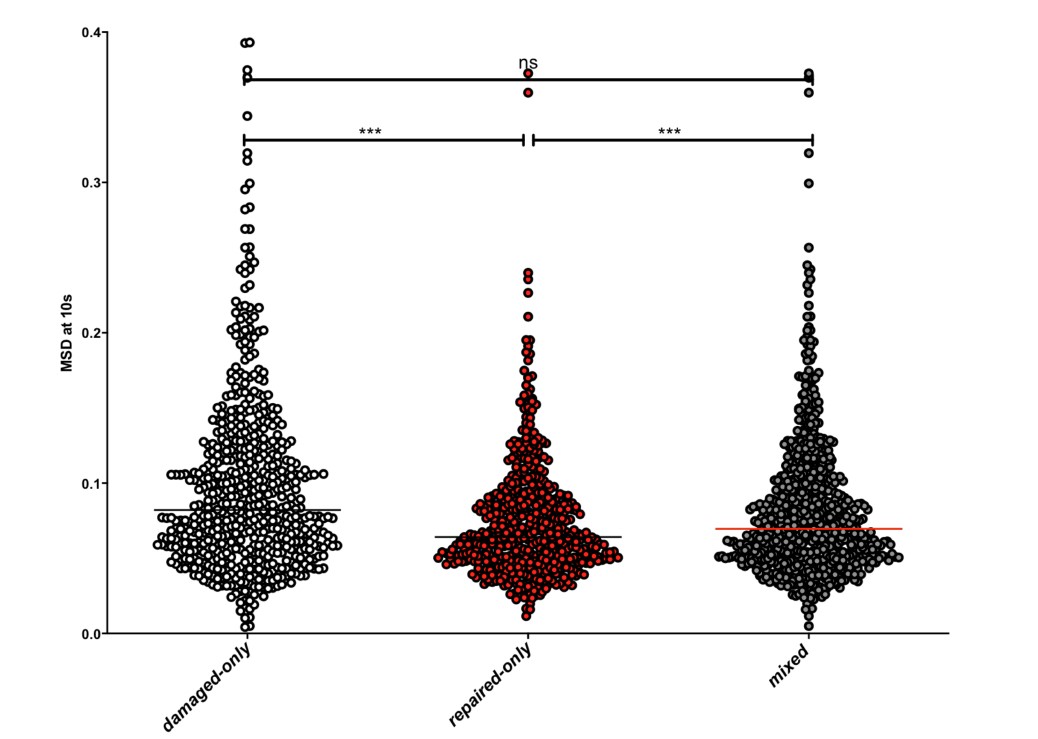
The second point concerns the important question of the heterogeneity of a population, a central challenge in biology. Here we wish to distinguish between undamaged and damaged cells. Even if a selection of the damaged cells had been made, this would not solve entirely the inherent cell to cell variation: at a given time, it is possible that a cell, although damaged, moves little and conversely that a cell moves more, even if not damaged. The question of heterogeneity is therefore important and the subject of intense research that goes beyond the framework of our work (Altschuler and Wu, 2010). However, in order to start to clarify if a bias could exist when considering a mixed population (20% undamaged and 80% damaged), we analyzed MSDs, using a scatter plot. We considered two population of cells where the damage is the best controlled, i.e. i) the red population which we know has been repaired and, importantly, has lost the cut site and will be not cut again (undamaged--‐only population) and ii) the white population, blocked in G2/M, because it is damaged and not repaired (damaged--‐only population). These two populations show very significant differences in their median MSDs. We artificially mixed the MSDs values obtained from these two populations at a rate of 20% of undamaged--‐only cells and 80% of damaged--‐only cells. We observed that the mean MSDs of the damaged--‐only and undamaged--‐only cells were significantly different. Yet, the mean MSD of damaged--‐only cells was not statistically different from the mean MSD from the 20%--‐80% mixed cell population. Thus, the conclusions based on the average MSDs of all cells remain consistent.
Scatter plot showing the MSD at 10 seconds of the damaged-‐only population (in white), the repaired-‐only population (in red), or the 20%-‐80% mixed population
- Perform the phospho-H2A ChIP-qPCR in the C and S strains in the absence of Rad51 and Rad9, to strengthen the painter model.
ChIP experiments in mutant backgrounds as well as phosphorylation/dephosphorylation kinetics would corroborate the mobility data described here, but are beyond the scope of this manuscript. Yet, a phospho--‐ H2A ChIP experiment was performed in a Δrad51 mutant in Renkawitz et al. 2013. In that case, γH2A propagation was restricted only to the region around the DSB, corroborating both the requirement for Rad51 in distal mobility and the lack of requirement for Rad51 in proximal mobility.
