Host casein kinase 1-mediated phosphorylation modulates phase separation of a rhabdovirus phosphoprotein and virus infection
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Recently liquid-liquid phase separation (LLPS) has emerged as a mechanism by which membraneless compartments are formed inside the cells to compartmentalize biomolecules. In this paper, the authors show that the P protein from a plant-infecting negative sense RNA virus undergoes LLPS to promote virus replication. The host casein kinase 1 phosphorylates P protein and inhibits phase separation and viral replication. This paper will be of interest to virologists and researchers who study LLPS.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Liquid-liquid phase separation (LLPS) plays important roles in forming cellular membraneless organelles. However, how host factors regulate LLPS of viral proteins during negative-sense RNA (NSR) virus infection is largely unknown. Here, we used barley yellow striate mosaic virus (BYSMV) as a model to demonstrate regulation of host casein kinase 1 (CK1) in phase separation and infection of NSR viruses. We first found that the BYSMV phosphoprotein (P) formed spherical granules with liquid properties and recruited viral nucleotide (N) and polymerase (L) proteins in vivo. Moreover, the P-formed granules were tethered to the ER/actin network for trafficking and fusion. BYSMV P alone formed droplets and incorporated the N protein and the 5′ trailer of genomic RNA in vitro. Interestingly, phase separation of BYSMV P was inhibited by host CK1-dependent phosphorylation of an intrinsically disordered P protein region. Genetic assays demonstrated that the unphosphorylated mutant of BYSMV P exhibited condensed phase, which promoted viroplasm formation and virus replication. Whereas, the phosphorylation-mimic mutant existed in diffuse phase state for virus transcription. Collectively, our results demonstrate that host CK1 modulates phase separation of the viral P protein and virus infection.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
In Figure 1A, the authors should show TEM images of control mock treated samples to show the difference between infected and healthy tissue. Based on the data shown in Figure 1B-E that the overexpression of GFP-P in N. benthamiana leads to formation of liquid-like granules. Does this occur during virus infection? Since authors have infectious clones, can it be used to show that the virally encoded P protein in infected cells does indeed exist as liquid-like granules? If the fusion of GFP to P protein affects its function, the authors could fuse just the spGFP11 and co-infiltrate with p35S-spGFP1-10. These experiments will show that the P protein when delivered from virus does indeed form liquid-like granules in plants cells. Authors should include controls in Figure 1H to show that the …
Author Response
Reviewer #1 (Public Review):
In Figure 1A, the authors should show TEM images of control mock treated samples to show the difference between infected and healthy tissue. Based on the data shown in Figure 1B-E that the overexpression of GFP-P in N. benthamiana leads to formation of liquid-like granules. Does this occur during virus infection? Since authors have infectious clones, can it be used to show that the virally encoded P protein in infected cells does indeed exist as liquid-like granules? If the fusion of GFP to P protein affects its function, the authors could fuse just the spGFP11 and co-infiltrate with p35S-spGFP1-10. These experiments will show that the P protein when delivered from virus does indeed form liquid-like granules in plants cells. Authors should include controls in Figure 1H to show that the interaction between P protein and ER is specific.
We agree with the reviewer and appreciate the helpful suggestion. As suggested, we added TEM images of control mock treated barley leaves. We also carried out immune-electron microscope to show the presence of BYSMV P protein in the viroplasms. Please see Figure 1–Figure supplement 1.
BYSMV is a negative-stranded RNA virus, and is strictly dependent on insect vector transmission for infecting barley plants. We have tried to fuse GFP to BYSMV P in the full-length infectious clones. Unfortunately, we could not rescue BYSMV-GFP-P into barley plants through insect transmission.
In Figure 1H, we used a PM localized membrane protein LRR84A as a negative control to show LRR84A-GS and BYSMV P could not form granules although they might associate at molecular distances. Therefore, the P granules were formed and tethered to the ER tubules. Please see Figure 1–Figure supplement 4
Data shown in Figure 2 do demonstrate that the purified P protein could undergo phase separation. Furthermore, it can recruit viral N protein and part of viral genomic RNA to P protein induced granules in vitro.
