Prolonged nicotine exposure reduces aversion to the drug in mice by altering nicotinic transmission in the interpeduncular nucleus
Curation statements for this article:-
Curated by eLife
eLife assessment
In the current study, Mondoloni and colleagues reveal how a selective nicotine receptor in the interpeduncular nucleus is involved in nicotine consumption, which is an important contribution to the understanding of individual differences in drug addiction. However, the preferred hypothesis would benefit from testing in additional experimental models, metabolic assessment, and cell-type specificity.
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Nicotine intake is likely to result from a balance between the rewarding and aversive properties of the drug, yet the individual differences in neural activity that control aversion to nicotine and their adaptation during the addiction process remain largely unknown. Using a two-bottle choice experiment, we observed considerable heterogeneity in nicotine-drinking profiles in isogenic adult male mice, with about half of the mice persisting in nicotine consumption even at high concentrations, whereas the other half stopped consuming. We found that nicotine intake was negatively correlated with nicotine-evoked currents in the interpeduncular nucleus (IPN), and that prolonged exposure to nicotine, by weakening this response, decreased aversion to the drug, and hence boosted consumption. Lastly, using knock-out mice and local gene re-expression, we identified β4-containing nicotinic acetylcholine receptors of IPN neurons as molecular and cellular correlates of nicotine aversion. Collectively, our results identify the IPN as a substrate for individual variabilities and adaptations in nicotine consumption.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
Nicotine preference is highly variable between individuals. The paper by Mondoloni et al. provided some insight into the potential link between IPN nAchR heterogeneity with male nicotine preference behavior. They scored mice using the amount of nicotine consumption, as well as the rats' preference of the drug using a two-bottle choice experiment. An interesting heterogeneity in nicotine-drinking profiles was observed in adult male mice, with about half of the mice ceasing nicotine consumption at high concentrations. They observed a negative association of nicotine intake with nicotine-evoked currents in the antiparticle nucleus (IPN). They also identified beta4-containing nicotine acetylcholine receptors, which exhibit an association with nicotine aversion. The behavioral differentiation …
Author Response
Reviewer #1 (Public Review):
Nicotine preference is highly variable between individuals. The paper by Mondoloni et al. provided some insight into the potential link between IPN nAchR heterogeneity with male nicotine preference behavior. They scored mice using the amount of nicotine consumption, as well as the rats' preference of the drug using a two-bottle choice experiment. An interesting heterogeneity in nicotine-drinking profiles was observed in adult male mice, with about half of the mice ceasing nicotine consumption at high concentrations. They observed a negative association of nicotine intake with nicotine-evoked currents in the antiparticle nucleus (IPN). They also identified beta4-containing nicotine acetylcholine receptors, which exhibit an association with nicotine aversion. The behavioral differentiation of av vs. n-avs and identification of IPN variability, both in behavioral and electrophysiological aspects, add an important candidate for analyzing individual behavior in addiction.
The native existence of beta4-nAchR heterogeneity is an important premise that supports the molecules to be the candidate substrate of variabilities. However, only knockout and re-expression models were used, which is insufficient to mimic the physiological state that leads to variability in nicotine preference.
We’d like to thank reviewer 1 for his/her positive remarks and for suggesting important control experiments. Regarding the reviewer’s latest comment on the link between b4 and variability, we would like to point out that the experiment in which mice were put under chronic nicotine can be seen as another way to manipulate the physiological state of the animal. Indeed, we found that chronic nicotine downregulates b4 nAChR expression levels (but has no effect on residual nAChR currents in b4-/- mice) and reduces nicotine aversion. Therefore, these results also point toward a role of IPN b4 nAChRs in nicotine aversion. We have now performed additional experiments and analyses to address these concerns and to reinforce our demonstration.
Reviewer #2 (Public Review):
In the current study, Mondoloni and colleagues investigate the neural correlates contributing to nicotine aversion and its alteration following chronic nicotine exposure. The question asked is important to the field of individual vulnerability to drug addiction and has translational significance. First, the authors identify individual nicotine consumption profiles across isogenic mice. Further, they employed in vivo and ex vivo physiological approaches to defining how antiparticle nuclei (IPn) neuronal response to nicotine is associated with nicotine avoidance. Additionally, the authors determine that chronic nicotine exposure impairs IPn neuronal normal response to nicotine, thus contributing to higher amounts of nicotine consumption. Finally, they used transgenic and viralmediated gene expression approaches to establish a causal link between b4 nicotine receptor function and nicotine avoidance processes.
The manuscript and experimental strategy are well designed and executed; the current dataset requires supplemental analyses and details to exclude possible alternatives. Overall, the results are exciting and provide helpful information to the field of drug addiction research, individual vulnerability to drug addiction, and neuronal physiology. Below are some comments aiming to help the authors improve this interesting study.
We would like to thank the reviewer for his/her positive remarks and we hope the new version of the manuscript will clarify his/her concerns.
- The authors used a two-bottle choice behavioral paradigm to investigate the neurophysiological substrate contributing to nicotine avoidance behaviors. While the data set supporting the author's interpretation is compelling and the experiments are well-conducted, a few supplemental control analyses will strengthen the current manuscript.
a) The bitter taste of nicotine might generate confounds in the data interpretation: are the mice avoiding the bitterness or the nicotine-induced physiological effect? To address this question, the authors mixed nicotine with saccharine, thus covering the bitterness of nicotine. Additionally, the authors show that all the mice exposed to quinine avoid it, and in comparison, the N-Av don't avoid the bitterness of the nicotine-saccharine solution. Yet it is unclear if Av and N-Av have different taste discrimination capacities and if such taste discrimination capacities drive the N-Av to consume less nicotine. Would Av and N-Av mice avoid quinine differently after the 20-day nicotine paradigm? Would the authors observe individual nicotine drinking behaviors if nicotine/quinine vs. quinine were offered to the mice?