- Their data at least partly run against previously published results, or fail to account for them. For instance, it is hard to see how their model (or the painter model), could explain the constitutively activated global mobility increase observed by Smith .. Rothstein 2018 in a rad51 rad52 mutant. Furthermore, the gasser lab linked the increased chromatin mobility to a general loss of histones genome-wide, which would be inconsistent with the more localized mechanism proposed here. Do they represent an independent mechanism? These conflicting observations need to be discussed in detail.
Apart from the fact that the mechanisms in place in a haploid or a diploid cell are not necessarily comparable, it is not clear to us that our data are inconsistent with that of Smith et al. (Smith et al., 2018). Indeed, it is not known by which mechanisms the increase in global mobility is constitutively activated in a Δrad51 Δrad52 mutant. But according to their hypothesis the induction of a checkpoint is likely and so is the phosphorylation of H2A. It would be interesting to verify γH2A in such a context. This question is now mentioned in the main text.
Concerning histone loss, it appears to be different depending on the number of DSBs. Upon multiple DNA damage following genotoxic treatment with Zeocin, Susan Gasser's group has clearly established that nucleosome loss occurs (Cheblal et al., 2020; Hauer et al., 2017). Nucleosome loss, like H2A phosphorylation as we have shown (Garcia Fernandez et al., 2021; Herbert et al., 2017), leads to increased global mobility. The state of chromatin following these histone losses or modifications is not yet fully understood, but could coexist. In the case of a single DSB by HO, it is the local mobility of the MAT locus that is examined (Fig3B in (Cheblal et al., 2020). In this case, the increase in mobility is indeed dependent on Arp8 which controls histone degradation and correlates with a polymer pattern consistent with normal chromatin. It is likely that histone degradation occurs locally when a single DSB occurs. Concerning histone loss genome wide, the question remains open. If histone eviction nevertheless occurred globally upon a single DSB, both types of modifications could be possible. This aspect is now mentioned in the discussion.
-

Evaluation Summary:
This study is of relevance to the field of DNA repair. It uses a cleverly designed new recombination assay in yeast to address the impact of DNA break position on global genome mobility. A centromere-proximal DNA double-strand break (DSB) induces an H2A(X) phosphorylation-dependent global mobility that accelerates but is not essential for DSB repair, while a centromere-distal DSB triggers global mobility that is essential for repair and which depends on H2A(X) phosphorylation, Rad9 and Rad51. Together, these data support a model where global genome mobility promotes homologous recombination repair, particularly for centromere-distal DSBs, and help settle some recent controversy in the field.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive …
Evaluation Summary:
This study is of relevance to the field of DNA repair. It uses a cleverly designed new recombination assay in yeast to address the impact of DNA break position on global genome mobility. A centromere-proximal DNA double-strand break (DSB) induces an H2A(X) phosphorylation-dependent global mobility that accelerates but is not essential for DSB repair, while a centromere-distal DSB triggers global mobility that is essential for repair and which depends on H2A(X) phosphorylation, Rad9 and Rad51. Together, these data support a model where global genome mobility promotes homologous recombination repair, particularly for centromere-distal DSBs, and help settle some recent controversy in the field.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
The data support the claims, and the manuscript does not have significant weaknesses in its present form. Key strengths of the paper include using a creative HR-based reporter system combining different inducible DSB positions along a chromosome arm and testing plasmid-based and chromosomal donor sequences. Combining that system with the visualization of specific chromosomal sites via microscopy is powerful. Overall, this work will constitute a timely and helpful contribution to the field of DSB/genome mobility in DNA repair, especially in yeast, and may inform similar mechanisms in other organisms. Importantly, this study also reconciles some of the apparent contradictions in the field.
-

Reviewer #2 (Public Review):
The authors are clarifying the role of global mobility in homologous recombination (HR). Global mobility is positively correlated with recombinant product formation in some reports. However, some studies argue the contrary and report that global mobility is not essential for HR. To characterize the role of global chromatin mobility during HR, the authors set up a system in haploid yeast cells that allows simultaneously tracking of HR at the single-cell level and allows the analysis of different positions of the DSB induction. By moving the position of the DSB within their system, the authors postulate that the chromosomal conformation surrounding a DNA break affects the global mobility response. Finally, the authors assessed the contributions of H2A(X) phosphorylation, checkpoint progression and Rad51 in the …
Reviewer #2 (Public Review):
The authors are clarifying the role of global mobility in homologous recombination (HR). Global mobility is positively correlated with recombinant product formation in some reports. However, some studies argue the contrary and report that global mobility is not essential for HR. To characterize the role of global chromatin mobility during HR, the authors set up a system in haploid yeast cells that allows simultaneously tracking of HR at the single-cell level and allows the analysis of different positions of the DSB induction. By moving the position of the DSB within their system, the authors postulate that the chromosomal conformation surrounding a DNA break affects the global mobility response. Finally, the authors assessed the contributions of H2A(X) phosphorylation, checkpoint progression and Rad51 in the mobility response.