Because the full-length BYSMV RNA has 12,706 nt and is difficult to be transcribed in vitro, we cannot show whether the BYSMV genome is recruited into the droplets. We have softened the claim and state that the P-N droplets can recruit 5′ trailer of BYSMV genome as shown in Figure 3B. Please see line 22, 177 and 190.
Based on the data shown in Figure 4 using phospho-null and phospho-mimetic mutants of P protein, the authors conclude that phosphorylation inhibits P protein phase separation. It is unclear based on the experiments, why endogenous NbCK1 fails to phosphorylate GFP-P-WT and inhibit formation of liquid-like granules similar to that of GFP-P-S5D mutant? Is this due to overexpression of GFP-P-WT? To overcome this, the authors should perform these experiments as suggested above using infectious clones and these P protein mutants.
As we known, phosphorylation and dephosphorylation are reversible processes in eukaryotic cells. Therefore, as shown in Figure 5B and 6B, the GFP-PWT protein have two bands, corresponding to P74 and P72, which represent hyperphosphorylation and hypophosphorylated forms, respectively. Only overexpression of NbCK1 induced high ratio of P74 to P72 in vivo, and then abolished phase separation of BYSMV.
In Figure 5, the authors overexpress NbCK1 in N. benthamiana or use an in vitro co-purification scheme to show that NbCK1 inhibits phase separation properties of P protein. These results show that overexpression of both GFP-P and NbCK1 proteins is required to induce liquid-like granules. Does this occur during normal virus infection? During normal virus infection, P protein is produced in the plant cells and the endogenous NbCK1 will regulate the phosphorylation state of P protein. These are reasons for authors to perform some of the experiments using infectious clones. Furthermore, the authors have antibodies to P protein and this could be used to show the level of P protein that is produced during the normal infection process.
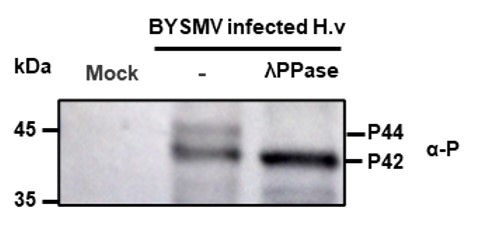
We detected the P protein existed as two phosphorylation forms in BYSMV-infected barley leaves, and λPPase treatment decreased the P44 phosphorylation form. Therefore, these results indicate that endogenous CK1 cannot phosphorylate BYSMV P completely.
Based on the data shown in Figure 6, the authors conclude that phase separated P protein state promotes replication but inhibits transcription by overexpressing P-S5A and P-S5D mutants. To directly show that the NbCK1 controlled phosphorylation state of P regulates this process, authors should knockdown/knockout NbCK1 and see if it increases P protein condensates and promote recruitment of viral proteins and genomic RNA to increase viral replication.
In our previous studies, BLAST searches showed that the N. benthamiana and barley genomes encode 14 CK1 orthologs, most of which can phosphorylated the SR region of BYSMV P. Therefore, it is difficult to make knockdown/knockout lines of all the CK1 orthologues. Accordingly, we generated a point mutant (K38R and D128N) in HvCK1.2, in which the kinase activity was abolished. Overexpression of HvCK1.2DN inhibit endogenous CK1-mediated phosphorylation of BYSMV P, indicating that HvCK1.2DN is a dominant-negative mutant.
It is important to note that both replication and transcription are required for efficient infection of negative-stranded RNA viruses. Therefore, our previous studies have revealed that both PS5A and PS5D are required for BYSMV infection. Therefore, expression of HvCK1.2DN in BYSMV vector inhibit virus infection by impairing the balance of endogenous CK1-mediated phosphorylation in BYSMV P.
