YAP1 activation by human papillomavirus E7 promotes basal cell identity in squamous epithelia
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The oncogenic virus Human Papillomavirus encodes the E7 protein which is an important contributor to carcinogenesis. Here, the authors show that E7-mediated degradation of the cellular tumor suppressor PTPN14 leads to activation of YAP1 in basal cells of the stratified squamous epithelium. They show that the ability of E7 to extend the lifespan of keratinocytes and facilitate basal cell retention are both activities mediated by the basal-cell specific activation of YAP1 and conclude that this newly discovered function of HPV E7 contributes to its carcinogenic properties. This report will be of great interest for researchers in the HPV and epithelial differentiation fields.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Persistent human papillomavirus (HPV) infection of stratified squamous epithelial cells causes nearly 5% of cancer cases worldwide. HPV-positive oropharyngeal cancers harbor few mutations in the Hippo signaling pathway compared to HPV-negative cancers at the same anatomical site, prompting the hypothesis that an HPV-encoded protein inactivates the Hippo pathway and activates the Hippo effector yes-associated protein (YAP1). The HPV E7 oncoprotein is required for HPV infection and for HPV-mediated oncogenic transformation. We investigated the effects of HPV oncoproteins on YAP1 and found that E7 activates YAP1, promoting YAP1 nuclear localization in basal epithelial cells. YAP1 activation by HPV E7 required that E7 binds and degrades the tumor suppressor protein tyrosine phosphatase non-receptor type 14 (PTPN14). E7 required YAP1 transcriptional activity to extend the lifespan of primary keratinocytes, indicating that YAP1 activation contributes to E7 carcinogenic activity. Maintaining infection in basal cells is critical for HPV persistence, and here we demonstrate that YAP1 activation causes HPV E7 expressing cells to be retained in the basal compartment of stratified epithelia. We propose that YAP1 activation resulting from PTPN14 inactivation is an essential, targetable activity of the HPV E7 oncoprotein relevant to HPV infection and carcinogenesis.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
This manuscript elegantly demonstrates that the degradation of PTPN14 by human papillomavirus (HPV) 16 and 18 E7 proteins previously reported by the authors is essential for E7-mediated YAP1 activation. This is important for E7-mediated maintenance of basal cell state and presumably persistence of HPV infection. The authors use a series of innovative tissue models combined with validation in clinical samples to demonstrate the importance of YAP1 activation in high-risk HPV pathogenesis.
The data are of high quality with excellent controls. The manuscript is well-written and the rationale of each experiment easy to follow. In general the results support the authors conclusions. I have the following suggestion to improve the manuscript: The enhanced nuclear expression of YAP in the basal …
Author Response:
Reviewer #1 (Public Review):
This manuscript elegantly demonstrates that the degradation of PTPN14 by human papillomavirus (HPV) 16 and 18 E7 proteins previously reported by the authors is essential for E7-mediated YAP1 activation. This is important for E7-mediated maintenance of basal cell state and presumably persistence of HPV infection. The authors use a series of innovative tissue models combined with validation in clinical samples to demonstrate the importance of YAP1 activation in high-risk HPV pathogenesis.
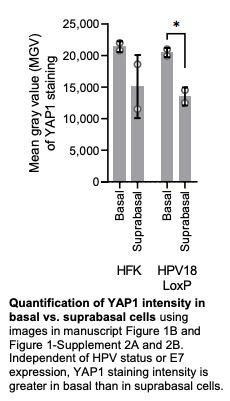
The data are of high quality with excellent controls. The manuscript is well-written and the rationale of each experiment easy to follow. In general the results support the authors conclusions. I have the following suggestion to improve the manuscript: The enhanced nuclear expression of YAP in the basal cells of epithelia expressing HPV16/18 E7 is difficult to see in the low resolution IF images shown. The magnified images do show enhanced expression compared to HFK cultures, but to remove any bias in selection of enhanced areas, could the authors include quantification of the distribution of IF signal in the basal cells, compared to the suprabasal cells, of the epithelia shown with statistical analysis? Figure 2 would also benefit from quantification as described above.
