Sleep EEG in young people with 22q11.2 deletion syndrome: A cross-sectional study of slow-waves, spindles and correlations with memory and neurodevelopmental symptoms
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The authors quantified sleep oscillations and their coordination in young people with 22q11.2 Deletion Syndrome and their siblings. This was done to identify potential biomarkers of later neurodevelopmental diagnoses in 22q11.2DS. The core findings demonstrate that sleep rhythms in 22q11.2DS are altered in comparison to the control group, as is their relationship with the behavioural expressions of memory consolidation.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Young people living with 22q11.2 Deletion Syndrome (22q11.2DS) are at increased risk of schizophrenia, intellectual disability, attention-deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD). In common with these conditions, 22q11.2DS is also associated with sleep problems. We investigated whether abnormal sleep or sleep-dependent network activity in 22q11.2DS reflects convergent, early signatures of neural circuit disruption also evident in associated neurodevelopmental conditions.
Methods:
In a cross-sectional design, we recorded high-density sleep EEG in young people (6–20 years) with 22q11.2DS (n=28) and their unaffected siblings (n=17), quantifying associations between sleep architecture, EEG oscillations (spindles and slow waves) and psychiatric symptoms. We also measured performance on a memory task before and after sleep.
Results:
22q11.2DS was associated with significant alterations in sleep architecture, including a greater proportion of N3 sleep and lower proportions of N1 and REM sleep than in siblings. During sleep, deletion carriers showed broadband increases in EEG power with increased slow-wave and spindle amplitudes, increased spindle frequency and density, and stronger coupling between spindles and slow-waves. Spindle and slow-wave amplitudes correlated positively with overnight memory in controls, but negatively in 22q11.2DS. Mediation analyses indicated that genotype effects on anxiety, ADHD and ASD were partially mediated by sleep EEG measures.
Conclusions:
This study provides a detailed description of sleep neurophysiology in 22q11.2DS, highlighting alterations in EEG signatures of sleep which have been previously linked to neurodevelopment, some of which were associated with psychiatric symptoms. Sleep EEG features may therefore reflect delayed or compromised neurodevelopmental processes in 22q11.2DS, which could inform our understanding of the neurobiology of this condition and be biomarkers for neuropsychiatric disorders.
Funding:
This research was funded by a Lilly Innovation Fellowship Award (UB), the National Institute of Mental Health (NIMH 5UO1MH101724; MvdB), a Wellcome Trust Institutional Strategic Support Fund (ISSF) award (MvdB), the Waterloo Foundation (918-1234; MvdB), the Baily Thomas Charitable Fund (2315/1; MvdB), MRC grant Intellectual Disability and Mental Health: Assessing Genomic Impact on Neurodevelopment (IMAGINE) (MR/L011166/1; JH, MvdB and MO), MRC grant Intellectual Disability and Mental Health: Assessing Genomic Impact on Neurodevelopment 2 (IMAGINE-2) (MR/T033045/1; MvdB, JH and MO); Wellcome Trust Strategic Award ‘Defining Endophenotypes From Integrated Neurosciences’ Wellcome Trust (100202/Z/12/Z MO, JH). NAD was supported by a National Institute for Health Research Academic Clinical Fellowship in Mental Health and MWJ by a Wellcome Trust Senior Research Fellowship in Basic Biomedical Science (202810/Z/16/Z). CE and HAM were supported by Medical Research Council Doctoral Training Grants (C.B.E. 1644194, H.A.M MR/K501347/1). HMM and UB were employed by Eli Lilly & Co during the study; HMM is currently an employee of Boehringer Ingelheim Pharma GmbH & Co KG. The views and opinions expressed are those of the author(s), and not necessarily those of the NHS, the NIHR or the Department of Health funders.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
This paper presents analysis of an impressive dataset acquired from sibling pairs, where one child had a specific gene mutation (22q11.2DS), whereas other child served as a blood-related, healthy control. The authors gathered rich, multi-faced data, including genetic profile, behavioral testing, neuropsychiatric questionnaires, and sleep PSG.
The analyses explore group differences (gene mutation vs. healthy controls) in terms of sleep architecture, sleep-specific brain oscillations and performance on a memory task.
The authors utilized a solid mix-model statistical approach, which not only controlled for the multi-comparison problem, but also accounted for between-subject and within-family variance. This was supplemented by mediation analysis, exploring the exact interaction between …
Author Response
Reviewer #1 (Public Review):
This paper presents analysis of an impressive dataset acquired from sibling pairs, where one child had a specific gene mutation (22q11.2DS), whereas other child served as a blood-related, healthy control. The authors gathered rich, multi-faced data, including genetic profile, behavioral testing, neuropsychiatric questionnaires, and sleep PSG.
