Septin7 is indispensable for proper skeletal muscle architecture and function
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The study of previously unexplored roles of a cytoskeleton protein termed Septin-7 in muscle could be a significant contribution to understand muscle development and regeneration. The majority of the data support the importance of Septin-7 protein in muscle physiology. With some additional experiments and data analysis to strengthen the mechanistic characterization of Septin-7 in muscle physiology, this manuscript will be of broad interest to readers in the skeletal muscle research field.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Today septins are considered as the fourth component of the cytoskeleton, with the Septin7 isoform playing a critical role in the formation of higher-order structures. While its importance has already been confirmed in several intracellular processes of different organs, very little is known about its role in skeletal muscle. Here, using Septin7 conditional knockdown (KD) mouse model, the C2C12 cell line, and enzymatically isolated adult muscle fibers, the organization and localization of septin filaments are revealed, and an ontogenesis-dependent expression of Septin7 is demonstrated. KD mice displayed a characteristic hunchback phenotype with skeletal deformities, reduction in in vivo and in vitro force generation, and disorganized mitochondrial networks. Furthermore, knockout of Septin7 in C2C12 cells resulted in complete loss of cell division while KD cells provided evidence that Septin7 is essential for proper myotube differentiation. These and the transient increase in Septin7 expression following muscle injury suggest that it may be involved in muscle regeneration and development.
Article activity feed
-

-

Autho Response
Reviewer #1 (Public Review):
Here the authors aimed to gain insight into the role of Septin-7 in skeletal muscle biology using a novel and powerful mouse model of inducible muscle specific septin-7 deletion. They combine this with CRISPR/Cas9 and shRNA mediated manipulation of Septin-7 in C2C12 cells in vitro to explore its role in muscle progenitor morphology and proliferation. There are a variety of interesting observations, with clear phenotypes induced by the Septin-7 manipulation, including effects on body weight, muscle force production, mitochondrial morphology, and cell proliferation. However each area is somewhat superficially examined, and certain conclusions require additional validation for robust support. Additionally, mechanistic insight into Septin 7's role is limited. Therefore, while the phenotypes are …
Autho Response
Reviewer #1 (Public Review):
Here the authors aimed to gain insight into the role of Septin-7 in skeletal muscle biology using a novel and powerful mouse model of inducible muscle specific septin-7 deletion. They combine this with CRISPR/Cas9 and shRNA mediated manipulation of Septin-7 in C2C12 cells in vitro to explore its role in muscle progenitor morphology and proliferation. There are a variety of interesting observations, with clear phenotypes induced by the Septin-7 manipulation, including effects on body weight, muscle force production, mitochondrial morphology, and cell proliferation. However each area is somewhat superficially examined, and certain conclusions require additional validation for robust support. Additionally, mechanistic insight into Septin 7's role is limited. Therefore, while the phenotypes are likely of intrigue to both the muscle and septin community, to significantly advance the field will require additional experimentation.
Specifically, it is currently difficult to distinguish between developmental and adult roles of Septin-7. The authors induce tamoxifen-mediated deletion at 1 month of age and examine muscle structure/function only at 4 months. By not studying early time points, it is difficult to determine whether particular phenotypes are directly due to Septin deletion or a secondary consequence of muscle atrophy and/or a decline in body weight. Further, by not inducing deletion at a later time point (i.e. after 2 months when muscle is generally matured), it is difficult to assess whether septin-7 plays a role in maintaining structure and function of mature muscle, or if its primary role is in muscle development.
We have conducted a number of trials for knocking-down of Septin-7 expression. These included Tamoxifen treatment of Cre- pregnant mothers, shorter treatments starting at early after birth, and treatments of adult animals. While the former led to still-born offsprings, the later resulted in only a minor – less than 20% - reduction of Septin-7 expression. These long trials led us to, on the one hand, concentrate on the protocol used throughout the manuscript (where a significant, up to 50%, reduction in the expression of the protein could be achieved) and to, on the other hand, focus also on myogenic cells in culture. This selection was also substantiated by the finding that Septin-7 expression is the highest in neonatal muscles and declines with age until adulthood (but remains essentially constant until an age of 18 months for the mice examined). As an identical Tamoxifen treatment of littermate Cre- mice did not result in any of the presented alterations (as demonstrated in the Supplementary material) we can conclude that they are the consequence of Septin-7 down-regulation. We, nonetheless, completely agree with the Reviewer that some observations are most likely indirect, i.e., are due to the loss of muscle mass. These include, e.g., the altered shape of the vertebra and the consequent “hunchback” phenotype. However, this observation further supports our claim that Septin-7 is essential for proper development of a normal musculature in these animals.
Further, the conclusion that septin-7 has an essential role in regeneration (seemingly based on expression increasing after injury) is unsupported and requires further experimentation where injury and regeneration is triggered in the absence of Septin-7 to establish a causative role.