As requested by all three reviewers, we have now performed a two-bottle choice experiment to verify whether different sensitivities to the bitterness of the nicotine solution could explain the different sensitivities to the aversive properties of nicotine. Indeed, even though we used saccharine to mask the bitterness of the nicotine solution, we cannot fully exclude the possibility that the taste capacity of the mice could affect their nicotine consumption. Reviewers 1 and 2 suggested to perform nicotine/quinine versus quinine preference tests, but we were afraid that forcing mice to drink an aversive, quinine-containing solution might affect the total volume of liquid consumed per day, and also might create a “generalized conditioned aversion to drinking water - detrimental to overall health and a confounding factor” as pointed out by reviewer 3. Therefore, we designed the experiment a little differently.
In this two-bottle choice experiment, mice were first proposed a high concentration of nicotine (100 µg/ml) which has previously been shown to induce avoidance behavior in mice (Figure 3C). Then, mice were offered three increasing concentrations of quinine: 30, 100 and 300 µM. Quinine avoidance was dose dependent, as expected: it was moderate for 30 µM but almost absolute for 300 µM quinine. We then investigated whether nicotine and quinine avoidances were linked. We found no correlation between nicotine and quinine preference (new Figure: Figure 1- supplementary figure 1D). This new experiment strongly suggests that aversion to the drug is not directly tied to the sensitivity of mice to the bitter taste of nicotine.
Other results reinforce this conclusion. First, none of the b4-/- mice (0/13) showed aversion to nicotine, whereas about half of the virally-rescued animals (8/17, b4 re-expressed in the IPN of b4-/- mice) showed nicotine aversion, a proportion similar to the one observed in WT mice. This experiment makes a clear, direct link between the expression of b4 nAChRs in the IPN and aversion to the drug.
Furthermore, we also verified that the sensitivity of b4-/- mice to bitterness is not different from that of WT mice (new Figure 4 – figure supplement 1B). This new result indicates that the reason why b4-/- mice consume more nicotine than WT mice is not because they have a reduced sensitivity bitterness.
Together, these new experiments strongly suggests that interindividual differences in sensitivity to the bitterness of nicotine play little role in nicotine consumption behavior in mice.
b) Metabolic variabilities amongst isogenic mice have been observed. Thus, while the mice consume different amounts of nicotine, changes in metabolic processes, thus blood nicotine concentrations, could explain differences in nicotine consumption and neurophysiology across individuals. The authors should control if the blood concentration of nicotine metabolites between N-Av and Av are similar when consuming identical amounts of nicotine (50ug/ml), different amounts (200ug/ml), and in response to an acute injection of a fixed nicotine quantity.
We agree with the reviewer that metabolic variabilities could explain (at least in part) the differences observed between avoiders and non-avoiders. But other factors could also play a role, such as stress level (there is a strong interaction between stress and nicotine addiction, as shown by our group (PMID: 29155800, PMID: 30361503) and others), hierarchical ranking, epigenetic factors etc… Our goal in this study is not to examine all possible sources of variability. What is striking about our results is that deletion of a single gene (encoding the nAChR b4 subunit) is sufficient to eliminate nicotine avoidance, and that re-expression of this receptor subunit in the IPN is sufficient to restore nicotine avoidance. In addition, we observe a strong correlation between the amplitude of nicotineinduced current in the IPN, and nicotine consumption. Therefore, the expression level of b4 in the IPN is sufficient to explain most of the behavioral variability we observe. We do not feel the need to explore variations in metabolic activities, which are (by the way) very expensive experiments. However, we have added a sentence in the discussion to mention metabolic variabilities as a potential source of variability in nicotine consumption.
- Av mice exposed to nicotine_200ug/ml display minimal nicotine_50ug/ml consumption, yet would Av mice restore a percent nicotine consumption >20 when exposed to a more extended session at 50ug/kg? Such a data set will help identify and isolate learned avoidance processes from dose-dependent avoidance behaviors.
We have now performed an additional two-bottle choice experiment to examine an extended time at 50 µg/ml. But we also performed the experiment a little differently. We directly proposed a high nicotine concentration to mice (200 µg/ml), followed by 8 days at 50 µg/ml. We found that, overall, mice avoided the 200 µg/ml nicotine solution, and that the following increase in nicotine preference was slow and gradual throughout the eight days at 50 µg/ml (Figure 2-figure supplement 1C). This slow adjustment to a lower-dose contrasts with the rapid (within a day) change in intake observed when nicotine concentration increases (Figure 1-figure supplement 1A). About half of the mice (6/13) retained a steady, low nicotine preference (< 20%) throughout the eight days at 50 µg/ml, resembling what was observed for avoiders in Figure 2D. Together, these results suggest that some of the mice, the non-avoiders, rapidly adjust their intake to adapt to changes in nicotine concentration in the bottle. For avoiders, aversion for nicotine seems to involve a learning mechanism that, once triggered, results in prolonged cessation of nicotine consumption.