The analyses explore group differences (gene mutation vs. healthy controls) in terms of sleep architecture, sleep-specific brain oscillations and performance on a memory task.
The authors utilized a solid mix-model statistical approach, which not only controlled for the multi-comparison problem, but also accounted for between-subject and within-family variance. This was supplemented by mediation analysis, exploring the exact interaction between the variables. Remarkably, the two subject groups were gender balanced, and were matched in terms of age and sex.
Thank you for this endorsement of our approach.
There are some aspects requiring clarification. In the discussion section, some claims come across as too general, or too speculative, and lack proper evidence in the current analysis of in the references.
We have extensively revised our discussion, including introducing more referencing and adding subheadings which we hope makes our conclusions both more structured and better evidenced (Discussion, pages 27 – 31)
Furthermore, the authors seem to treat their (child) participants with the gene mutation as forerunners of (adult) schizophrenic patients, to whom their repeatedly compare the findings. However, less than half of these children with 22q11.2DS are expected to develop psychotic disorders. In fact, they are at risk of many other neuropsychiatric conditions (incl. intellectual disability, ASD, ADHD, epilepsy) (cf. introduction section).
We have revised our introduction (page 4 -5) and discussion to clarify the significant comorbidity in 22q11.2DS. We discuss the limitations and future directions section of our work in the discussion (page 30)
Furthermore, the liberal criteria for detecting slow-waves, along with odd topography of the detections, limit the credibility of the slow-wave-related results.
As there is no single common method for SW detection, as noted on page 37, we prioritised rate of detection in order to provide a robust dataset for spindle-SW coupling analysis. We considered the use of an absolute detection threshold (e.g. – 75 microVolts) – however, because our participants were of a wide range of ages (6 to 20 years), and it is established that the absolute amplitude of the EEG decreases across childhood (e.g. Hahn et al 2020), our view is that the use of an absolute detection threshold would potentially bias the detection of slow waves by age. We have added comments on this matter to the methods section (page 37)
Lastly, we cannot be sure whether the presented memory effects reflect between-group difference in general cognitive capacities, or, as claimed, in overnight memory consolidation.
We have added statistical analysis of the overnight change in performance (results, page 6) to explore this point. We clarify that although 22q11.2DS is associated with slower learning and worse accuracy in the test session, there is not a difference in overnight change in 22q11.2DS.
Generally, the current study introduces dataset connecting various aspects of 22q11.2DS. It has a great potential for complementing the current state of knowledge not only in the clinical, but also in sleep-science field.
Thank you
Reviewer #2 (Public Review):
This study examines 22q11.2 microdeletion syndrome in 28 individuals and their unaffected siblings. Though the sample size is small, it is on par with many neuroimaging studies of the syndrome. Part of the interest in this disorder arises from the risk this syndrome confers for neuropsychiatric disorders in general and psychosis specifically. The authors examine sleep neurophysiology in 22q11.2DS and their siblings. Principal findings include increase slow wave and spindle amplitudes in deletion carriers as compared to controls.
Strengths of this manuscript include:
- The inclusion of siblings as a control group, which minimizes environmental and (other) genetic confounds
- The data analyses of the sleep EEG are appropriate and in-depth
- High-density sleep EEG allows for topographic mapping
We thank the reviewer for this positive endorsement of our work
Weaknesses of this manuscript include:
- The manuscript is framed as an investigation of the psychosis and schizophrenia; however, psychotic experiences did not differ between 22q11.2DS and healthy controls. Therefore, the emphasis on schizophrenia and psychosis does not pertain to this sample and the manuscript introduction and discussion should be carefully reframed. The final sentence of the abstract is also not supported by the data: "... out findings may therefore reflect delayed or compromised neurodevelopmental processes which precede, and may be biomarkers for, psychotic disorders".
We have expanded our abstract, introduction and discussion to reflect the complex neurodevelopment phenotype observed in 22q11.2DS, and discuss the links between our findings, and elements of this phenotype
- What is the rationale for using a mediation model to test for the association between genotype and psychiatric symptoms? Given the modest sample size would a regression to test the association between genotype and psychiatric symptoms be more appropriate?