Reviewer #2 (Public Review):
The manuscript by Fang et al. details the ability of the P protein from Barley yellow striate mosaic virus (BYSMV) to form phase-separated droplets both in vitro and in vivo. The authors demonstrate P droplet formation using recombinant proteins and confocal microscopy, FRAP to demonstrate fluidity, and observed droplet fusion. The authors also used an elaborate split-GFP system to demonstrate that P droplets associate with the tubulur ER network. Next, the authors demonstrate that the N protein and a short fragment of viral RNA can also partition into P droplets. Since Rhabdovirus P proteins have been shown to phase separate and form "virus factories" (see https://doi.org/10.1038/s41467-017-00102-9), the novelty from this work is the rigorous and conclusive demonstration that the P droplets only exist in the unphosphorylated form. The authors identify 5 critical serine residues in IDR2 of P protein that when hyper-phosphorylated /cannot form droplets. Next, the authors conclusively demonstrate that the host kinase CK1 is responsible for P phosphorylation using both transient assays in N. benthamiana and a co-expression assay in E. coli. These findings will likely lead to future studies identifying cellular kinases that affect phase separation of viral and cellular proteins and increases our understanding of regulation of condensate formation. Next, the authors investigated whether P droplets regulated virus replication and transcription using a minireplicon system. The minireplicon system needs to be better described as the results were seemingly conflicting. The authors also used a full-length GFP-reporter virus to test whether phase separation was critical for virus fitness in both barley and the insect vector. The authors used 1, 6-hexanediol which broadly suppresses liquid-liquid phase separation and concluded that phase separation is required for virus fitness (based on reduced virus accumulation with 1,6 HD). However, this conclusion is flawed since 1,6-hexanediol is known to cause cell toxicity and likely created a less favorable environment for virus replication, independent of P protein phase separation. These with other issues are detailed below:
- In Figure 3B, the authors display three types of P-N droplets including uniform, N hollow, and P-N hollow droplets. The authors do not state the proportion of droplets observed or any potential significance of the three types. Finally, as "hollow" droplets are not typically observed, is there a possibility that a contaminating protein (not fluorescent) from E. coli is a resident client protein in these droplets? The protein purity was not >95% based on the SDS-PAGE gels presented in the supplementary figures. Do these abnormalities arise from the droplets being imaged in different focal planes? Unless some explanation is given for these observations, this reviewer does not see any significance in the findings pertaining to "hollow" droplets.
Thanks for your constructive suggestions. We removed the "hollow" droplets as suggested. We think that the hollow droplets might be an intermediate form of LLPS. Please see PAGE 7 and 8 of revised manuscript.
- Pertaining to the sorting of "genomic" RNA into the P-N droplets, it is unlikely that RNA sorting is specific for BYSMV RNA. In other words, if you incubate a non-viral RNA with P-N droplets, is it sorted? The authors conclusion that genomic RNA is incorporated into droplets is misleading in a sense that a very small fragment of RNA was used. Cy5 can be incorporated into full-length genomic RNAs during in vitro transcription and would be a more suitable approach for the conclusions reached.
Thanks for your constructive suggestions. Unfortunately, we could not obtain the in vitro transcripts of the full-length genomic RNAs (12706 nucleotides). We have softened the claim and state that the P-N droplets can recruit the 5′ trailer of BYSMV genome as shown in Figure 3B. Please see line 22, 177 and 190.
According to previous studies (Ivanov, et al., 2011), the Rhabdovirus P protein can bind to nascent N moleculaes, forming a soluble N/P complex, to prevent from encapsidating cellular RNAs. Therefore, we suppose that the P-N droplets can incorporate viral genomic RNA specifically.
Reference: Ivanov I, Yabukarski F, Ruigrok RW, Jamin M. 2011. Structural insights into the rhabdovirus transcription/ replication complex. Virus Research 162:126–137. DOI: https://doi.org/10.1016/j.virusres.2011.09.025
- In Figure 4C, it is unclear how the "views" were selected for granule counting. The methods should be better described as this reviewer would find it difficult to select fields of view in an unbiased manner. This is especially true as expression via agroinfiltration can vary between cells in agroinfiltrated regions. The methods described for granule counting and granule sizes are not suitable for publication. These should be expanded (i.e. what ImageJ tools were used?).
