Dopamine D2Rs coordinate cue-evoked changes in striatal acetylcholine levels
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The study addressed interactions between two key striatal transmitters, dopamine and acetylcholine during an appetitive behavioral task. Helping to reconcile conflicting evidence in the literature, the data show that changes in both transmitters are correlated and that decreases in acetylcholine with reward and reward cues is only partially a consequence of elevated dopamine release acting at D2 dopamine receptors on striatal cholinergic interneurons. The behavioral significance of this correlation remains to be fully clarified. This manuscript will be of interest to those interested in the neural correlates of appetitive behavior and dopamine and striatal function.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
In the striatum, acetylcholine (ACh) neuron activity is modulated co-incident with dopamine (DA) release in response to unpredicted rewards and reward-predicting cues and both neuromodulators are thought to regulate each other. While this co-regulation has been studied using stimulation studies, the existence of this mutual regulation in vivo during natural behavior is still largely unexplored. One long-standing controversy has been whether striatal DA is responsible for the induction of the cholinergic pause or whether DA D2 receptors (D2Rs) modulate a pause that is induced by other mechanisms. Here, we used genetically encoded sensors in combination with pharmacological and genetic inactivation of D2Rs from cholinergic interneurons (CINs) to simultaneously measure ACh and DA levels after CIN D2R inactivation in mice. We found that CIN D2Rs are not necessary for the initiation of cue-induced decrease in ACh levels. Rather, they prolong the duration of the decrease and inhibit ACh rebound levels. Notably, the change in cue-evoked ACh levels is not associated with altered cue-evoked DA release. Moreover, D2R inactivation strongly decreased the temporal correlation between DA and ACh signals not only at cue presentation but also during the intertrial interval pointing to a general mechanism by which D2Rs coordinate both signals. At the behavioral level D2R antagonism increased the latency to lever press, which was not observed in CIN-selective D2R knock out mice. Press latency correlated with the cue-evoked decrease in ACh levels and artificial inhibition of CINs revealed that longer inhibition shortens the latency to press compared to shorter inhibition. This supports a role of the ACh signal and it’s regulation by D2Rs in the motivation to initiate actions.
Article activity feed
-