Our rationale for mediation analysis was to expand on making simple group comparisons for various measures by asking if genotype effects on particular psychiatric/behavioural measures were potentially mediated by EEG measures. This is of considerable interest because, as noted above, the behavioural and psychiatric phenotype in 22q11.2DS is complex, and therefore dissection of links between particular EEG features and phenotypes, and asking if EEG measures can be biomarkers for these phenotypes, may give insight into this complexity.
- From Table 1, which presents means, standard deviations and statistics, it is hard to tell if there is a range of symptoms or if there are some participants with 22q11.2DS who met diagnostic criteria for a the listed disorder while others who have no or sub-threshold symptoms. This is important and informs the statistical analysis. Given the broad range of psychiatric symptoms, I also wonder if a composite score of psychopathology may be more appropriate. What about other psychiatric symptoms such as depression?
We have added a supplementary figure to figure 1 to provide individual participants scores on psychiatric measures and FSIQ to fully inform the reader about individual data.
We have taken the approach of using symptom scores, rather than using binary cut offs for diagnosis, to maximise the use of our dataset, and given many/all psychiatric phenotypes exist on a spectrum, to reflect the difference between clinical and research diagnoses.
Regarding depression, it has been previously demonstrated in 22q11.2DS that mood disorders are rare at young ages (Chawner et al 2019), therefore given the low frequency, we have not included depression in this dataset
We have considered the utility of a composite psychopathology score; however, it is already established that 22q11.2DS can be associated with a broad range of psychiatric/behavioural difficulties; in this study we were primarily interested in exploring the links (if any) between specific groups of symptoms, and specific features of the sleep phenotype. Therefore, we feel a composite psychopathology score would not add to the overall clarity of the manuscript
- The age range is very broad spanning 6 to 20 years. As there are marked changes in the sleep EEG with age, it is important to understand the influence of age. The small sample size precludes investigating age by group interactions meaningfully, but the presentation of the ages of 22q11.2DS and controls, rather than means, standard deviations and ranges, would be helpful for the reader to understand the sample.
We have added scatter plots of EEG measures and age to each figure supplement to allow the reader to see changes with age
Also, a figure showing individual data (e.g., spindle power) as a function of age and group would be informative. The authors should also discuss the possibility that the difference between the groups may vary as a function of age as has been shown for cortical grey matter volume (Bagaiutdinova et al., Molecular Psychiatry, 2021).
We have provided plots of individual data with age for our main figures, in the figure supplements. We also note we have included age as a covariate in all main statistical models (methods, page 39). We thank the reviewer for the additional reference, this has been added to the discussion (page 29)
- There is a large group difference with regards to full scale IQ. IQ is associated with sleep spindles (e.g., Gruber et al., Int J of Psychphsy, 2013; Geiger et al., SLEEP, 2011). For this reason, the authors should control for IQ in all analyses.
We note that the relationship between spindle characteristics and IQ has been questioned (e.g. Reynolds et al 2018 performed a meta-analysis which suggests no correlation with FSIQ, which would suggest against the suggested approach). We also note that genotype effects on FSIQ were not mediated by spindle properties. Furthermore, the phenotype in 22q11.2DS is complex, while lower IQ is a well evidenced part, it is only one component. We are unclear if it would be justified to regress out only one component of a phenotype.
- The authors find greater power in the delta and sigma bands in 22q11.2DS compared to their siblings. Looking at the Figure 2, it appears power is elevated across frequencies. If this were the case, this would likely change the interpretation of the findings, and suggest that the sleep EEG likely reflects changes in cortical thickness between controls and 22q11.2DS participants.
We thank the review for this interesting comment. We have now altered the approach taken to our analysis of spectral data in order to probe overall differences in overall power, using the IRASA approach described by Hahn et al 2020. We present these results on page 13, and use measures derived from this analysis in the mediation and behavioural analyses, and discuss these findings in the discussion (page 29)
- Along the same lines as the above comment, it would be interesting to examine REM sleep and test how specific to sleep spindles and slow waves these findings are.
We have now added analysis of REM-derived spectral measures, which we believe complement our finding of altered proportions of REM sleep in 22q11.2DS compared to controls (page 13)
Reviewer #3 (Public Review):
In this study, Donnelly and colleagues quantified sleep oscillations and their coordination in in young people with 22q11.2 Deletion Syndrome and their siblings. They demonstrate that 22q11.2DS was associated with enhanced power the in slow wave and sleep spindle range, elevated slow-wave and spindle amplitudes and altered coupling between spindles and slow-waves. In addition, spindle and slow-wave amplitudes in 22q11.2DS correlated negatively with the outcomes of a memory test. Overall, the topic and the results of the present study are interesting and timely. The authors employed many thoughtful analyses, making sense out of complicated data. However, some features of the manuscript need further clarification.