We agree with the reviewer that it is important to select fields of view in an unbiased manner. We selected the representative views and provided large views in the new Supplement Figures. In addition, we added new detail methods in revision. Please see Figure 4–Figure supplement 1, Figure 5–Figure supplement 1, and method (line 489-498).
- In Figure 4F, the authors state that they expected P-S5A to only be present in the pellet fraction since it existed in the condensed state. However, WT P also forms condensates and was not found in the pellet, but rather exclusively in the supernatant. Therefore, the assumption of condensed droplets only being found in the pellet appears to be incorrect.
Many thanks for pointing this out. This method is based on a previous study (Hubstenberger et al., 2017). The centrifugation method might efficiently precipitate large granules more than small granules. As shown in Figure 4B, GFP-PS5A formed large granules, therefore GFP-PS5A mainly existed in the pellet. In contrast, GFP-PWT only existed in small granule and fusion state, thus most of GFP-PWT protein was existed in supernatant, and only little GFP-PWT protein in the pellet. These results also indicate the increased phase separation activity of GFP-PS5A compared with GFP-PWT. Please see the new Figure 4F.
Reference: Hubstenberger A, Courel M, Benard M, Souquere S, Ernoult-Lange M, Chouaib R, Yi Z, Morlot JB, Munier A, Fradet M, et al. 2017. P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Molecular Cell 68(1): 144-157 e145.
- The authors conclude that P-S5A has enhanced phase separation based on confocal microscopy data (Fig S6A). The data presented is not convincing. Microscopy alone is difficult for comparing phase separation between two proteins. Quantitative data should be collected in the form of turbidity assays (a common assay for phase separation). If P-S5A has enhanced phase separation compared to WT, then S5A should have increased turbidity (OD600) under identical phase separation conditions. The microscopy data presented was not quantified in any way and the authors could have picked fields of view in a biased manner.
Thanks for your constructive suggestions. As suggested, turbidity assays were performed to show both GFP-PWT and GFP-PS5A had increased turbidity (OD600) compared with GFP. Please see Figure 4–Figure supplement 3.
- The authors constructed minireplicons to determine whether mutant P proteins influence RNA replication using trans N and L proteins. However, this reviewer finds the minireplicon design confusing. How is DsRFP translated from the replicon? If a frameshift mutation was introduced into RsGFP, wouldn't this block DsRFP translation as well? Or is start/stop transcription used? Second, the use of the 2x35S promoter makes it difficult to differentiate between 35S-driven transcription and replication by L. How do you know the increased DsRFP observed with P5A is not due to increased transcription from the 35S promoter? The RT-qPCR data is also very confusing. It is not clear that panel D is only examining the transcription of RFP (I assume via start/stop transcription) whereas panel C is targeting the minireplicon.
Thank you for your questions and we are sorry for the lack of clarity regarding to the mini-replicon vectors. Here, we updated the Figure supplement 14 to show replication and transcription of BYSMV minireplicon, a negative-stranded RNA virus derivative. In addition, we insert an A after the start codon to abolish the translation of GFP mRNA, which allow us to observe phase separation of GFP-PWT, GFP-PS5A, and GFP-PS5D during virus replication. Use this system, we wanted to show the localization and phase separation of GFP-PWT, GFP-PS5A, and GFP-PS5D during replication and transcription of BYS-agMR. Please see Figure 6–Figure supplement 1.
- Pertaining to the replication assay in Fig. 6, transcription of RFP mRNA was reduced by S5A and increased by S5D. However, the RFP translation (via Panel A microscopy) is reversed. How do you explain increased RFP mRNA transcription by S5D but very low RFP fluorescence? The data between Panels A, C, and D do not support one another.
Many thanks for pointing this out! We also noticed the interesting results that have been repeated independently. As shown the illustration of BYSMV-agMR system in Figure 6–Figure supplement 1, the relative transcriptional activities of different GFP-P mutants were calculated from the normalized RFP transcript levels relative to the gMR replicate template (RFP mRNA/gMR), because replicating minigenomes are templates for viral transcription.