We agree with the Reviewer that a clear causative role of Septin-7 in muscle regeneration would require a substantial amount of further experimentation on Septin-7 knock-down animals. We, however, believe that this – detailed description of the changes in transcription factors and key regulatory proteins together with changes in morphology in Septin-7 KD animals following muscle injury – is beyond the scope of the present manuscript and should be presented as a separate study. In this manuscript, however, we provide the essential background to substantiate this claim. We describe that fusion of myogenic cells is severely hindered if Septin-7 expression is suppressed while Septin-7 is upregulated following muscle injury to the extent which is significantly more than what would be expected if it would be simply due to the production of new muscle fibers.
Finally, there are intriguing observations in mitochondrial and myofiber organization and mitochondrial content; however further interrogation into additional relevant metrics of each, and at different time points of Septin-7 deletion, are needed to better understand these phenotypes and gain insight into Septin-7's role in their regulation.
Accepting the concern of the Reviewer we have conducted additional experiments to enable the proper characterization of the morphology. Additional relevant metrics – Aspect Ratios and Form Factors – have been calculated and are now incorporated into the revised MS and are presented in Figure 5.
Reviewer #2 (Public Review):
This is a comprehensive work describing for the first time the location and importance of the cytoskeletal protein Septin-7 in skeletal muscle. The authors, using a Septin-7 conditional knockdown mouse model, the C2C12 cell line, and enzymatically isolated adult muscle fibers, explore the normal location of this protein in muscle fibers, the morphological alterations in conditioned knockdown conditions, the developmental alterations, and the functional alterations in terms of force production. The global picture that emerges shows Septin-7 as a fundamental brick in both muscle construction, development, and regeneration; all this leads to reinforcing the basically structural nature of this protein role.
We thank the Reviewer for the appreciative words. We indeed believe that Septin-7 plays and important role in the proper organization and development of skeletal muscle. Even a partial knock-down of the protein at the early stages of life results in a severe loss in muscle mass accompanied by skeletal deformities. A complete knock-out of the protein results, at the myoblast level, in the inability of the cells to proliferate and form multinucleated cells confirming the essential role of this structural protein.
Reviewer #3 (Public Review):
This is an original study to explore the role of Septin-7, a cytoskeleton protein, in skeletal muscle physiology. The authors produced a unique mouse model with Septin-7 conditional knockdown specifically in skeletal muscle, which allowed them to examine the structure and function changes of skeletal muscle in response to the reduced protein expression level of Septin-7 in vivo and ex vivo at different development stages without the influence of other body parts with reduced Septin-7 expression. The study on the cellular model, C2C12 myoblast/myotubes with knockdown of Septin-7 expression, provided additional evidence of the importance of this cytoskeleton protein in regulating myoblast proliferation and differentiation. Majority of the data are supportive of the the major claim in this manuscript. However, additional key experiments and data analysis are needed to provide more mechanistic characterization of Septin-7 in muscle physiology.
We would like to express our thanks to the Reviewer for the critical comments on our manuscript and for the valuable suggestions that help substantiate our claim, that Septin-7 is an essential part of the cytoskeletal network in skeletal muscle and plays an important role in muscle differentiation as well as in myoblast proliferation and fusion.
A number of additional experiments were carried out to answer the comments/concerns of the Reviewer. Immunostaining of critical proteins (actin, myosin, and the L-type calcium channel) are now presented in Figure S4 for Cre+ animals. The T-tubules of enzymatically isolated fibers from these Septin-7 knock-down mice were also stained using Di-8-ANEPPS and the corresponding images are presented below. We describe how different Tamoxifen treatments at different time-points in the intra- and extra-uterine life of the animals resulted in the deletion of the SEPTIN 7 gene which ultimately led us to use the protocol (largest reduction with still viable mice) described in this manuscript. A more detailed description on how the fusion index, a clear marker a myotube differentiation, was conducted using desmin staining is now included and additional experiments (immunostaining and western blot) with MYH as suggested by the Reviewer are also presented. We carried out a thorough analysis of mitochondrial morphology (in line with the requirements of another Reviewer) and modified the corresponding figure in the revised MS accordingly.
Major Concerns:
- The Septin-7 knockdown mouse model, the EM and IHC techniques are all established in the research group. It is a surprise to see that authors missed the opportunity to characterize the morphological changes in the T-tubule network, triad structure, the distribution of Ca release units (i.e., IHC of DHPR and RyR), and its co-localization with other key cytoskeletal proteins (i.e. actin) etc., in the muscle section or isolated muscle fibers.
We appreciate the reviewer's valuable critical comments. Even if we were not able to fully comply with all the requests, we corrected as many of the mentioned shortcomings as possible, by correcting the errors and to prove our claims with further experiments. Please find our responses to each critical remark below.