1.) Several interesting results of the manuscript are related to altered sleep spindle characteristics in 22q11.2DS (increased power, increased amplitudes and altered coupling with slow waves). On top of that the authors report, that the spindle frequency was correlated with age. I was wondering whether the authors might want to take these individual (age-related) differences into account in their analyses. The authors could detect the peak spindle frequency per participant and inform their spindle detection procedure accordingly. This procedure might lead to an even more clear cut picture concerning altered spindle activity in 22q11.2DS.
We thank the review for this informative suggestion. We have now implemented this method, detecting spindles for each individual at a frequency defined through IRASA analysis of the EEG (results, page 13; methods, page 35), and then using the properties of spindles detected through this method in further analysis.
We have included age as a covariate in all main models (methods, page 39), and present individual data scattered with age in our figure supplements.
2.) The authors state in the methods section that EEG data was re-referenced to a common average during pre-processing. Did the authors take into account that this reference scheme will lead to a polarity inversion of the signal, potentially over parietal/occipital areas? This inversion will not affect spindle related analyses, but might misguide the detection of slow waves and hence confound related analyses and results.
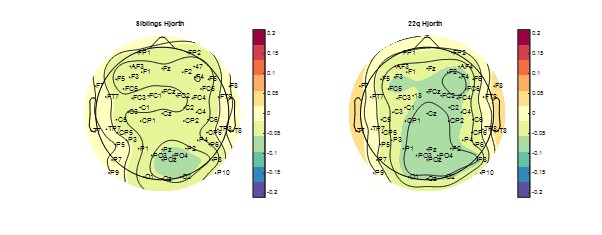
We have reviewed our data preprocessing pipeline, and updated it based on the latest methods suggested from the EEGlab authors (methods, page 33). As a supplementary analysis we applied a heuristic signal polarity measure described by the authors of the luna software package https://zzz.bwh.harvard.edu/luna/vignettes/nsrr-polarity/ and did not observe any inversion of polarity in our sample.
In the included figure (below) we calculated the Hjorth measure of signal polarity as described in luna, at every electrode and plotted a topoplot of the measure. In the figure numbers > 0 represent signals with a positive polarity, values < 0 a negative polarity. As demonstrated in the figure, there were no electrodes with a positive polarity, although we note that the most peripheral electrodes had an approximately neutral polarity, whereas more central electrodes had a slight negative bias.
We also note that we only detected negative half waves with our slow wave detection algorithm, using a threshold set for each channel based on its own characteristics, so would not necessarily expect alterations in slow waves detection. Further, other authors have suggested that average referencing does not impact SW detection (e.g. Wennberg 2010)
3.) I have some issues understanding the reported slow wave - spindle coupling results. Figure 5A indicates that ~100 degrees correspond to the down-state of the slow wave. Figure 5E shows that spindles preferentially clustered at fronto-central electrodes between 0 and 90 degrees, hence they seem to peak towards the slow wave downstate. This finding is rather puzzling given the prototypical grouping of sleep spindles by slow wave up-states (Staresina, 2015; Helfrich, 2018; Hahn, 2020). Could it be that the majority of detected spindles represent slow spindles (9-12 Hz; Mölle, 2011)?
We observed peaks of spindle activity in the range of 9 – 24 degrees (so on the descending slope from the positive peak of the slow wave), but an average spindle frequencies in the 12 – 13 Hz range. Given we allowed each individual to have an individual spindle detection frequency, as above, and did not observe bimodal distributions of power in the sigma frequency band (Figure 2 Supplement 1), we do not believe our spindles specifically represent slow spindles
Slow spindles are known to peak rather at the up- to down-state transition (which would fit the reported results) and show a frontal distribution (which again would fit to the spindle amplitude topographies in Fig 3E). If that was the case, it would make sense to specifically look at fast spindles (12-16 Hz) as well, given their presumed role in memory consolidation (Klinzing, 2019).
We agree with the reviewer’s assessment of the distribution of the putative spindles we have detected. However, as we and other authors (Hahn et al 2020) have noted, we did not observe discrete fast and slow spindle frequency peaks in our analysis of the PSD (as has been observed by other authors e.g. Cox et al 2017). For this reason, and to reduce the complexity of the manuscript, we believe the best approach with our dataset is to focus on spindles at large, rather than detecting spindles in arbitrary frequency bands.