Since GFP-PS5D supported decreased replication, the ratio of RFP mRNA/gMR increased although the RFP mRNA of GFP-PS5D is not increased. In addition, the foci number of GFP-PS5D is much less than GFP-PWT and GFP-PS5A, indicating mRNAs in GFP-PS5D samples may contain aberrant transcripts those cannot be translated the RFP protein. In contrast, mRNAs in GFP-PS5A samples are translated efficiently. These results were in consistent with our previous studies using the free PWT, PS5A, and PS5D.
Reference: Gao Q, et al. 2020. Casein kinase 1 regulates cytorhabdovirus replication and transcription by phosphorylating a phosphoprotein serine-rich motif. The Plant Cell 32(9): 2878-2897.
- The authors relied on 1,6-hexanediol to suppress phase separation in both insect vectors and barley. However, the authors disregarded several publications demonstrating cellular toxicity by 1,6-hexanediol and a report that 1,6-HD impairs kinase and phosphatase activities (see below). doi: 10.1016/j.jbc.2021.100260,
We agree with the reviewer that 1, 6-hexanediol induced cellular toxicity. Therefore, we removed these results, which does not affect the main conclusion of our results.
- The authors state that reduced accumulation of BYSMV-GFP in insects and barley under HEX treatment "indicate that phase separation is important for cross-kingdom infection of BYSMV in insect vectors and host plants." The above statement is confounded by many factors, the most obvious being that HEX treatment is most likely toxic to cells and as a result cannot support efficient virus accumulation. Also, since HEX treatment interferes with phosphorylation (see REF above) its use here should be avoided since P phase separation is regulated by phosphorylation.
We agree with the reviewer that 1, 6-hexanediol induced cellular toxicity and hereby affected infections of BYSMV and other viruses. In addition, 1, 6-hexanediol would inhibit LLPS of cellular membraneless organelles, such as P-bodies, stress granules, cajal bodies, and the nucleolus, which also affect different virus infections directly or indirectly. Therefore, we removed these results, which does not affect the main conclusion of our results.
Reviewer #3 (Public Review):
Membrane-less organelles formed through liquid-liquid phase separation (LLPS) provide spatiotemporal control of host immunity responses and other cellular processes. Viruses are obligate pathogens proliferating in host cells which lead their RNAs and proteins are more likely to be targeted by immune-related membrane-less organelles. To successfully infect and proliferate in host cells, virus need to efficiently suppressing the immune function of those immune-related membrane-less organelles. Moreover, viruses also generate exogenous membrane-less organelles/RNA granules to facilitate their proliferation. Accordingly, host cells also need to target and suppress the functions of exogenous membrane-less organelles/RNA granules generated by viruses, the underlying mechanisms of which are still mysterious.
In this study, Fang et al. investigated how plant kinase confers resistance against viruses via modulating the phosphorylation and phase separation of BYSMV P protein. They firstly characterized the phase separation feature of BYSMV P protein. They also discovered that droplets formed by P protein recruit viral RNA and other viral protein in vivo. The phase separation activity of P protein is inhibited by the phosphorylation on its intrinsically disordered region. Combined with their previous study, this study demonstrated that host casein kinase (CK1) decreases the phase separation of P protein via increasing the phosphorylation of P protein. Finally, the author claimed that the phase separation of P protein facilitates BYSMV replication but decreases its transcription. Taking together, this study uncovered the molecular mechanism of plant regulating viral proliferation via decreasing the formation of exogenous RNA granules/membraneless organelles. Overall, this paper tells an interesting story about the host immunity targeting viruses via modulating the dynamics of exogenous membraneless organelles, and uncovers the modulation of viral protein phase separation by host protein, which is a hotspot in plant immunity, and the writing is logical.
Thanks for your positive comment on our studies.