- The author should further investigate the basal properties of IPn neuron in vivo firing rate activity recorded and establish if their spontaneous activity determines their nicotine responses in vivo, such as firing rate, ISI, tonic, or phasic patterns. These analyses will provide helpful information to the neurophysiologist investigating the function of IPn neurons and will also inform how chronic nicotine exposure shapes the IPn neurophysiological properties.
We have performed additional analyses of the in vivo recordings. First, we have built maps of the recorded neurons, and we show that there is no anatomical bias in our sampling between the different groups. The only condition for which we did not sample neurons similarly is when we compare the responses to nicotine in vivo in WT and b4-/- mice (Figure 4E). The two groups were not distributed similarly along the dorso-ventral axis (Figure 4-figure supplement 2B). Yet, we do not think that the difference in nicotine responses observed between WT and b4-/- mice is due to a sampling bias. Indeed, we found no link between the response to nicotine and the dorsoventral coordinates of the neurons, in any of the groups (MPNic and MP Sal in Figure 3-figure supplement 1D; WT and b4-/- mice in Figure 4-figure supplement 2C). Therefore, our different groups are directly comparable, and the conclusions drawn in our study fully justified.
As requested, we have looked at whether the basal firing rate of IPN neurons determines the response to nicotine and indeed, neurons with higher firing rate show greater change in firing frequency upon nicotine injection (Figure 3 -figure supplement 1G and Figure 4-figure supplement 2F). We have also looked at the effect of chronic nicotine on the spontaneous firing rate of IPN neurons (Figure 3 -figure supplement 1F) but found no evidence for a change in basal firing properties. Similarly, the deletion of b4 had no effect on the spontaneous activity of the recorded neurons (Figure 4-figure supplement 2F). Finally, we found no evidence for any link between the anatomical coordinates of the neurons and their basal firing rate (Figure 3-figure supplement 1E and Figure 4figure supplement 2D).
Reviewer #3 (Public Review):
The manuscript by Mondoloni et al characterizes two-bottle choice oral nicotine consumption and associated neurobiological phenotypes in the antiparticle nucleus (IPN) using mice. The paper shows that mice exhibit differential oral nicotine consumption and correlate this difference with nicotine-evoked inward currents in neurons of the IPN. The beta4 nAChR subunit is likely involved in these responses. The paper suggests that prolonged exposure to nicotine results in reduced nAChR functional responses in IPN neurons. Many of these results or phenotypes are reversed or reduced in mice that are null for the beta4 subunit. These results are interesting and will add a contribution to the literature. However, there are several major concerns with the nicotine exposure model and a few other items that should be addressed.
Strengths:
Technical approaches are well-done. Oral nicotine, electrophysiology, and viral re-expression methods were strong and executed well. The scholarship is strong and the paper is generally well-written. The figures are high-quality.
We would like to thank the reviewer for his/her comments and suggestions on how to improve the manuscript.
Weaknesses:
Two bottle choice (2BC) model. 2BC does not examine nicotine reinforcement, which is best shown as a volitional preference for the drug over the vehicle. Mice in this 2BC assay (and all such assays) only ever show indifference to nicotine at best - not preference. This is seen in the maximal 50% preference for the nicotine-containing bottle. 2BC assays using tastants such as saccharin are confounded. Taste responses can very likely differ from primary reinforcement and can be related to peripheral biology in the mouth/tongue rather than in the brain reward pathway.
The two-bottle nicotine drinking test is a commonly used method to study addiction in mice (Matta, S. G. et al. 2006. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190, 269–319). Like all methods, it has its limitations, but it also allows for different aspects to be addressed than those covered by selfadministration protocols. The two-bottle nicotine drinking test simply measures the animals' preference for a solution containing nicotine over a control solution without nicotine: the animals are free to choose nicotine or not, which allows to evaluate sensitivity and avoidance thresholds. What we show in this paper is precisely that despite interindividual differences in the way the drug is used (passively or actively), a significant proportion of the animals avoids the nicotine bottle at a certain concentration, suggesting that we are dealing with individual characteristics that are interesting to identify in the context of addiction and vulnerability. We agree that the twobottle choice test cannot provide as much information about the reinforcing effects of the drug as selfadministration procedures. We are aware of the limitations of the method and were careful not to interpret our data in terms of reinforcement to the drug. For instance, mice that consume nicotine were called “non-avoiders” and not “consumers”. We added a few sentences at the beginning of the discussion to highlight these limitations.
The reviewer states that the mice in this 2BC assay (and all such assays) “only ever show indifference to nicotine at best - not preference”. This is seen in the maximal 50% preference for the nicotine-containing bottle. While this is true on average, it isn’t when we look at individual profiles, as we did here. We clearly observed that some mice have a strong preference for nicotine and, conversely, that some mice actively avoid nicotine after a certain concentration is proposed in the bottle.