We conducted IHC staining on individual FDB fibers of C57Bl/6 mice presenting the distribution of skeletal muscle specific α-actinin, and RyR1 alongside with Septin-7 proteins (Figure 1E and F). As demonstrated in Figure 5E and F of the original MS (Figure 5 F and G in the revised version) normal triad structures were present both in Cre- and Cre+ muscle samples using EM analysis. However, the sarcomeres were distorted at places where large mitochondria appeared in Cre+ samples.
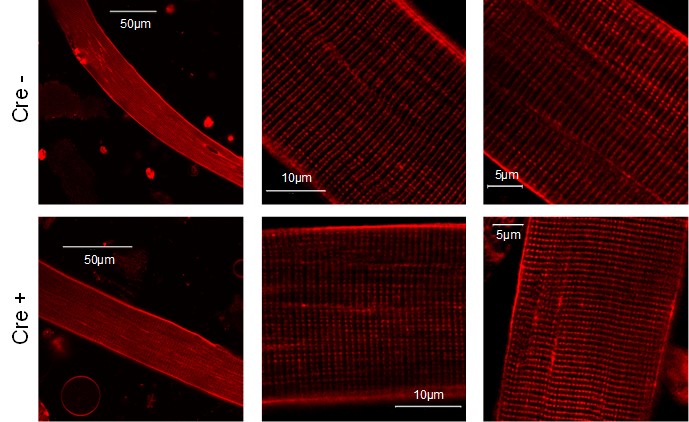
As suggested, T-tubule staining by Di-8-ANEPPS was carried out on isolated FDB fibers from Cre- and Cre+ animals, which revealed no considerable differences between the two groups.
Images present the T-tubule system of a single muscle fibers isolated from Cre- and Cre+ FDB muscle. Di-8-ANEPPS staining reveals no considerable difference between the two type of animals suggesting that the reduced Septin-7 expression does not alter the T-tubular system of skeletal muscle cells.
To further investigate the key components of muscle contraction and EC coupling, we carried out immunostaining in isolated single fibers from FDB muscle originating from Cre+ and Cre- mice. Immunocytochemistry revealed no significant alteration of actin, myosin 4, and L-type calcium channel labeling comparing the two mouse strains (see Figure S4 in the revised version).
- The authors only studied one time point following the Tamoxifen treatment (4-month old with 3-month treatment). Based on Fig 2D, a significant body weight reduction was achieved after one month of the Tamoxifen treatment (at the age of 7 weeks), indicating a potential reduced muscle development at this age. Mice are considered fully matured at the age of 2 months. It will be more informative if the muscle samples and the in vivo and in vitro muscle activity are analyzed at this time point (7 or 8-week old), which should provide a direct answer if the knockdown of Septin-7 affects the muscle development. Additionally, a time dependent correlation of the level of Septin-7 knockdown with muscle function/morphology analysis should better define the role of Septin-7 in muscle development and function.
We agree with the Reviewer that Septin-7 has presumably more pronounced effect in the early stage of muscle development, since we detected higher expression level of the protein in muscle samples isolated from newborn and young as compared with adult animals. We conducted preliminarily in vivo and in vitro force experiments on 2-month-old mice after 1 month of Tamoxifen treatment. The grip force already decreased significantly in Cre+ mice but the decrease in twitch and tetanic force of EDL and Sol did not reach significance. These experiments were followed by the analysis of Septin-7 level in the muscle samples which showed less than 20% of reduction on average in the samples of Cre+ mice. This suggested that a more robust suppression of Septin-7 is needed to reach significant reduction in in vitro force thus we decided to extend the Tamoxifen treatment to 3 months.
- Although the expression level of Septin-7 reduced during muscle development (Fig 1C), but its expression is still evident at the age of 4 months (Fig 1C and Fig S1F), indicating a potential role of Septin-7 in maintaining normal muscle function. It is important to examine whether the Tomaxifen treatment started after the muscle maturation at the age of 2-month old would affect the muscle structure and function. Particularly, these type of KD mice will be critical to answer if the KD will affect the regeneration rate following the muscle injury. The outcome will further test or support their claim of the essential roles of Septin-7 in muscle regeneration.
We agree with the Reviewer opinion that Septin-7 presumably plays an essential role not only during the early development of skeletal muscle but also in the matured tissue. In our preliminary studies Septin-7 protein expression was determined in skeletal muscle samples from mice at different developmental stage. As presented in Figure 1C we observed decrease in Septin-7 protein expression from newborn to adult stages. The expression profile of Septin-7 was also investigated in samples from 2, 4, 6, 9, and 18-month-old mice and a significant decrease was observed in samples isolated from mice of 4, 6, 9, and 18 months of age (58±8; 48±9; 66±16; 54±9% relative to the 2-month-old muscles, respectively), however there were no considerable changes between samples after 4 months of age.