-

Author Response
Reviewer #3 (Public Review):
The authors showed that D2R antagonism did not affect the initial dip amplitude but shortened the temporal length of the dip and the rebound ACh levels. In addition, by using both ACh and DA sensors, the authors showed DA levels correlate with ACh dip length and rebound level, not the dip amplitude. Both pieces of evidence support their conclusion that DA does not evoke the dip but controls the overall shape of ACh dip. Overall the current study provides solid data and interpretation. The combination of D2R antagonist and CIN-specific Drd2 KO further support a causal relationship between DA and ACh dip. Overall, the experiments are well-designed, carefully conducted and the manuscript is well-written.
At the behavioral level, the author found a positive correlation between total AUC (of …
Author Response
Reviewer #3 (Public Review):
The authors showed that D2R antagonism did not affect the initial dip amplitude but shortened the temporal length of the dip and the rebound ACh levels. In addition, by using both ACh and DA sensors, the authors showed DA levels correlate with ACh dip length and rebound level, not the dip amplitude. Both pieces of evidence support their conclusion that DA does not evoke the dip but controls the overall shape of ACh dip. Overall the current study provides solid data and interpretation. The combination of D2R antagonist and CIN-specific Drd2 KO further support a causal relationship between DA and ACh dip. Overall, the experiments are well-designed, carefully conducted and the manuscript is well-written.
At the behavioral level, the author found a positive correlation between total AUC (of ACh signal dip) and press latency in Figure 10, indicating cholinergic levels contributes to the motivation. The next logic experiment would be to compare the press latency between control and ChAT-Drd2KO mice, since KO mice have smaller AUC while not affecting DA. However, this piece of information was missing in the manuscript. The author instead showed the correlation between AUC and latency was disrupted, which is indirectly related to the conclusion and hard to interpret. Figure 10 showed that eticlopride elongates the press latency, in a dose-dependent manner. However, it is not clear what this press latency means and how it was measured in this CRF task (Since there is no initial cue in the CRF test, how can we define the press latency?).
We did compare the press latency between control and ChATDrd2KO mice (Figure 10B). At baseline (saline), there is no difference between press latency between these two groups. We measured press latency as the time to press the lever after the lever has been extended. When the lever extends, it makes a sound (cue), which signals to the mice that a new trial has started. The fact that press latency is not enhanced in ChATDrd2KO mice was surprising to us. It is possibly due to compensation via other neuronal mechanisms that regulate press latency (see discussion to comment 6 of public review).
Pearson r<0.5 is normally defined as a weak correlation. It is better to state r values and discuss that in the manuscript.
A valid comment. We clarified our correlation analyses in the methods section (line 717):
“We used a variance explained statistical analysis (R2) to determine the % of variance in our correlation analyses (example: a correlation of 0.5 means 0.52 X 100= 25% of the variance in Y is “explained” or predicted by the X variable. When comparing correlation values, Fisher’s transformation was used to convert Pearson correlation coefficients to z-scores.”
We also added this to the result section: e.g., line 256: “which accounts for 22% of the variance in the ACh decrease explained by the DA peak.
Is there any correlation between ACh AUC and other behavior indexes such as press speed or the time between press and reward licking?
We don’t have the ability to measure press speed and there is no press rate because the lever retracts after the first lever press. We quantified the correlation between time to press until head entry (press to reward latency) and ACh AUC and the results are difficult to interpret. For Drd2f/fl control mice we determined a weak negative correlation (the larger the ACh dip the lower the press to reward latency). In contrast, in ChATDrd2KO mice we found a weak positive correlation between ACh AUC and press to reward latency (the smaller the dip, the lower the press to reward latency). Given these conflicting results, it is difficult to determine how the ACh AUC affects press to reward latency.
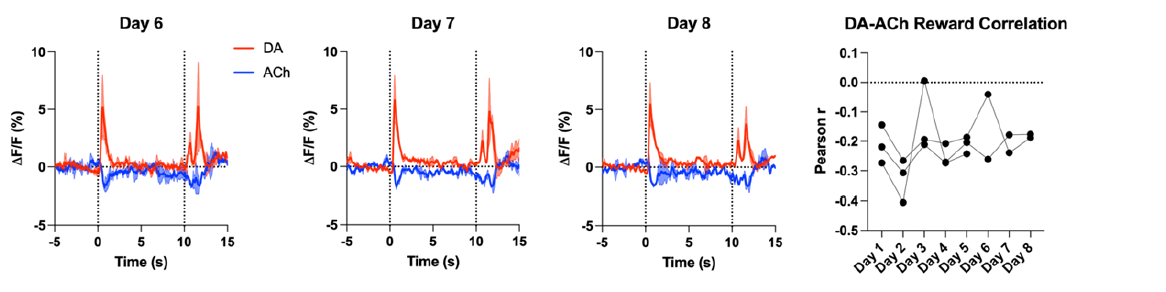
In figure 2B CS+ group, the author was focusing on the responses at CS+, however, the ACh dips at reward delivery seem to persist even after in this particular example. This might be an interesting phenomenon in which ACh got dissociated from DA signals, which needs further analysis from the author.
We see a persistent signal at reward delivery in both DA and ACh up to the 8 days of testing. However, 1 mouse lost its optical fiber for the GACh signal so the data from Days 6-8 is from 2 mice. We also measured the correlation between DA and ACh at reward delivery for all 8 days of testing (see below). The correlation data is variable with the strongest correlation being observed on Day 2. It is possible that these signals could get dissociated after even more days of testing, but we do not have this data available.
-

Evaluation Summary:
The study addressed interactions between two key striatal transmitters, dopamine and acetylcholine during an appetitive behavioral task. Helping to reconcile conflicting evidence in the literature, the data show that changes in both transmitters are correlated and that decreases in acetylcholine with reward and reward cues is only partially a consequence of elevated dopamine release acting at D2 dopamine receptors on striatal cholinergic interneurons. The behavioral significance of this correlation remains to be fully clarified. This manuscript will be of interest to those interested in the neural correlates of appetitive behavior and dopamine and striatal function.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with …
Evaluation Summary:
The study addressed interactions between two key striatal transmitters, dopamine and acetylcholine during an appetitive behavioral task. Helping to reconcile conflicting evidence in the literature, the data show that changes in both transmitters are correlated and that decreases in acetylcholine with reward and reward cues is only partially a consequence of elevated dopamine release acting at D2 dopamine receptors on striatal cholinergic interneurons. The behavioral significance of this correlation remains to be fully clarified. This manuscript will be of interest to those interested in the neural correlates of appetitive behavior and dopamine and striatal function.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
The authors have used a classical behavioral task (learning to press a lever for a food reward in food-restricted mice) to address complementary changes in dopamine (DA) and acetylcholine (ACh). They have used the latest methods to monitor DA and ACh and have chosen a behavioral test that elicits reliable changes in both. The paper adds to existing literature on which behavioral aspects are influenced by these transmitters. A particular strength is examination of how a reward-related drop in ACh levels is altered after conditional knockout of D2 DA receptors in striatal cholinergic interneurons (ChIs). ChIs are the primary source of striatal ACh, and show a decrease in firing rate (a pause) as DA levels rise - an effect that has been linked to D2 activation. Here, the authors show that the duration of the …
Reviewer #1 (Public Review):
The authors have used a classical behavioral task (learning to press a lever for a food reward in food-restricted mice) to address complementary changes in dopamine (DA) and acetylcholine (ACh). They have used the latest methods to monitor DA and ACh and have chosen a behavioral test that elicits reliable changes in both. The paper adds to existing literature on which behavioral aspects are influenced by these transmitters. A particular strength is examination of how a reward-related drop in ACh levels is altered after conditional knockout of D2 DA receptors in striatal cholinergic interneurons (ChIs). ChIs are the primary source of striatal ACh, and show a decrease in firing rate (a pause) as DA levels rise - an effect that has been linked to D2 activation. Here, the authors show that the duration of the decrease in ACh, as an index of ACh activity, is indeed D2 dependent, although a drop in ACh is still seen, implying other regulators besides DA.
Despite these strengths, there are a few limitations, as well. First, there is no discussion of other regulators that might lead to a decrease in ACh amplitude that is DA-independent, which is somewhat unsatisfying. Second, much of the report examines correlations between ACh and DA levels at different points in the behavior, with a focus on how DA regulates ACh levels. Not addressing the opposite question of DA regulation by ACh, as well, seems a missed opportunity. Lastly, the paper frames the changes in transmitter concentration monitored with genetically encoded probes as though these changes cause the behaviors examined. This seems simplistic, given that there are many steps between the data reported and understanding how DA and ACh influence the circuitry underlying the behaviors. The discussion considers some of these, but the general framing is on the observed changes in transmitter levels, rather than underlying cellular activity, narrowing the implications of the results.
-