Regarding tastants, we indeed used saccharine to hide the bitter taste of nicotine and prevent taste-related side bias. This is a classical (though not perfect) paradigm in the field of nicotine research (Matta, S. G. et al. 2006. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190, 269–319). To evaluate whether different sensitivities to the bitterness of nicotine may explain the interindividual differences in nicotine consumption we performed new experiments (as suggested by all three reviewers). In this two-bottle choice experiment, mice were first proposed a high concentration of nicotine (100 µg/ml) which has previously been shown to induce avoidance behavior in mice (Figure 3C). Then, mice were offered three increasing concentrations of quinine: 30, 100 and 300 µM. Quinine avoidance was dose dependent, as expected: it was moderate for 30 µM but almost absolute for 300 µM quinine. We then investigated whether nicotine and quinine avoidances were linked. We found no correlation between nicotine and quinine preference (new Figure: Figure 1- supplementary figure 1D). This new experiment strongly suggests that aversion to the drug is not directly tied to the sensitivity of mice to the bitter taste of nicotine. Other results reinforce this conclusion. First, none of the b4-/- mice (0/13) showed aversion to nicotine, whereas about half of the virally-rescued animals (8/17, b4 re-expressed in the IPN of b4-/- mice) showed nicotine aversion, a proportion similar to the one observed in WT mice. This experiment makes a clear, direct link between the expression of b4 nAChRs in the IPN and aversion to the drug. Furthermore, we also verified that the sensitivity of b4-/- mice to bitterness is not different from that of WT mice (new Figure 4 - figure supplement 1B). This new result indicates that the reason why b4-/- mice consume more nicotine than WT mice is not because they have a reduced sensitivity bitterness. Together, these new experiments strongly suggests that interindividual differences in sensitivity to the bitterness of nicotine play little role in nicotine consumption behavior in mice.
Moreover, this assay does not test free choice, as nicotine is mixed with water which the mice require to survive. Since most concentrations of nicotine are aversive, this may create a generalized conditioned aversion to drinking water - detrimental to overall health and a confounding factor.
Mice are given a choice between two bottles, only one of which contains nicotine. Hence, even though their choices are not fully free (they are being presented with a limited set of options), mice can always decide to avoid nicotine and drink from the bottle containing water only. We do not understand how this situation may create a generalized aversion to drinking. In fact, we have never observed any mouse losing weight or with deteriorated health condition in this test, so we don’t think it is a confounding factor.
What plasma concentrations of nicotine are achieved by 2BC? When nicotine is truly reinforcing, rodents and humans titrate their plasma concentrations up to 30-50 ng/mL. The Discussion states that oral self-administration in mice mimics administration in human smokers (lines 388-389). This is unjustified and should be removed. Similarly, the paragraph in lines 409-423 is quite speculative and difficult or impossible to test. This paragraph should be removed or substantially changed to avoid speculation. Overall, the 2BC model has substantial weaknesses, and/or it is limited in the conclusions it will support.
The reviewer must have read another version of our article, because these sentences and paragraphs are not present in our manuscript.
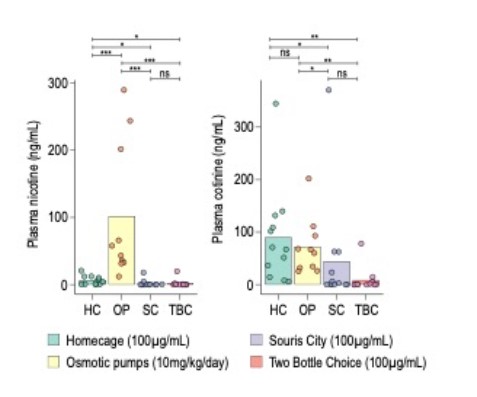
Regarding the actual concentration of nicotine in the plasma, this is indeed a good question. We have actually measured the plasma concentrations of nicotine for another study (article in preparation). The results from this experiment can be found below. The half-life of nicotine is very short in the blood and brain of mice (about 6 mins, see Matta, S. G. et al. 2006. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190, 269–319), making it very hard to assess. Therefore, we also assessed the plasma concentration of cotinine, the main metabolite of nicotine. We compared 4 different conditions: home-cage (forced drinking of 100 ug/ml nicotine solution); osmotic minipump (OP, 10 mg/kg/d, as in our current study); Souris-city (a large social environment developed by our group, see Torquet et al. Nat. Comm. 2018); and the two-bottle choice procedure (when a solution of nicotine 100 ug/ml was proposed). The concentrations of plasma nicotine found were very low for all groups that drank nicotine, but not for the group that received nicotine through the osmotic minipump group. This is most likely because mice did not drink any nicotine in the hour prior to being sampled and all nicotine was metabolized. Indeed, when we look at the plasma concentration of cotinine, we see that cotinine was present in all of the groups. The plasma concentration of cotinine was similar in the groups for which “consumption” was forced: forced drinking in the home cage (HC) or infusion through osmotic minipump. This indicates that the plasma concentration of cotinine is similar whether mice drink nicotine (100 ug/ml) or whether nicotine is infused with the minipump (10 mg/kg/d). For Souris city and the two-bottle choice procedure, the cotinine concentrations were in the same range (mostly between 0-100 ng/ml). Globally, the concentrations of nicotine and cotinine found in the plasma of mice that underwent the two-bottle choice procedure are in the range of what has been previously described (Matta, S. G. et al. 2006. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190, 269–319).
Regarding the limitations of the two-bottle choice test, we discuss them more extensively in the current version of the manuscript.
Statistical testing on subgroups. Mice are run through an assay and assigned to subgroups based on being classified as avoiders or non-avoiders. The authors then perform statistical testing to show differences between the avoiders and non-avoiders. It is circular to do so. When the authors divided the mice into avoiders and non-avoiders, this implies that the mice are different or from different distributions in terms of nicotine intake. Conducting a statistical test within the null hypothesis framework, however, implies that the null hypothesis is being tested. The null hypothesis, by definition, is that the groups do NOT differ. Obviously, the authors will find a difference between the groups in a statistical test when they pre-sorted the mice into two groups, to begin with. Comparing effect sizes or some other comparison that does not invoke the null hypothesis would be appropriate.