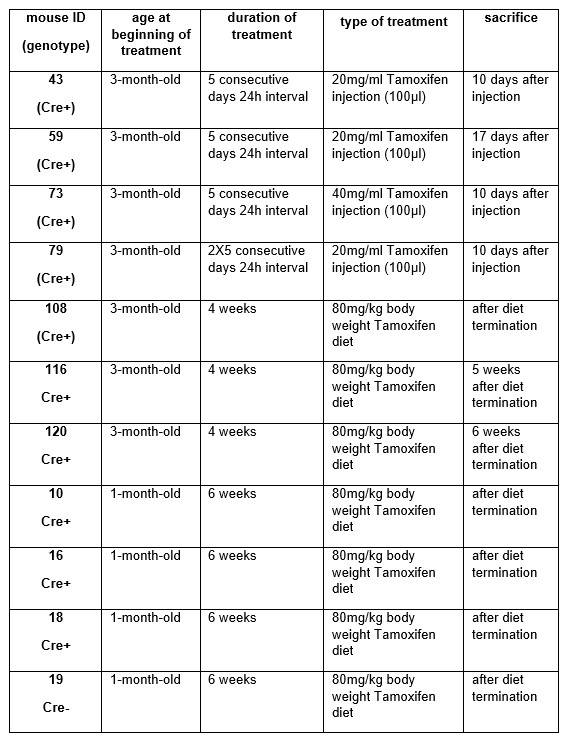
In order to generate skeletal muscle specific, conditional Septin-7 knock-down animals, we applied Tamoxifen treatment at different developmental stages in our preliminary studies (see the table and figures below). When Cre- pregnant females were fed with Tamoxifen in the third trimester of pregnancy, it caused intrauterin lethality independent of the genotype. According to the animal ethics requirements we did not continue this experimental protocol. In the next stage of our initial experiments, 3 month-old mice were treated with both intraperitoneal injections for 5 consecutive days or Tamoxifen diet for 4 weeks. Here, only a moderate deletion of the exon4 was detected in SEPTIN 7 gene in Cre+ animals (data obtained from these mice are shown below).
These findings and the observation of ontogenesis dependent expression of Septin-7 indicated its significance at the early stage of development and suggested that we should try to modify the gene expression at earlier age. Six weeks of diet supplemented with Tamoxifen generated well detectable exon deletion in younger (1-month-old) mice. Regarding these observations we decided to start the Tamoxifen-supplemented diet in younger (4-week-old) animals immediately after separation from the mother and we continued the treatment for a longer period (3 months) to be sure that exon deletion will be prominent in all Cre+ animals.
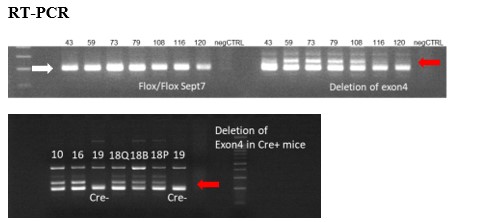
Genetic modification of SEPTIN 7 gene following Tamoxifen treatment in mice mentioned above. RT-PCR
Figure presents the presence of floxed sites at SEPTIN 7 gene (white arrow) and the deletion of exon4 (red arrows) in the appropriate DNA samples isolated from mice treated with Tamoxifen from different age and using different methods and period of Tamoxifen application. Exon4 deletions were less than 20%, therefore these trials were not continued. Numbers above each lane correspond to the animal ID-s presented in the table above. Q – m. quadriceps, B- m. biceps femoris, P – m. pectoralis.
The knock-down of Septin-7 in the adult animals (where its expression is already low; see above) did not result in an appreciable further reduction. This led us to conclude that the role of Septin-7 is most pronounced in muscle development. In this framework, at the adult stage a possible function of Septin-7 in muscle regeneration following injury could be envisioned. This is demonstrated in Fiure 6 where we present that Septin-7 is upregulated following a mild injury. However, we believe, that a detailed examination of the role of Septin-7 in the regeneration is beyond the scope of the current manuscript and should be the basis of further studies.
- Regarding the impact of Septin-7 on differentiation, it could be problematic if the images with the resolution shown in Figure S4A-C were used for fusion index calculation. If those are just zoomed in representative images and the authors used other lower resolution, global view images for quantification, those images are needed to be shown. The authors may also need to elaborate on why they stained Desmin instead of MYH for quantification of the fusion index of myotubes (page 27). Desmin also marks mesenchymal cells.