-

Evaluation Summary:
Recently liquid-liquid phase separation (LLPS) has emerged as a mechanism by which membraneless compartments are formed inside the cells to compartmentalize biomolecules. In this paper, the authors show that the P protein from a plant-infecting negative sense RNA virus undergoes LLPS to promote virus replication. The host casein kinase 1 phosphorylates P protein and inhibits phase separation and viral replication. This paper will be of interest to virologists and researchers who study LLPS.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
In Figure 1A, the authors should show TEM images of control mock treated samples to show the difference between infected and healthy tissue. Based on the data shown in Figure 1B-E that the overexpression of GFP-P in N. benthamiana leads to formation of liquid-like granules. Does this occur during virus infection? Since authors have infectious clones, can it be used to show that the virally encoded P protein in infected cells does indeed exist as liquid-like granules? If the fusion of GFP to P protein affects its function, the authors could fuse just the spGFP11 and co-infiltrate with p35S-spGFP1-10. These experiments will show that the P protein when delivered from virus does indeed form liquid-like granules in plants cells. Authors should include controls in Figure 1H to show that the interaction between P …
Reviewer #1 (Public Review):
In Figure 1A, the authors should show TEM images of control mock treated samples to show the difference between infected and healthy tissue. Based on the data shown in Figure 1B-E that the overexpression of GFP-P in N. benthamiana leads to formation of liquid-like granules. Does this occur during virus infection? Since authors have infectious clones, can it be used to show that the virally encoded P protein in infected cells does indeed exist as liquid-like granules? If the fusion of GFP to P protein affects its function, the authors could fuse just the spGFP11 and co-infiltrate with p35S-spGFP1-10. These experiments will show that the P protein when delivered from virus does indeed form liquid-like granules in plants cells. Authors should include controls in Figure 1H to show that the interaction between P protein and ER is specific.
Data shown in Figure 2 do demonstrate that the purified P protein could undergo phase separation. Furthermore, it can recruit viral N protein and part of viral genomic RNA to P protein induced granules in vitro.
Based on the data shown in Figure 4 using phospho-null and phospho-mimetic mutants of P protein, the authors conclude that phosphorylation inhibits P protein phase separation. It is unclear based on the experiments, why endogenous NbCK1 fails to phosphorylate GFP-P-WT and inhibit formation of liquid-like granules similar to that of GFP-P-S5D mutant? Is this due to overexpression of GFP-P-WT? To overcome this, the authors should perform these experiments as suggested above using infectious clones and these P protein mutants.
In Figure 5, the authors overexpress NbCK1 in N. benthamiana or use an in vitro co-purification scheme to show that NbCK1 inhibits phase separation properties of P protein. These results show that overexpression of both GFP-P and NbCK1 proteins is required to induce liquid-like granules. Does this occur during normal virus infection? During normal virus infection, P protein is produced in the plant cells and the endogenous NbCK1 will regulate the phosphorylation state of P protein. These are reasons for authors to perform some of the experiments using infectious clones. Furthermore, the authors have antibodies to P protein and this could be used to show the level of P protein that is produced during the normal infection process.
Based on the data shown in Figure 6, the authors conclude that phase separated P protein state promotes replication but inhibits transcription by overexpressing P-S5A and P-S5D mutants. To directly show that the NbCK1 controlled phosphorylation state of P regulates this process, authors should knockdown/knockout NbCK1 and see if it increases P protein condensates and promote recruitment of viral proteins and genomic RNA to increase viral replication.