Our analysis, which can be summarized as follows, is fairly standard (see Krishnan, V. et al. (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404). Firstly, the mice are segregated into two groups based on their consumption profile, using the variability in their behavior. The two groups are obviously statistically different when comparing their consumption. This first analytical step allows us to highlight the variability and to establish the properties of each sub-population in terms of consumption. Our analysis could support the reviewer's comment if it ended at this point. However, our analysis doesn't end here and moves on to the second step. The separation of the mice into two groups (which is now a categorical variable) is used to compare the distribution of other variables, such as mouse choice strategy and current amplitude, based on the 2 categories. The null hypothesis tested is that the value of these other variables is not different between groups. There is no a priori obvious reason for the currents recorded in the IPN to be different in the two groups. These approaches allow us to show correlations between the variables. Finally, in the third and last step, one (or several) variable(s) are manipulated to check whether nicotine consumption is modified accordingly. Manipulation was performed by exposing mice to chronic nicotine, by using mutant mice with decreased nicotinic currents, and by re-expressing the deleted nAChR subunit only in the IPN. This procedure is fairly standard, and cannot be considered as a circular analysis with data selection problem, as explained in (Kriegeskorte, N., Simmons, W. K., Bellgowan, P. S. F. & Baker, C. I. (2009) Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience 12, 535-540).
Decreased nicotine-evoked currents following passive exposure to nicotine in minipumps are inconsistent with published results showing that similar nicotine exposure enhances nAChR function via several measures (Arvin et al, J Neurosci, 2019). The paper does acknowledge this previous paper and suggests that the discrepancy is explained by the fact that they used a higher concentration of nicotine (30 uM) that was able to recruit the beta4containing receptor (whereas Arvin et al used a caged nicotine that was unable to do so). This may be true, but the citation of 30 uM nicotine undercuts the argument a bit because 30 uM nicotine is unlikely to be achieved in the brain of a person using tobacco products; nicotine levels in smokers are 100-500 nM. It should be noted in the paper that it is unclear whether the down-regulated receptors would be active at concentrations of nicotine found in the brain of a smoker.
We indeed find opposite results compared to Arvin et al., and we give possible explanations for this discrepancy in the discussion. To be honest we don’t fully understand why we have opposite results. However, we clearly observed a decreased response to nicotine, both in vitro (with 30 µM nicotine on brain slices) and in vivo (with a classical dose of 30 µg/kg nicotine i.v.), while Arvin et al. only tested nicotine in vitro.
Regarding the reviewer’s comment about the nicotine concentration used (30 µM): we used that concentration in vitro to measure nicotine-induced currents (it’s a concentration close to the EC50 for heteromeric receptors, which will likely recruit low affinity a3b4 receptors) and to evaluate the changes in nAChR current following nicotine exposure. We did not use that concentration to induce nAChR desensitization, so we don’t really understand the argument regarding the levels of nicotine in smokers. For inducing desensitization, we used a minipump that delivers a daily dose of 10 mg/kg/day, which is the amount of nicotine mice drink in our assay.
The statement in lines 440-41 ("we show that concentrations of nicotine as low as 7.5 ug/kg can engage the IPN circuitry") is misleading, as the concentration in the water is not the same as the concentration in the CSF since the latter would be expected to build up over time. The paper did not provide measurements of nicotine in plasma or CSF, so concluding that the water concentration of nicotine is related to plasma concentrations of nicotine is only speculative.
The sentence “we show that concentrations of nicotine as low as 7.5 ug/kg can engage the IPN circuitry" is not in the manuscript so the reviewer must have read another version of the paper.
The results in Figure 2E do not appear to be from a normal distribution. For example, results cluster at low (~100 pA) responses, and a fraction of larger responses drive the similarities or differences.
Indeed, that is why we performed a non-parametric Mann-Whitney test for comparing the two groups, as indicated in the legend of figure 2E.
10 mg/kg/day in mice or rats is likely a non-physiological exposure to nicotine. Most rats take in 1.0 to 1.5 mg/kg over a 23-hour self-administration period (O'Dell, 2007). Mice achieve similar levels during SA (Fowler, Neuropharmacology 2011). Forced exposure to 10 mg/kg/day is therefore 5 to 10-fold higher than rodents would ever expose themselves to if given the choice. This should be acknowledged in a limitations section of the Discussion.
The two-bottle choice task is very different from nicotine self-administration procedures in terms of administration route: oral versus injected (in the blood or in the brain), respectively. Therefore, the quantities of drug consumed cannot be directly compared. In our manuscript, mice consume on average 10 mg/kg/day of nicotine at the highest nicotine concentration tested, which is fully consistent with what was already published in many studies (20 mg/kg/day in Frahm et al. Neuron 2013, 5-10 mg/kg/day in Bagdas et al., NP 2020, 10-20 mg/kg/day in Bagdas et al. NP2019, to cite a few...). Hence, we used that concentration of nicotine (10 mg/kg/d) for chronic administration of nicotine using minipumps. This is also a nicotine concentration that is classically used in osmotic minipumps for chronic administration of nicotine: 10 mg/kg/d in Dongelmans et al. Nat. Com 2021 (our lab), 12 mg/kg/d in Arvin et al. J. Neuro. 2019 (Drenan lab), 12 mg/kg/d in Lotfipour et al. J. Neuro. 2013 (Boulter lab) etc… Therefore, we do not see the issue here.