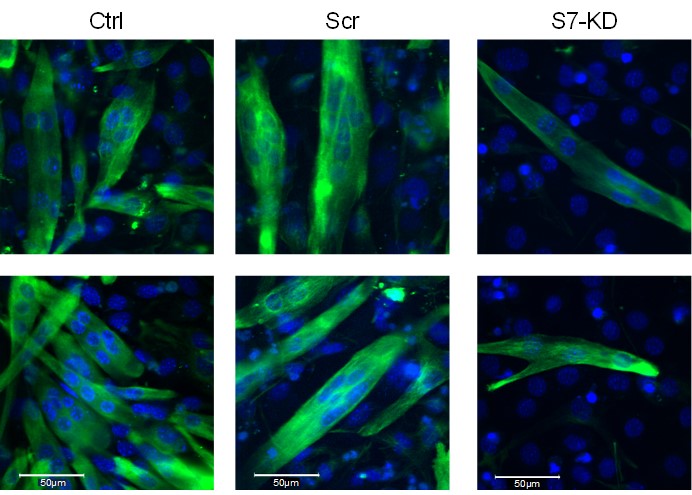
We apologize that the method used for fusion index calculation was not clear enough. Images in Figure S4A-C present the Septin-7 and actin cytoskeletal structure in proliferating myoblasts, before the induction of differentiation. Fusion index was determined in cultures where myotube differentiation was induced by reduced serum content (as described in Methods). We used desmin staining as the expression of this protein is present only in myotubes with 2 or more nuclei, where fusion of myoblasts has already started (see representative images below). Representative desmin-labeling images from control, scrambled and KD cultures are now included in Figure S5G at 5 days differentiated stage.
Figure presents two examples (bottom row is now added to Figure S5 as panel G) of the desmin-specific immunostaining used for the calculation of fusion index in the different C2C12 cultures. Specific signals of desmin are present following the fusion of single nuclei myoblast into myotubes (green), while non-differentiated myoblasts did not show immunolabeling for desmin. Nuclei are stained with DAPI (blue).
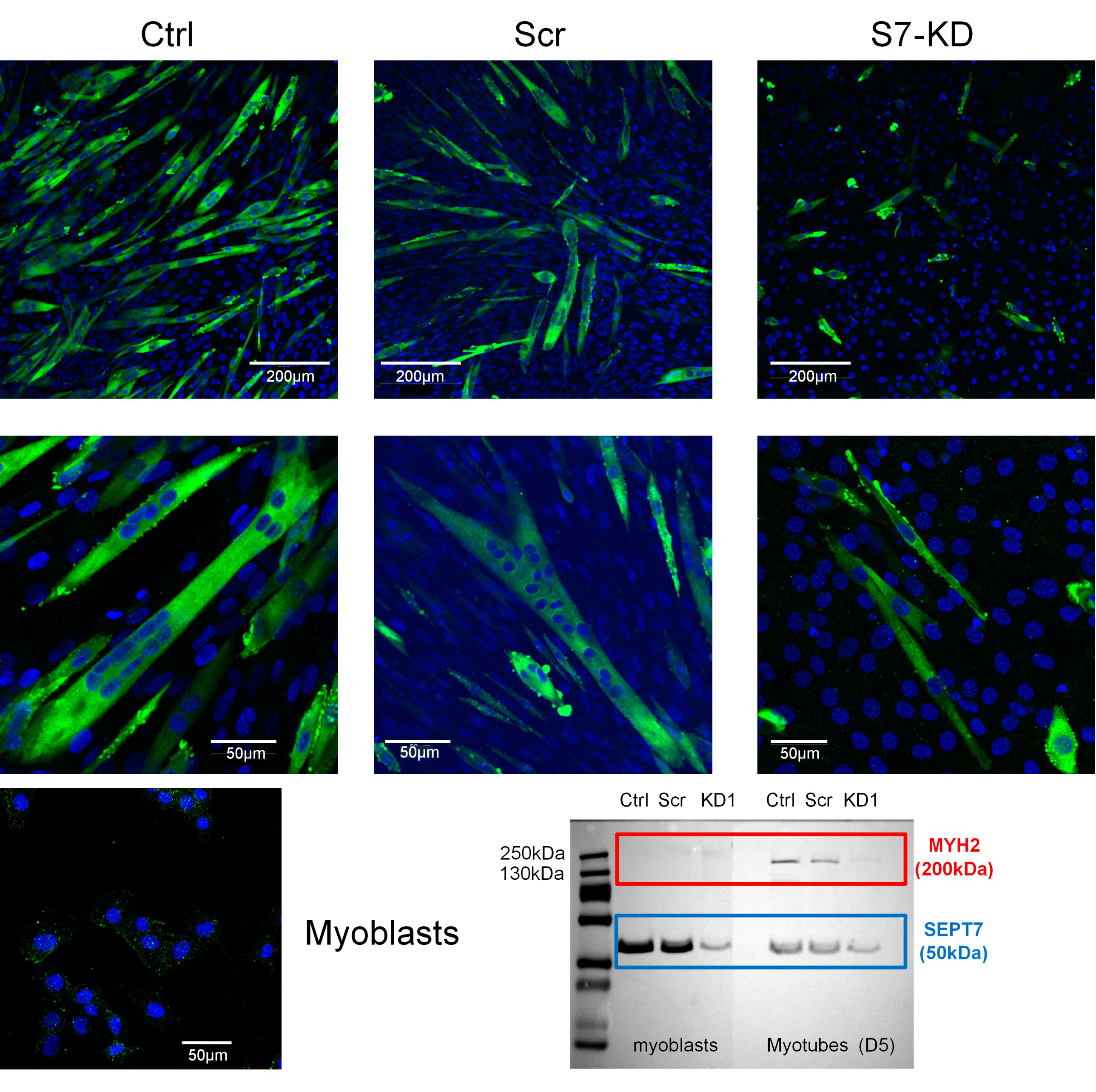
If Septin-7 is truly affecting differentiation, a decrease of MYH 2 expression can be readily detected by IHC or WB.