One of the strengths of the manuscript is the development of "THRIV" as an efficient method for tracking homologous recombination in vivo. The authors take advantage of the power of yeast genetics and use gene deletions and as well as mutations to test the contribution of H2A(X) phosphorylation, checkpoint progression and Rad51 to the mobility response in their THRIV system.
A major weakness in the manuscript is the lack of a marker to indicate that DSB formation has occurred (or is occurring)? Although at 6 hours there is 80% I-SceI cutting, around 20% of the cells are uncut and cannot be distinguished from the ones that are cut (or have already been repaired). Thus, the MSD analysis is done in the blind with respect to cells actually undergoing DSB repair.
The authors clearly outlined their aims and have substantial evidence to support their conclusions. They discovered new features of global mobility that may clear up some of the controversies in the field. They overinterpreted some of their observations, but these criticisms can be easily addressed.
The authors addressed conflicting results concerning the importance of global mobility to HR and their results aid in reconciling some of the controversies in the field. A key strength of this manuscript is the analysis of global mobility in response to breaks at different locations within chromosomes? They identified two types of DSB-induced global chromatin mobility involved in HR and postulate that they differ based on the position of the DSB. For example, DSBs close to the centromere exhibit increased global mobility that is not essential for repair and depends solely on H2A(X) phosphorylation. However, if the DSB is far away from the centromere, then global mobility is essential for HR and is dependent on H2A(X) phosphorylation, checkpoint progression as well as the Rad51 recombinase.
The Bloom lab had previously identified differences in mobility based on the position of the tracked site. However, in the study reported here, the mobility response is analyzed after inducing DSBs located at different positions along the chromosome.
They also addressed the question of the importance of the Rad51 protein in increased global mobility in haploid cells. Previous studies used DNA damaging agents that induce DSBs randomly throughout the genome, where it would have been rare to induce DSBs near the centromere. In the studies reported in this manuscript, they find no increase in global mobility in a rad51∆ background for breaks induced near the centromere (proximal), but find that breaks induced near the telomeres (distal), are dependent on both gamma-H2A(X) spreading and the Rad51 recombinase.
-

Reviewer #3 (Public Review):
In this study, Garcia Fernandez et al. employ a variety of genetic constructs to define the mechanism underlying the global chromatin mobility elicited in response to a single DNA double-strand break (DSB). Such local and global chromatin mobility increases have been described a decade ago by the Gasser and Rothstein laboratories, and a number of determinants have been identified: one epistasis group results in H2A-S129 phosphorylation via Rad9 and Mec1 activation. The mechanism is thought to be due to chromatin rigidification (Herbert 2017; Miné-Hattab 2017) or general eviction of histones (Cheblal 2020). More enigmatic, global chromatin mobility increase also depends on Rad51, a central recombination protein downstream of checkpoint activation (Smith & Rothstein 2017), which is also required for local DSB …
Reviewer #3 (Public Review):
In this study, Garcia Fernandez et al. employ a variety of genetic constructs to define the mechanism underlying the global chromatin mobility elicited in response to a single DNA double-strand break (DSB). Such local and global chromatin mobility increases have been described a decade ago by the Gasser and Rothstein laboratories, and a number of determinants have been identified: one epistasis group results in H2A-S129 phosphorylation via Rad9 and Mec1 activation. The mechanism is thought to be due to chromatin rigidification (Herbert 2017; Miné-Hattab 2017) or general eviction of histones (Cheblal 2020). More enigmatic, global chromatin mobility increase also depends on Rad51, a central recombination protein downstream of checkpoint activation (Smith & Rothstein 2017), which is also required for local DSB mobility (Dion .. Gasser 2012). The authors set out to address this difficulty in the field.