Are the in vivo recordings in IPN enriched or specific for cells that have a spontaneous firing at rest? If so, this may or may not be the same set/type of cells that are recorded in patch experiments. The results could be biased toward a subset of neurons with spontaneous firing. There are MANY different types of neurons in IPN that are largely intermingled (see Ables et al, 2017 PNAS), so this is a potential problem.
It is true that there are many types of neurons in the IPN. In-vivo electrophysiology and slice electrophysiology should be considered as two complementary methods to obtain detailed properties of IPN neurons. The populations sampled by these two methods are certainly not identical (IPR in patch -clamp versus mostly IPR and IPC in vivo), and indeed only spontaneously active neurons are recorded in in-vivo electrophysiology. The question is whether this is or not a potential problem. The results we obtained using in-vivo and brain-slice electrophysiology are consistent (i.e., a decreased response to nicotine), which indicates that our results are robust and do not depend on the selection of a particular subpopulation. In addition, we now provide the maps of the neurons recorded both in slices and in vivo (see supplementary figures, and response to the other two referees). We show that, overall, there is no bias sampling between the different groups. Together, these new analyses strongly suggest that the differences we observe between the groups are not due to sampling issues. We have added the Ables 2017 reference and are discussing neuron variability more extensively in the revised manuscript.
Related to the above issue, which of the many different IPN neuron types did the group re-express beta4? Could that be controlled or did beta4 get re-expressed in an unknown set of neurons in IPN? There is insufficient information given in the methods for verification of stereotaxic injections.
Re-expression of b4 was achieved with a strong, ubiquitous promoter (pGK), hence all cell types should in principle be transduced. This is now clearly stated in the result section, the figure legend and the method section. Unfortunately, we had no access to a specific mouse line to restrict expression of b4 to b4-expressing cells, since the b4-Cre line of GENSAT is no more alive. This mouse line was problematic anyways because expression levels of the a3, a5 and b4 nAChR subunits, which belong to the same gene cluster, were reported to be affected. Yet, we show in this article that deleting b4 leads to a strong reduction of nicotine-induced currents in the IPR (80%, patch-clamp), and of the response to nicotine in vivo (65%). These results indicate that b4 is strongly expressed in the IPN, likely in a large majority of IPR and IPC neurons (see also our response to reviewer 1). In addition, we show that our re-expression strategy restores nicotine-induced currents in patch-clamp experiments and also the response to nicotine in vivo (new Figure 5C). Non-native expression levels could potentially be achieved (e.g. overexpression) but this is not what we observed: responses to nicotine were restored to the WT levels (in slices and in vivo). And importantly this strategy rescued the WT phenotype in terms of nicotine consumption. Expression of b4 alone in cells that do not express any other nAChR subunit (as, presumably, in the lateral parts of the IPN, see GENSAT images above) should not produce any functional nAChR, since alpha subunits are mandatory to produce functional receptors. As specified in the manuscript, proper transduction of the IPN was verified using post-hoc immunochemistry, and mice with transduction of b4 in the VTA were excluded from the analyses.
Data showing that alpha3 or beta4 disruption alters MHb/IPN nAChR function and nicotine 2BC intake is not novel. In fact, some of the same authors were involved in a paper in 2011 (Frahm et al., Neuron) showing that enhanced alpha3beta4 nAChR function was associated with reduced nicotine consumption. The present paper would therefore seem to somewhat contradict prior findings from members of the research group.
Frahm et al used a transgenic mouse line (called TABAC) in which the expression of a3b4 receptor is increased, and they observed reduced nicotine consumption. We do the exact opposite: we reduce (a3)b4 receptor expression (using the b4 knock-out line, or by putting mice under chronic nicotine), and observe increased consumption. There is thus no contradiction. In fact, we discuss our findings in the light of Frahm et al. in the discussion section.
Sex differences. All studies were conducted in male mice, therefore nothing was reported regarding female nicotine intake or physiology responses. Nicotine-related biology often shows sex differences, and there should be a justification provided regarding the lack of data in females. A limitations section in the Discussion section is a good place for this.
We agree with the reviewer. We added a sentence in the discussion.
-

eLife assessment
In the current study, Mondoloni and colleagues reveal how a selective nicotine receptor in the interpeduncular nucleus is involved in nicotine consumption, which is an important contribution to the understanding of individual differences in drug addiction. However, the preferred hypothesis would benefit from testing in additional experimental models, metabolic assessment, and cell-type specificity.
-

Reviewer #1 (Public Review):
Nicotine preference is highly variable between individuals. The paper by Mondoloni et al. provided some insight into the potential link between IPN nAchR heterogeneity with male nicotine preference behavior. They scored mice using the amount of nicotine consumption, as well as the rats' preference of the drug using a two-bottle choice experiment. An interesting heterogeneity in nicotine-drinking profiles was observed in adult male mice, with about half of the mice ceasing nicotine consumption at high concentrations. They observed a negative association of nicotine intake with nicotine-evoked currents in the antiparticle nucleus (IPN). They also identified beta4-containing nicotine acetylcholine receptors, which exhibit an association with nicotine aversion. The behavioral differentiation of av vs. n-avs and …
Reviewer #1 (Public Review):
Nicotine preference is highly variable between individuals. The paper by Mondoloni et al. provided some insight into the potential link between IPN nAchR heterogeneity with male nicotine preference behavior. They scored mice using the amount of nicotine consumption, as well as the rats' preference of the drug using a two-bottle choice experiment. An interesting heterogeneity in nicotine-drinking profiles was observed in adult male mice, with about half of the mice ceasing nicotine consumption at high concentrations. They observed a negative association of nicotine intake with nicotine-evoked currents in the antiparticle nucleus (IPN). They also identified beta4-containing nicotine acetylcholine receptors, which exhibit an association with nicotine aversion. The behavioral differentiation of av vs. n-avs and identification of IPN variability, both in behavioral and electrophysiological aspects, add an important candidate for analyzing individual behavior in addiction.