We are grateful for the Reviewer´s suggestion. We have conducted immunocytochemistry and WB experiments in proliferating myoblasts and myotubes at day 5 of differentiation. As the figure below demonstrates, myosin heavy chain-specific immunolabeling could be detected only in differentiated samples, while myoblasts did not show positive signal. However, there is a significantly lower number of MYH2-positive myotubes in Septin-7 KD cultures as compared with the control and scrambled samples. In addition, we detected decreased WB signal for MYH2 in Septin-7 KD protein samples compared with their control counterparts.
Figure presents the MYH2-specific immunostaining in the different C2C12 cultures. Specific signals of myosin heavy chain 2 (green) are present during myotube formation of differentiating cultures, however, less MYH2-positive myotubes are present in the Septin-7 KD cultures as a result of reduced capability of cells to fuse, here the DAPI-stained nuclei were only present. Proliferating myoblasts did not show specific immunolabeling for MYH2, as the confocal image and the appropriate part of the WB membranes show. We could also detect a decreased MYH2-specific labeling in Septin-7 KD samples as compared with the control ones using WB.
Additionally, Septin-7 may also affect the migration or fusion of myoblasts instead of differentiation. The observation of altered cell morphology and filopodia/lamellipodia formation (Figure 3C) in Septin7-KD cells before differentiation also implies a potential role of Septin-7 in migration. This possibility should be at least discussed.
We appreciate the Reviewer´s comment and suggestion. There are a few publication showing that alteration of septin (in some cases Septin-7) expression modifies the migration of different eukaryotic cell types, like in microvascular endothelial cells (PMID: 24451259), in human epithelial cells (PMID: 31905721), in neural crest cells (PMID: 2881782), and in human breast cancer or lung cancer cells (PMID: 27557506, 31558699, and 32516969). In the work of Li et al. (PMID:32382971) their findings revealed that miR-127-3p regulates myoblast proliferation by targeting Septin-7. In the present manuscript we described that Septin-7 modification alters myoblast fusion (Figure 3J), which is the accompanying phenomenon of differentiation. On the other hand, the effect of Septin-7 gene silencing on cell migration has been studied in detail and was presented to The Biophysical Society. The results are intended to be submitted as a separate manuscript.
- The image shown in Figure 5F does not support the pooled data showed in Figure 5C. The size of mitochondria is remarkably lager in Cre+ muscle (Fig 5E and 5F). The morphology of mitochondria in Cre+ muscle are apparently normal (Fig 5F), while the mitochondrial DNA content are drastically reduced (Figure 5H), which is an important discovery and deserved to be further confirmed by WB and/or qPCR for critical mitochondrial proteins (i.e. MTCOX, COXV, etc.).
We thank the Reviewer for pointing out that the interpretation of images in Figure 5 was not clear enough. Based on this, and the on the clear request from the other Reviewer, a detailed evaluation of mitochondrial morphology was carried out and the panels of Figure 5 were redrawn and reorganized. The revised Figure 5 now presents the average Perimeter, the average Aspect Ratio, and the average Form Factor (panels C & H, for cross- & horizontal-sections, respectively), the relative distributions of the areas (panels D & I, for cross- & horizontal-sections, respectively), and the number of mitochondria normalized to fiber area (panel E, cross-sections). The mitochondrial DNA content is presented in panel J. As evidenced from these figures (and from the representative EM micro graphs), larger mitochondria, sometimes in large associations, are present in the muscles of Cre+ animals.
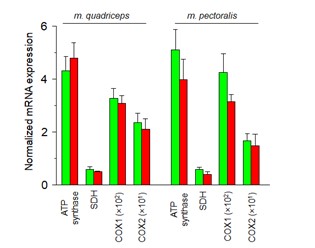
Furthermore, gene expression of four essential mitochondrial proteins cytochrome oxidase 1 (COX1), cytochrome oxidase 2 (COX2), succinate dehydrogenase (SDH), and ATP synthase) were determined in RNA samples from different skeletal muscles of Cre- and Cre+ animals using qPCR. As the figure below demonstrates there was a tendency of decreased expression of the aforementioned genes in Cre+ muscle samples, however, significant difference between the Cre- and Cre+ data could not be detected.