We appreciate the positive feedback and constructive suggestions from Reviewer #1. We used widefield images with the goal of presenting as many cells in organotypic cultures as possible, but at low magnification. We have further analyzed the imaging data and updated the manuscript as follows:
We assessed YAP1 intensity in basal and suprabasal layers as suggested by the reviewer. Consistent with literature reports, YAP1 is expressed predominantly in basal cells in each of our organotypic cultures, independent of E7 status (see figure below).
Because YAP1 is always more highly expressed in basal cells than in suprabasal cells and YAP1 is regulated at the level of nuclear/cytoplasmic localization, we anticipated that quantification of YAP1 nuclear localization in our organotypic cultures may be more useful to readers than basal/suprabasal quantification.
Consequently, we conducted classification-based analyses to quantify YAP1 nuclear localization (a surrogate for YAP1 activity) in the cultures. Each image to be analyzed was deidentified and assigned a coded name. Each cell in the basal layer was then classified as having either predominantly nuclear YAP1 staining, predominantly cytoplasmic YAP1 staining, or YAP1 staining that is comparably distributed between the nucleus and cytoplasm. At least three fields were analyzed per raft. We assessed YAP1 localization in 8,323 cells (average 378.3 cells/culture shown in the text for almost all cultures). The quantifications are now included in Figure 1-figure supplement 2C-E, Figure 1-Figure supplement 5A-C, and Figure 2-figure supplement 1D-F.
The new quantifications do not change our interpretations of the results nor our conclusion that HPV E7 degrades PTPN14 to activate YAP1 in basal cells. We noted that HPV E6 may promote YAP1 nuclear localization to some degree and have updated the text accordingly.
Reviewer #2 (Public Review):
Strengths: A major strength of this report is the use of several different technical approaches, the results from which converge to provide several types of data supporting their conclusions. These various techniques include genetic knockdown/overexpression in primary keratinocytes, organotypic raft cultures, laser-capture microdissection, cell fate monitoring assays, and analysis of publicly available datasets. The manuscript is well-written and the figures are well-made. Weaknesses: Overall, there are only a few minor weaknesses related to figure quality and presentation (which will be conveyed in the private recommendations to the authors).
We appreciate the positive feedback and these thoughtful comments from reviewer #2.
Are claims/conclusions justified by data? Overall, the authors' conclusions are adequately justified by the data. However, there were a few interpretations I felt were somewhat overstated given the experiments performed and data provided.
- The first issue relates to the interpretation/conclusion of the results from experiments analyzing basal cell number. In Figure 2, the basal cell number was indeed reduced in R84S compared to WT E7. However, it was not reduced to parental HFK levels, suggesting other E7 activities are involved in increasing basal cell number. A similar observation is presented in Figure 7 (E-F), where the R84S E7 mutant still had significantly higher basal cell retention than the empty vector control, albeit lower than WT E7. While their data certainly indicates that the binding and subsequent degradation of PTPN14 is an E7 function important to increasing basal cell number and retention, there are clearly other E7 functions involved. While the authors don't necessarily overinterpret these findings, the possibility that other E7 functions are involved is not explicitly acknowledged or explored in the Discussion.
Indeed, cells expressing HPV18 E7 R84S retain some capacity to increase basal cell number (Figure 2) and promote basal cell retention (Figure 7). It is possible that an activity of HPV E7 in addition to PTPN14 degradation influences these phenotypes. HPV18 E7 R84S retains the capacity to bind and degrade RB1 (Hatterschide et al., 2020). The basal cells in the HPV18 E7 R84S cell fate experiment were predominantly found in clusters indicative of possible clonal expansion. We hypothesize that such clusters reflect proliferation induced by RB1 inactivation and cause the ratio of basal to suprabasal cells to remain high even in the R84S mutant condition. Our hypothesis is now described in further detail in the text.