The native existence of beta4-nAchR heterogeneity is an important premise that supports the molecules to be the candidate substrate of variabilities. However, only knockout and re-expression models were used, which is insufficient to mimic the physiological state that leads to variability in nicotine preference.
-

Reviewer #2 (Public Review):
In the current study, Mondoloni and colleagues investigate the neural correlates contributing to nicotine aversion and its alteration following chronic nicotine exposure. The question asked is important to the field of individual vulnerability to drug addiction and has translational significance. First, the authors identify individual nicotine consumption profiles across isogenic mice. Further, they employed in vivo and ex vivo physiological approaches to defining how antiparticle nuclei (IPn) neuronal response to nicotine is associated with nicotine avoidance. Additionally, the authors determine that chronic nicotine exposure impairs IPn neuronal normal response to nicotine, thus contributing to higher amounts of nicotine consumption. Finally, they used transgenic and viral-mediated gene expression …
Reviewer #2 (Public Review):
In the current study, Mondoloni and colleagues investigate the neural correlates contributing to nicotine aversion and its alteration following chronic nicotine exposure. The question asked is important to the field of individual vulnerability to drug addiction and has translational significance. First, the authors identify individual nicotine consumption profiles across isogenic mice. Further, they employed in vivo and ex vivo physiological approaches to defining how antiparticle nuclei (IPn) neuronal response to nicotine is associated with nicotine avoidance. Additionally, the authors determine that chronic nicotine exposure impairs IPn neuronal normal response to nicotine, thus contributing to higher amounts of nicotine consumption. Finally, they used transgenic and viral-mediated gene expression approaches to establish a causal link between b4 nicotine receptor function and nicotine avoidance processes.
The manuscript and experimental strategy are well designed and executed; the current dataset requires supplemental analyses and details to exclude possible alternatives. Overall, the results are exciting and provide helpful information to the field of drug addiction research, individual vulnerability to drug addiction, and neuronal physiology. Below are some comments aiming to help the authors improve this interesting study.
1. The authors used a two-bottle choice behavioral paradigm to investigate the neurophysiological substrate contributing to nicotine avoidance behaviors. While the data set supporting the author's interpretation is compelling and the experiments are well-conducted, a few supplemental control analyses will strengthen the current manuscript.
a. The bitter taste of nicotine might generate confounds in the data interpretation: are the mice avoiding the bitterness or the nicotine-induced physiological effect? To address this question, the authors mixed nicotine with saccharine, thus covering the bitterness of nicotine. Additionally, the authors show that all the mice exposed to quinine avoid it, and in comparison, the N-Av don't avoid the bitterness of the nicotine-saccharine solution. Yet it is unclear if Av and N-Av have different taste discrimination capacities and if such taste discrimination capacities drive the N-Av to consume less nicotine. Would Av and N-Av mice avoid quinine differently after the 20-day nicotine paradigm? Would the authors observe individual nicotine drinking behaviors if nicotine/quinine vs. quinine were offered to the mice?
b. Metabolic variabilities amongst isogenic mice have been observed. Thus, while the mice consume different amounts of nicotine, changes in metabolic processes, thus blood nicotine concentrations, could explain differences in nicotine consumption and neurophysiology across individuals. The authors should control if the blood concentration of nicotine metabolites between N-Av and Av are similar when consuming identical amounts of nicotine (50ug/ml), different amounts (200ug/ml), and in response to an acute injection of a fixed nicotine quantity.2. Av mice exposed to nicotine_200ug/ml display minimal nicotine_50ug/ml consumption, yet would Av mice restore a percent nicotine consumption >20 when exposed to a more extended session at 50ug/kg? Such a data set will help identify and isolate learned avoidance processes from dose-dependent avoidance behaviors.
3. The author should further investigate the basal properties of IPn neuron in vivo firing rate activity recorded and establish if their spontaneous activity determines their nicotine responses in vivo, such as firing rate, ISI, tonic, or phasic patterns. These analyses will provide helpful information to the neurophysiologist investigating the function of IPn neurons and will also inform how chronic nicotine exposure shapes the IPn neurophysiological properties.
-

Reviewer #3 (Public Review):
The manuscript by Mondoloni et al characterizes two-bottle choice oral nicotine consumption and associated neurobiological phenotypes in the antiparticle nucleus (IPN) using mice. The paper shows that mice exhibit differential oral nicotine consumption and correlate this difference with nicotine-evoked inward currents in neurons of the IPN. The beta4 nAChR subunit is likely involved in these responses. The paper suggests that prolonged exposure to nicotine results in reduced nAChR functional responses in IPN neurons. Many of these results or phenotypes are reversed or reduced in mice that are null for the beta4 subunit. These results are interesting and will add a contribution to the literature. However, there are several major concerns with the nicotine exposure model and a few other items that should be …
Reviewer #3 (Public Review):
The manuscript by Mondoloni et al characterizes two-bottle choice oral nicotine consumption and associated neurobiological phenotypes in the antiparticle nucleus (IPN) using mice. The paper shows that mice exhibit differential oral nicotine consumption and correlate this difference with nicotine-evoked inward currents in neurons of the IPN. The beta4 nAChR subunit is likely involved in these responses. The paper suggests that prolonged exposure to nicotine results in reduced nAChR functional responses in IPN neurons. Many of these results or phenotypes are reversed or reduced in mice that are null for the beta4 subunit. These results are interesting and will add a contribution to the literature. However, there are several major concerns with the nicotine exposure model and a few other items that should be addressed.