Figure represents the normalized mRNA expression of ATP synthase, SDH, COX1, and COX2 in Cre- (green) and Cre+ (red) samples isolated from m. quadriceps and m. pectoralis. Each gene expression was determined from 3 individual animals and a technical duplicate was used during the qPCR analysis. 36B4 gene encoding an acidic ribosomal phosphoprotein P0 was used as a normalizing gene.
- Figure 2 H & I: It is unclear whether the muscle force was normalized to the individual muscle weight.
We are sorry about the incomplete representation and explanation of muscle force values. Figure 2F-I presents absolute force values without normalization to the cross sectional area. In order to answer the Reviewer´s comment the averages of normalized values are given in Table S3 in the modified manuscript.
- The IHC results in Figure 6B are confusing. There are no centrally located nuclei in the Pax7 alone image of Figure 6B but abundant in the Pax7 + H&E image. The brown color of DAB and the purple color of hematoxylin are hard to be distinguished.
Images presenting the labeling of Pax7 (a transcription factor expressed in activated satellite cells) alone could not show centrally located nuclei, as the nuclei could only be visible when HE staining is applied. As the Reviewer mentioned brown color of DAB and the purple color of hematoxylin are sometimes difficult to distinguish, therefore, we first presented PAX7 expression visualized by DAB staining (localization was near the sarcolemma). In the next step we performed a double staining for PAX7 and HE to show both the cytoplasm and nuclei.
-

Evaluation Summary:
The study of previously unexplored roles of a cytoskeleton protein termed Septin-7 in muscle could be a significant contribution to understand muscle development and regeneration. The majority of the data support the importance of Septin-7 protein in muscle physiology. With some additional experiments and data analysis to strengthen the mechanistic characterization of Septin-7 in muscle physiology, this manuscript will be of broad interest to readers in the skeletal muscle research field.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #3 (Public Review):
This is an original study to explore the role of Septin-7, a cytoskeleton protein, in skeletal muscle physiology. The authors produced a unique mouse model with Septin-7 conditional knockdown specifically in skeletal muscle, which allowed them to examine the structure and function changes of skeletal muscle in response to the reduced protein expression level of Septin-7 in vivo and ex vivo at different development stages without the influence of other body parts with reduced Septin-7 expression. The study on the cellular model, C2C12 myoblast/myotubes with knockdown of Septin-7 expression, provided additional evidence of the importance of this cytoskeleton protein in regulating myoblast proliferation and differentiation. Majority of the data are supportive to the major claim in this manuscript. However, …
Reviewer #3 (Public Review):
This is an original study to explore the role of Septin-7, a cytoskeleton protein, in skeletal muscle physiology. The authors produced a unique mouse model with Septin-7 conditional knockdown specifically in skeletal muscle, which allowed them to examine the structure and function changes of skeletal muscle in response to the reduced protein expression level of Septin-7 in vivo and ex vivo at different development stages without the influence of other body parts with reduced Septin-7 expression. The study on the cellular model, C2C12 myoblast/myotubes with knockdown of Septin-7 expression, provided additional evidence of the importance of this cytoskeleton protein in regulating myoblast proliferation and differentiation. Majority of the data are supportive to the major claim in this manuscript. However, additional key experiments and data analysis are needed to provide more mechanistic characterization of Septin-7 in muscle physiology.
Major Concerns:
1. The Septin-7 knockdown mouse model, the EM and IHC techniques are all established in the research group. It is a surprise to see that authors missed the opportunity to characterize the morphological changes in the T-tubule network, triad structure, the distribution of Ca release units (i.e., IHC of DHPR and RyR), and its co-localization with other key cytoskeletal proteins (i.e. actin) etc., in the muscle section or isolated muscle fibers.
2. The authors only studied one time point following the Tomaxifen treatment (4-month old with 3-month treatment). Based on Fig 2D, a significant body weight reduction was achieved after one month of the Tamoxifen treatment (at the age of 7 weeks), indicating a potential reduced muscle development at this age. Mice are considered fully matured at the age of 2 months. It will be more informative if the muscle samples and the in vivo and in vitro muscle activity are analyzed at this time point (7 or 8-week old), which should provide a direct answer if the knockdown of Septin-7 affects the muscle development. Additionally, a time dependent correlation of the level of Septin-7 knockdown with muscle function/morphology analysis should better define the role of Septin-7 in muscle development and function.
3. Although the expression level of Septin-7 reduced during muscle development (Fig 1C), but its expression is still evident at the age of 4 months (Fig 1C and Fig S1F), indicating a potential role of Septin-7 in maintaining normal muscle function. It is important to examine whether the Tomaxifen treatment started after the muscle maturation at the age of 2-month old would affect the muscle structure and function. Particularly, these type of KD mice will be critical to answer if the KD will affect the regeneration rate following the muscle injury. The outcome will further test or support their claim of the essential roles of Septin-7 in muscle regeneration.