- The second issue pertains to the findings related to the effect on differentiation upon modulation of key Hippo pathway components (Figure 4). It does not appear that the authors performed these studies in the presence of any well-known stimuli that induce the differentiation process in keratinocytes grown in 2D culture (high calcium, high serum, etc) nor did they use these cells in organotypic rafts wherein differentiation occurs during the raft stratification process. This is particularly true in the studies exploring PTPN14 plus LATS1/2 silencing and the effect on repression of keratinocyte differentiation. Whereas it seems PTPN14 itself was serving as the differentiation stimuli in earlier experiments (Figure 4C/D), it does not appear any differentiation stimuli were provided in the experiments shown in Figures 4E-I. For these reasons, the interpretation drawn by the authors that "...inactivation of three different YAP1 inhibitors dampens differentiation gene expression" (Line 220-221) and "inactivation of LATS1 or LATS2...also repressed differentiation genes" (Lines 349-350) seems specific to endogenous levels of differentiation genes. It seems difficult to conclude that inactivation of the Hippo pathway is actively repressing the induction of differentiation if the cells are not being treated with stimuli to induce differentiation.
Indeed, no differentiation stimuli were used in these experiments. We previously observed that PTPN14 knockout or E7 expression reduced differentiation gene expression both in undifferentiated cells and in cells stimulated to differentiate (Hatterschide et al., 2019, 2020). We anticipate that gene expression in unstimulated cells is reflective of gene expression in cells stimulated to differentiate. We altered the results and discussion text to emphasize that the experiment measures differentiation gene expression in unstimulated cells.
-

Evaluation Summary:
The oncogenic virus Human Papillomavirus encodes the E7 protein which is an important contributor to carcinogenesis. Here, the authors show that E7-mediated degradation of the cellular tumor suppressor PTPN14 leads to activation of YAP1 in basal cells of the stratified squamous epithelium. They show that the ability of E7 to extend the lifespan of keratinocytes and facilitate basal cell retention are both activities mediated by the basal-cell specific activation of YAP1 and conclude that this newly discovered function of HPV E7 contributes to its carcinogenic properties. This report will be of great interest for researchers in the HPV and epithelial differentiation fields.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with …
Evaluation Summary:
The oncogenic virus Human Papillomavirus encodes the E7 protein which is an important contributor to carcinogenesis. Here, the authors show that E7-mediated degradation of the cellular tumor suppressor PTPN14 leads to activation of YAP1 in basal cells of the stratified squamous epithelium. They show that the ability of E7 to extend the lifespan of keratinocytes and facilitate basal cell retention are both activities mediated by the basal-cell specific activation of YAP1 and conclude that this newly discovered function of HPV E7 contributes to its carcinogenic properties. This report will be of great interest for researchers in the HPV and epithelial differentiation fields.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This manuscript elegantly demonstrates that the degradation of PTPN14 by human papillomavirus (HPV) 16 and 18 E7 proteins previously reported by the authors is essential for E7-mediated YAP1 activation. This is important for E7-mediated maintenance of basal cell state and presumably persistence of HPV infection. The authors use a series of innovative tissue models combined with validation in clinical samples to demonstrate the importance of YAP1 activation in high-risk HPV pathogenesis.
The data are of high quality with excellent controls. The manuscript is well-written and the rationale of each experiment easy to follow. In general the results support the authors conclusions. I have the following suggestion to improve the manuscript:
The enhanced nuclear expression of YAP in the basal cells of epithelia …
Reviewer #1 (Public Review):
This manuscript elegantly demonstrates that the degradation of PTPN14 by human papillomavirus (HPV) 16 and 18 E7 proteins previously reported by the authors is essential for E7-mediated YAP1 activation. This is important for E7-mediated maintenance of basal cell state and presumably persistence of HPV infection. The authors use a series of innovative tissue models combined with validation in clinical samples to demonstrate the importance of YAP1 activation in high-risk HPV pathogenesis.