-

Reviewer #2 (Public Review):
The manuscript by Fang et al. details the ability of the P protein from Barley yellow striate mosaic virus (BYSMV) to form phase-separated droplets both in vitro and in vivo. The authors demonstrate P droplet formation using recombinant proteins and confocal microscopy, FRAP to demonstrate fluidity, and observed droplet fusion. The authors also used an elaborate split-GFP system to demonstrate that P droplets associate with the tubulur ER network. Next, the authors demonstrate that the N protein and a short fragment of viral RNA can also partition into P droplets. Since Rhabdovirus P proteins have been shown to phase separate and form "virus factories" (see https://doi.org/10.1038/s41467-017-00102-9), the novelty from this work is the rigorous and conclusive demonstration that the P droplets only exist in …
Reviewer #2 (Public Review):
The manuscript by Fang et al. details the ability of the P protein from Barley yellow striate mosaic virus (BYSMV) to form phase-separated droplets both in vitro and in vivo. The authors demonstrate P droplet formation using recombinant proteins and confocal microscopy, FRAP to demonstrate fluidity, and observed droplet fusion. The authors also used an elaborate split-GFP system to demonstrate that P droplets associate with the tubulur ER network. Next, the authors demonstrate that the N protein and a short fragment of viral RNA can also partition into P droplets. Since Rhabdovirus P proteins have been shown to phase separate and form "virus factories" (see https://doi.org/10.1038/s41467-017-00102-9), the novelty from this work is the rigorous and conclusive demonstration that the P droplets only exist in the unphosphorylated form. The authors identify 5 critical serine residues in IDR2 of P protein that when hyper-phosphorylated cannot form droplets. Next, the authors conclusively demonstrate that the host kinase CK1 is responsible for P phosphorylation using both transient assays in N. benthamiana and a co-expression assay in E. coli. These findings will likely lead to future studies identifying cellular kinases that affect phase separation of viral and cellular proteins and increases our understanding of regulation of condensate formation. Next, the authors investigated whether P droplets regulated virus replication and transcription using a minireplicon system. The minireplicon system needs to be better described as the results were seemingly conflicting. The authors also used a full-length GFP-reporter virus to test whether phase separation was critical for virus fitness in both barley and the insect vector. The authors used 1,6-hexanediol which broadly suppresses liquid-liquid phase separation and concluded that phase separation is required for virus fitness (based on reduced virus accumulation with 1,6 HD). However, this conclusion is flawed since 1,6-hexanediol is known to cause cell toxicity and likely created a less favorable environment for virus replication, independent of P protein phase separation. These with other issues are detailed below:
1. In Figure 3B, the authors display three types of P-N droplets including uniform, N hollow, and P-N hollow droplets. The authors do not state the proportion of droplets observed or any potential significance of the three types. Finally, as "hollow" droplets are not typically observed, is there a possibility that a contaminating protein (not fluorescent) from E. coli is a resident client protein in these droplets? The protein purity was not >95% based on the SDS-PAGE gels presented in the supplementary figures. Do these abnormalities arise from the droplets being imaged in different focal planes? Unless some explanation is given for these observations, this reviewer does not see any significance in the findings pertaining to "hollow" droplets.
2. Pertaining to the sorting of "genomic" RNA into the P-N droplets, it is unlikely that RNA sorting is specific for BYSMV RNA. In other words, if you incubate a non-viral RNA with P-N droplets, is it sorted? The authors conclusion that genomic RNA is incorporated into droplets is misleading in a sense that a very small fragment of RNA was used. Cy5 can be incorporated into full-length genomic RNAs during in vitro transcription and would be a more suitable approach for the conclusions reached.
3. In Figure 4C, it is unclear how the "views" were selected for granule counting. The methods should be better described as this reviewer would find it difficult to select fields of view in an unbiased manner. This is especially true as expression via agroinfiltration can vary between cells in agroinfiltrated regions. The methods described for granule counting and granule sizes should be expanded (i.e. what ImageJ tools were used?).
4. In Figure 4F, the authors state that they expected P-S5A to only be present in the pellet fraction since it existed in the condensed state. However, WT P also forms condensates and was not found in the pellet, but rather exclusively in the supernatant. Therefore, the assumption of condensed droplets only being found in the pellet appears to be incorrect.
5. The authors conclude that P-S5A has enhanced phase separation based on confocal microscopy data (Fig S6A). The data presented is not convincing. Microscopy alone is difficult for comparing phase separation between two proteins. Quantitative data should be collected in the form of turbidity assays (a common assay for phase separation). If P-S5A has enhanced phase separation compared to WT, then S5A should have increased turbidity (OD600) under identical phase separation conditions. The microscopy data presented was not quantified in any way and the authors could have picked fields of view in a biased manner.