4. Regarding the impact of Septin-7 on differentiation, it could be problematic if the images with the resolution shown in Figure S4A-C were used for fusion index calculation. If those are just zoomed in representative images and the authors used other lower resolution, global view images for quantification, those images are needed to be shown. The authors may also need to elaborate on why they stained Desmin instead of MYH for quantification of the fusion index of myotubes (page 27). Desmin also marks mesenchymal cells. If Septin-7 is truly affecting differentiation, a decrease of MYH expression can be readily detected by IHC or WB. Additionally, Septin-7 may also affect the migration or fusion of myoblasts instead of differentiation. The observation of altered cell morphology and filopodia/lamellipodia formation (Figure 3C) in Septin7-KD cells before differentiation also implies a potential role of Septin-7 in migration. This possibility should be at least discussed.
5. The image shown in Figure 5F does not support the pooled data showed in Figure 5C. The size of mitochondria is remarkably lager in Cre+ muscle (Fig 5E and 5F). The morphology of mitochondria in Cre+ muscle are apparently normal (Fig 5F), while the mitochondrial DNA content are drastically reduced (Figure 5H), which is an important discovery and deserved to be further confirmed by WB and/or qPCR for critical mitochondrial proteins (i.e. MTCOX, COXV, etc.).
6. Figure 2 H & I: It is unclear whether the muscle force was normalized to the individual muscle weight.
7. The IHC results in Figure 6B are confusing. There are no centrally located nuclei in the Pax7 alone image of Figure 6B but abundant in the Pax7 + H&E image. The brown color of DAB and the purple color of hematoxylin are hard to be distinguished.
-

Reviewer #2 (Public Review):
This is a comprehensive work describing for the first time the location and importance of the cytoskeletal protein Septin-7 in skeletal muscle. The authors, using a Septin-7 conditional knockdown mouse model, the C2C12 cell line, and enzymatically isolated adult muscle fibers, explore the normal location of this protein in muscle fibers, the morphological alterations in conditioned knockdown conditions, the developmental alterations, and the functional alterations in terms of force production.
The global picture that emerges shows Septin-7 as a fundamental brick in both muscle construction, development, and regeneration; all this leads to reinforcing the basically structural nature of this protein role. -

Reviewer #1 (Public Review):
Here the authors aimed to gain insight into the role of Septin-7 in skeletal muscle biology using a novel and powerful mouse model of inducible muscle specific septin-7 deletion. They combine this with CRISPR/Cas9 and shRNA mediated manipulation of Septin-7 in C2C12 cells in vitro to explore its role in muscle progenitor morphology and proliferation. There are a variety of interesting observations, with clear phenotypes induced by the Septin-7 manipulation, including effects on body weight, muscle force production, mitochondrial morphology, and cell proliferation. However each area is somewhat superficially examined, and certain conclusions require additional validation for robust support. Additionally, mechanistic insight into Septin 7's role is limited. Therefore, while the phenotypes are likely of …
Reviewer #1 (Public Review):
Here the authors aimed to gain insight into the role of Septin-7 in skeletal muscle biology using a novel and powerful mouse model of inducible muscle specific septin-7 deletion. They combine this with CRISPR/Cas9 and shRNA mediated manipulation of Septin-7 in C2C12 cells in vitro to explore its role in muscle progenitor morphology and proliferation. There are a variety of interesting observations, with clear phenotypes induced by the Septin-7 manipulation, including effects on body weight, muscle force production, mitochondrial morphology, and cell proliferation. However each area is somewhat superficially examined, and certain conclusions require additional validation for robust support. Additionally, mechanistic insight into Septin 7's role is limited. Therefore, while the phenotypes are likely of intrigue to both the muscle and septin community, to significantly advance the field will require additional experimentation.
Specifically, it is currently difficult to distinguish between developmental and adult roles of Septin-7. The authors induce tamoxifen-mediated deletion at 1 month of age and examine muscle structure/function only at 4 months. By not studying early time points, it is difficult to determine whether particular phenotypes are directly due to Septin deletion or a secondary consequence of muscle atrophy and/or a decline in body weight. Further, by not inducing deletion at a later time point (i.e. after 2 months when muscle is generally matured), it is difficult to assess whether septin-7 plays a role in maintaining structure and function of mature muscle, or if its primary role is in muscle development.
Further, the conclusion that septin-7 has an essential role in regeneration (seemingly based on expression increasing after injury) is unsupported and requires further experimentation where injury and regeneration is triggered in the absence of Septin-7 to establish a causative role.
Finally, there are intriguing observations in mitochondrial and myofiber organization and mitochondrial content; however further interrogation into additional relevant metrics of each, and at different time points of Septin-7 deletion, are needed to better understand these phenotypes and gain insight into Septin-7's role in their regulation.
-