Reviewer #2 (Public Review):
The authors nicely demonstrate a tight correlation between striatal ACh and dopamine release, and its regulation by CIN-D2 receptors. The strong correlation was demonstrated through pharmacological and selective genetic manipulations and extensively verified. Nevertheless, while the authors conclude that their data supports a role of CIN-D2 receptor control of ACh release in motivated behavior, data supporting the role of this signaling mechanisms in motivated behavior is lacking.
This study would benefit from further analyses on the behavioral effects of pharmacological and genetic manipulations. The inclusion of additional behavioral tests would greatly strengthen the study by identifying clear behavioral repercussions of CIN-D2 receptor control of ACh release. -

Reviewer #3 (Public Review):
The authors showed that D2R antagonism did not affect the initial dip amplitude but shortened the temporal length of the dip and the rebound ACh levels. In addition, by using both ACh and DA sensors, the authors showed DA levels correlate with ACh dip length and rebound level, not the dip amplitude. Both pieces of evidence support their conclusion that DA does not evoke the dip but controls the overall shape of ACh dip.
Overall the current study provides solid data and interpretation. The combination of D2R antagonist and CIN-specific Drd2 KO further support a causal relationship between DA and ACh dip. Overall, the experiments are well-designed, carefully conducted and the manuscript is well-written.At the behavioral level, the author found a positive correlation between total AUC (of ACh signal dip) and …
Reviewer #3 (Public Review):
The authors showed that D2R antagonism did not affect the initial dip amplitude but shortened the temporal length of the dip and the rebound ACh levels. In addition, by using both ACh and DA sensors, the authors showed DA levels correlate with ACh dip length and rebound level, not the dip amplitude. Both pieces of evidence support their conclusion that DA does not evoke the dip but controls the overall shape of ACh dip.
Overall the current study provides solid data and interpretation. The combination of D2R antagonist and CIN-specific Drd2 KO further support a causal relationship between DA and ACh dip. Overall, the experiments are well-designed, carefully conducted and the manuscript is well-written.At the behavioral level, the author found a positive correlation between total AUC (of ACh signal dip) and press latency in Figure 10, indicating cholinergic levels contributes to the motivation. The next logic experiment would be to compare the press latency between control and ChAT-Drd2KO mice, since KO mice have smaller AUC while not affecting DA. However, this piece of information was missing in the manuscript. The author instead showed the correlation between AUC and latency was disrupted, which is indirectly related to the conclusion and hard to interpret. Figure 10 showed that eticlopride elongates the press latency, in a dose-dependent manner. However, it is not clear what this press latency means and how it was measured in this CRF task (Since there is no initial cue in the CRF test, how can we define the press latency?).
Pearson r<0.5 is normally defined as a weak correlation. It is better to state r values and discuss that in the manuscript.
Is there any correlation between ACh AUC and other behavior indexes such as press speed or the time between press and reward licking?
In figure 2B CS+ group, the author was focusing on the responses at CS+, however, the ACh dips at reward delivery seem to persist even after in this particular example. This might be an interesting phenomenon in which ACh got dissociated from DA signals, which needs further analysis from the author.
-