6. The authors constructed minireplicons to determine whether mutant P proteins influence RNA replication using trans N and L proteins. However, this reviewer finds the minireplicon design confusing. How is DsRFP translated from the replicon? If a frameshift mutation was introduced into RsGFP, wouldn't this block DsRFP translation as well? Or is start/stop transcription used? Second, the use of the 2x35S promoter makes it difficult to differentiate between 35S-driven transcription and replication by L. How do you know the increased DsRFP observed with P5A is not due to increased transcription from the 35S promoter? The RT-qPCR data is also very confusing. It is not clear that panel D is only examining the transcription of RFP (I assume via start/stop transcription) whereas panel C is targeting the minireplicon.
7. Pertaining to the replication assay in Fig. 6, transcription of RFP mRNA was reduced by S5A and increased by S5D. However, the RFP translation (via Panel A microscopy) is reversed. How do you explain increased RFP mRNA transcription by S5D but very low RFP fluorescence? The data between Panels A, C, and D do not support one another.
8. The authors relied on 1,6-hexanediol to suppress phase separation in both insect vectors and barley. However, the authors disregarded several publications demonstrating cellular toxicity by 1,6-hexanediol and a report that 1,6-HD impairs kinase and phosphatase activities (see below). doi: 10.1016/j.jbc.2021.100260, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6445271/
9. The authors state that reduced accumulation of BYSMV-GFP in insects and barley under HEX treatment "indicate that phase separation is important for cross-kingdom infection of BYSMV in insect vectors and host plants."
The above statement is confounded by many factors, the most obvious being that HEX treatment is most likely toxic to cells and as a result cannot support efficient virus accumulation. Also, since HEX treatment interferes with phosphorylation (see REF above) its use here should be avoided since P phase separation is regulated by phosphorylation.
-

Reviewer #3 (Public Review):
Membrane-less organelles formed through liquid-liquid phase separation (LLPS) provide spatiotemporal control of host immunity responses and other cellular processes. Viruses are obligate pathogens proliferating in host cells which lead their RNAs and proteins are more likely to be targeted by immune-related membrane-less organelles. To successfully infect and proliferate in host cells, virus need to efficiently suppressing the immune function of those immune-related membrane-less organelles. Moreover, viruses also generate exogenous membrane-less organelles/RNA granules to facilitate their proliferation. Accordingly, host cells also need to target and suppress the functions of exogenous membrane-less organelles/RNA granules generated by viruses, the underlying mechanisms of which are still mysterious.
In …
Reviewer #3 (Public Review):
Membrane-less organelles formed through liquid-liquid phase separation (LLPS) provide spatiotemporal control of host immunity responses and other cellular processes. Viruses are obligate pathogens proliferating in host cells which lead their RNAs and proteins are more likely to be targeted by immune-related membrane-less organelles. To successfully infect and proliferate in host cells, virus need to efficiently suppressing the immune function of those immune-related membrane-less organelles. Moreover, viruses also generate exogenous membrane-less organelles/RNA granules to facilitate their proliferation. Accordingly, host cells also need to target and suppress the functions of exogenous membrane-less organelles/RNA granules generated by viruses, the underlying mechanisms of which are still mysterious.
In this study, Fang et al. investigated how plant kinase confers resistance against viruses via modulating the phosphorylation and phase separation of BYSMV P protein. They firstly characterized the phase separation feature of BYSMV P protein. They also discovered that droplets formed by P protein recruit viral RNA and other viral protein in vivo. The phase separation activity of P protein is inhibited by the phosphorylation on its intrinsically disordered region. Combined with their previous study, this study demonstrated that host casein kinase (CK1) decreases the phase separation of P protein via increasing the phosphorylation of P protein. Finally, the author claimed that the phase separation of P protein facilitates BYSMV replication but decreases its transcription. Taking together, this study uncovered the molecular mechanism of plant regulating viral proliferation via decreasing the formation of exogenous RNA granules/membraneless organelles. Overall, this paper tells an interesting story about the host immunity targeting viruses via modulating the dynamics of exogenous membraneless organelles, and uncovers the modulation of viral protein phase separation by host protein, which is a hotspot in plant immunity, and the writing is logical.
-