The data are of high quality with excellent controls. The manuscript is well-written and the rationale of each experiment easy to follow. In general the results support the authors conclusions. I have the following suggestion to improve the manuscript:
The enhanced nuclear expression of YAP in the basal cells of epithelia expressing HPV16/18 E7 is difficult to see in the low resolution IF images shown. The magnified images do show enhanced expression compared to HFK cultures, but to remove any bias in selection of enhanced areas, could the authors include quantification of the distribution of IF signal in the basal cells, compared to the suprabasal cells, of the epithelia shown with statistical analysis?
Figure 2 would also benefit from quantification as described above.
-

Reviewer #2 (Public Review):
Strengths:
A major strength of this report is the use of several different technical approaches, the results from which converge to provide several types of data supporting their conclusions. These various techniques include genetic knockdown/overexpression in primary keratinocytes, organotypic raft cultures, laser-capture microdissection, cell fate monitoring assays, and analysis of publicly available datasets. The manuscript is well-written and the figures are well-made.
Weaknesses:
Overall, there are only a few minor weaknesses related to figure quality and presentation.
Are claims/conclusions justified by data?
Overall, the authors' conclusions are adequately justified by the data. However, there were a few interpretations I felt were somewhat overstated given the experiments performed and data provided.Reviewer #2 (Public Review):
Strengths:
A major strength of this report is the use of several different technical approaches, the results from which converge to provide several types of data supporting their conclusions. These various techniques include genetic knockdown/overexpression in primary keratinocytes, organotypic raft cultures, laser-capture microdissection, cell fate monitoring assays, and analysis of publicly available datasets. The manuscript is well-written and the figures are well-made.
Weaknesses:
Overall, there are only a few minor weaknesses related to figure quality and presentation.
Are claims/conclusions justified by data?
Overall, the authors' conclusions are adequately justified by the data. However, there were a few interpretations I felt were somewhat overstated given the experiments performed and data provided.1. The first issue relates to the interpretation/conclusion of the results from experiments analyzing basal cell number. In Figure 2, the basal cell number was indeed reduced in R84S compared to WT E7. However, it was not reduced to parental HFK levels, suggesting other E7 activities are involved in increasing basal cell number. A similar observation is presented in Figure 7 (E-F), where the R84S E7 mutant still had significantly higher basal cell retention than the empty vector control, albeit lower than WT E7. While their data certainly indicates that the binding and subsequent degradation of PTPN14 is an E7 function important to increasing basal cell number and retention, there are clearly other E7 functions involved. While the authors don't necessarily over-interpret these findings, the possibility that other E7 functions are involved is not explicitly acknowledged or explored in the Discussion.
2. The second issue pertains to the findings related to the effect on differentiation upon modulation of key Hippo pathway components (Figure 4). It does not appear that the authors performed these studies in the presence of any well-known stimuli that induce the differentiation process in keratinocytes grown in 2D culture (high calcium, high serum, etc) nor did they use these cells in organotypic rafts wherein differentiation occurs during the raft stratification process. This is particularly true in the studies exploring PTPN14 plus LATS1/2 silencing and the effect on repression of keratinocyte differentiation. Whereas it seems PTPN14 itself was serving as the differentiation stimuli in earlier experiments (Figure 4C/D), it does not appear any differentiation stimuli were provided in the experiments shown in Figures 4E-I. For these reasons, the interpretation drawn by the authors that "...inactivation of three different YAP1 inhibitors dampens differentiation gene expression" (Line 220-221) and "inactivation of LATS1 or LATS2...also repressed differentiation genes" (Lines 349-350) seems specific to endogenous levels of differentiation genes. It seems difficult to conclude that inactivation of the Hippo pathway is actively repressing the induction of differentiation if the cells are not being treated with stimuli to induce differentiation.
-