A premise of their study is the convergence of two types of observations: First, the H2A phosphorylation ChIP profile matches that of Rad51, with both spreading in trans on other chromosomes at the level of centromeres when a DSB occurs in the vicinity of one of them (Renkawitz 2014). Second, global mobility depends on H2A phosphorylation and on Rad51 (their previous study Herbert 2017). They thus address whether the Rad51-ssDNA filament (and associated proteins) marks the chromatin engaged during the homology search. They found that the extent of the mobility depends on the residency time of the filament in a particular genomic and nuclear region, which can be induced at an initially distant trans site by providing a region of homology. Unfortunately, these findings are not clearly apparent from the title and the abstract, and in fact somewhat misrepresented in the manuscript, which would call for a rewrite (see points below).
To this end, they induce the formation of a site-specific DSB in either of two regions: a centromere-proximal region and a telomere-proximal region, and measure the mobility of an undamaged site near the centromere on another chromosome (with a LacO-LacI-GFP system). This system reveals that only the centromere-proximal DSB induces the mobility of the centromere-proximal undamaged site, in a Rad9- and Rad51-independent manner. Providing a homologous donor in the vicinity of the LacO array (albeit in trans) restores its mobility when the DSB is located in a subtelomeric region, in a Rad9- and Rad51-dependent fashion. These genetic requirements are the same as those described for local DSB mobility (Dion & Gasser 2012), drawing a link between the two types of mobility, which to my knowledge was not described. The authors should focus their message (too scattered in the current manuscript), on these key findings and the diffusive "painting" model, in which the canvas is H2A, the moving paintbrush Mec1, and the hand the Rad51-ssDNA filament whose movement depends on Rad9. In the absence of Rad51-Rad9 the hand stays still, only decorating H2A in its immediate environment. The amount of paint deposited depends on the residency time of the Rad51-ssDNA-Mec1 filament in a given nuclear region. This synthesis is in agreement with the data presented and contrasts with their proposal that "two types of global mobility" exist.
The rest of the manuscript attempts to define a role in DSB repair of this phosphor-H2A-dependent mobility, using a fluorescence recovery assay upon DSB repair. They correlate a defect in the centromere-proximal mobility (in the rad9 or h2a-s129a mutant) when a DSB is distantly induced in the subtelomere with a defect in repairing the DSB. Repair efficiency is not affected by these mutations when the donor is located initially close to the DSB site. This part is less convincing, as repair failure specifically at a distant donor in the rad9 and H2A-S129A mutants may result from other defects relating to chromatin than its mobility (i.e. affecting homology sampling, DNA strand invasion, D-loop extension, D-loop disruption, etc), which could be partially alleviated by repeated DSB-donor encounters when the two are spatially close. In fact, suggesting that undamaged site mobility is required for the early step of the homology search directly contradicts the fact that the centromere-proximal mobility induced by a subtelomeric DSB depends on the presence of a donor near the centromere: mobility is thus a product of homology identification and increased Rad51-ssDNA filament residency in the vicinity of the centromere, and so downstream of homology search. This is a major pitfall in their interpretation and model.
In conclusion, I think the data presented are of importance, as they identify a link between local and global chromatin mobility. The authors should rewrite their manuscript and reorganize the figures to focus on the painter model that their data support. I propose experiments that will help bolster the manuscript conclusions.
1. Attempt dual-color tracking of the DSB (i.e. Rad52-mCherry or Ddc1-mCherry) and the donor site, and track MSD as a function of proximity between the DSB and the Lac array (with DSB +/-dCen). The expectation is that only upon contact (or after getting in close range) should the MSD at the centromere-proximal LacO array increase with a DSB at a subtelomere. Furthermore, this approach will help distinguish MSDs in cells bearing a DSB (Rad52 foci) from undamaged ones (no Rad52 foci)(see Mine-Hattab & Rothstein 2012). This would help overcome the inefficient DSB induction of their system (less than 50% at 1 hr post-galactose addition, and reaching 80% at 6 hr). For the reader to have a better appreciation of the data distribution, replace the whisker plots of MSD at 10 seconds with either scatter dot plot or violin plots, whichever conveys most clearly the distribution of the data: indeed, a bimodal distribution is expected in the current data, with undamaged cells having lower, and damaged cells having higher MSDs.
2. Perform the phospho-H2A ChIP-qPCR in the C and S strains in the absence of Rad51 and Rad9, to strengthen the painter model.
3. Their data at least partly run against previously published results, or fail to account for them. For instance, it is hard to see how their model (or the painter model), could explain the constitutively activated global mobility increase observed by Smith .. Rothstein 2018 in a rad51 rad52 mutant. Furthermore, the gasser lab linked the increased chromatin mobility to a general loss of histones genome-wide, which would be inconsistent with the more localized mechanism proposed here. Do they represent an independent mechanism? These conflicting observations need to be discussed in detail. -