Strengths:
Technical approaches are well-done. Oral nicotine, electrophysiology, and viral re-expression methods were strong and executed well.
The scholarship is strong and the paper is generally well-written. The figures are high-quality.Weaknesses:
Two bottle choice (2BC) model. 2BC does not examine nicotine reinforcement, which is best shown as a volitional preference for the drug over the vehicle. Mice in this 2BC assay (and all such assays) only ever show indifference to nicotine at best - not preference. This is seen in the maximal 50% preference for the nicotine-containing bottle. 2BC assays using tastants such as saccharin are confounded. Taste responses can very likely differ from primary reinforcement and can be related to peripheral biology in the mouth/tongue rather than in the brain reward pathway. Moreover, this assay does not test free choice, as nicotine is mixed with water which the mice require to survive. Since most concentrations of nicotine are aversive, this may create a generalized conditioned aversion to drinking water - detrimental to overall health and a confounding factor. What plasma concentrations of nicotine are achieved by 2BC? When nicotine is truly reinforcing, rodents and humans titrate their plasma concentrations up to 30-50 ng/mL. The Discussion states that oral self-administration in mice mimics administration in human smokers (lines 388-389). This is unjustified and should be removed. Similarly, the paragraph in lines 409-423 is quite speculative and difficult or impossible to test. This paragraph should be removed or substantially changed to avoid speculation. Overall, the 2BC model has substantial weaknesses, and/or it is limited in the conclusions it will support.Statistical testing on subgroups. Mice are run through an assay and assigned to subgroups based on being classified as avoiders or non-avoiders. The authors then perform statistical testing to show differences between the avoiders and non-avoiders. It is circular to do so. When the authors divided the mice into avoiders and non-avoiders, this implies that the mice are different or from different distributions in terms of nicotine intake. Conducting a statistical test within the null hypothesis framework, however, implies that the null hypothesis is being tested. The null hypothesis, by definition, is that the groups do NOT differ. Obviously, the authors will find a difference between the groups in a statistical test when they pre-sorted the mice into two groups, to begin with. Comparing effect sizes or some other comparison that does not invoke the null hypothesis would be appropriate.
Decreased nicotine-evoked currents following passive exposure to nicotine in minipumps are inconsistent with published results showing that similar nicotine exposure enhances nAChR function via several measures (Arvin et al, J Neurosci, 2019). The paper does acknowledge this previous paper and suggests that the discrepancy is explained by the fact that they used a higher concentration of nicotine (30 uM) that was able to recruit the beta4-containing receptor (whereas Arvin et al used a caged nicotine that was unable to do so). This may be true, but the citation of 30 uM nicotine undercuts the argument a bit because 30 uM nicotine is unlikely to be achieved in the brain of a person using tobacco products; nicotine levels in smokers are 100-500 nM. It should be noted in the paper that it is unclear whether the down-regulated receptors would be active at concentrations of nicotine found in the brain of a smoker. The statement in lines 440-41 ("we show that concentrations of nicotine as low as 7.5 ug/kg can engage the IPN circuitry") is misleading, as the concentration in the water is not the same as the concentration in the CSF since the latter would be expected to build up over time. The paper did not provide measurements of nicotine in plasma or CSF, so concluding that the water concentration of nicotine is related to plasma concentrations of nicotine is only speculative.
The results in Figure 2E do not appear to be from a normal distribution. For example, results cluster at low (~100 pA) responses, and a fraction of larger responses drive the similarities or differences.
10 mg/kg/day in mice or rats is likely a non-physiological exposure to nicotine. Most rats take in 1.0 to 1.5 mg/kg over a 23-hour self-administration period (O'Dell, 2007). Mice achieve similar levels during SA (Fowler, Neuropharmacology 2011). Forced exposure to 10 mg/kg/day is therefore 5 to 10-fold higher than rodents would ever expose themselves to if given the choice. This should be acknowledged in a limitations section of the Discussion.
Are the in vivo recordings in IPN enriched or specific for cells that have a spontaneous firing at rest? If so, this may or may not be the same set/type of cells that are recorded in patch experiments. The results could be biased toward a subset of neurons with spontaneous firing. There are MANY different types of neurons in IPN that are largely intermingled (see Ables et al, 2017 PNAS), so this is a potential problem.
Related to the above issue, which of the many different IPN neuron types did the group re-express beta4? Could that be controlled or did beta4 get re-expressed in an unknown set of neurons in IPN? There is insufficient information given in the methods for verification of stereotaxic injections.
Data showing that alpha3 or beta4 disruption alters MHb/IPN nAChR function and nicotine 2BC intake is not novel. In fact, some of the same authors were involved in a paper in 2011 (Frahm et al., Neuron) showing that enhanced alpha3beta4 nAChR function was associated with reduced nicotine consumption. The present paper would therefore seem to somewhat contradict prior findings from members of the research group.
Sex differences. All studies were conducted in male mice, therefore nothing was reported regarding female nicotine intake or physiology responses. Nicotine-related biology often shows sex differences, and there should be a justification provided regarding the lack of data in females. A limitations section in the Discussion section is a good place for this.
-