In addition, is it possible that the rather strong phase shift from fronto-central to occipital sites is driven by a polarity inversion due to using a common reference (see comment 2)?
As noted above, we do not observe significant polarity inversion in our signals using the luna heuristic measure. We were not able to identify published literature to inform our investigation of this suggestion, but would be happy to consider any specific suggestions from the reviewer
Apart from that I would suggest to statistically evaluate non-uniformity using e.g. the Rayleigh test (both within and across participants).
We have added an analysis of non-uniformity to the results section (results, page 20).
4.) Somewhat related to the point raised above. The authors state that in the methods that slow wave spindle events were defined as time-windows were the peaks of spindles overlapped with slow waves. How was the duration of slow waves defined in this scenario? If it was up- to up-state the authors might miss spindles which lock briefly after the post down-state upstate, thereby overrepresenting spindles that lock to early phases of slow waves. Why not just defining a clear slow wave related time-window, such as slow wave down-state {plus minus} 1.5 seconds?
We have implemented this suggestion (methods, page 38)
5.) The authors correlated the NREM sleep features with the outcomes of a post-sleep memory test (both encoding and an initial memory test took place pre-sleep). If the authors want to show a clear association between sleep-related oscillations and the behavioural expressions of memory consolidation, taking just the post sleep memory task is probably not the best choice. The post-sleep test will, as the pre-sleep test, in isolation rather reflect general memory related abilities. To uncover the distinct behavioural effects of consolidation the authors should assess the relative difference between the pre- and post-sleep memory performance and correlate this metric with their EEG outcomes.
We have added evening-morning performance difference as a measure to the results (page 6); however as there was no difference between groups in overnight change in performance, we focus on morning performance in relating behaviour to EEG outcomes (explored in results, page 6)
-

Evaluation Summary:
The authors quantified sleep oscillations and their coordination in young people with 22q11.2 Deletion Syndrome and their siblings. This was done to identify potential biomarkers of later neurodevelopmental diagnoses in 22q11.2DS. The core findings demonstrate that sleep rhythms in 22q11.2DS are altered in comparison to the control group, as is their relationship with the behavioural expressions of memory consolidation.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their names with the authors.)
-

Reviewer #1 (Public Review):
This paper presents analysis of an impressive dataset acquired from sibling pairs, where one child had a specific gene mutation (22q11.2DS), whereas other child served as a blood-related, healthy control. The authors gathered rich, multi-faced data, including genetic profile, behavioral testing, neuropsychiatric questionnaires, and sleep PSG.
The analyses explore group differences (gene mutation vs. healthy controls) in terms of sleep architecture, sleep-specific brain oscillations and performance on a memory task.
The authors utilized a solid mix-model statistical approach, which not only controlled for the multi-comparison problem, but also accounted for between-subject and within-family variance. This was supplemented by mediation analysis, exploring the exact interaction between the variables.
Reviewer #1 (Public Review):
This paper presents analysis of an impressive dataset acquired from sibling pairs, where one child had a specific gene mutation (22q11.2DS), whereas other child served as a blood-related, healthy control. The authors gathered rich, multi-faced data, including genetic profile, behavioral testing, neuropsychiatric questionnaires, and sleep PSG.
The analyses explore group differences (gene mutation vs. healthy controls) in terms of sleep architecture, sleep-specific brain oscillations and performance on a memory task.
The authors utilized a solid mix-model statistical approach, which not only controlled for the multi-comparison problem, but also accounted for between-subject and within-family variance. This was supplemented by mediation analysis, exploring the exact interaction between the variables.
Remarkably, the two subject groups were gender balanced, and were matched in terms of age and sex.There are some aspects requiring clarification. In the discussion section, some claims come across as too general, or too speculative, and lack proper evidence in the current analysis of in the references. Furthermore, the authors seem to treat their (child) participants with the gene mutation as forerunners of (adult) schizophrenic patients, to whom their repeatedly compare the findings. However, less than half of these children with 22q11.2DS are expected to develop psychotic disorders. In fact, they are at risk of many other neuropsychiatric conditions (incl. intellectual disability, ASD, ADHD, epilepsy) (cf. introduction section). Furthermore, the liberal criteria for detecting slow-waves, along with odd topography of the detections, limit the credibility of the slow-wave-related results. Lastly, we cannot be sure whether the presented memory effects reflect between-group difference in general cognitive capacities, or, as claimed, in overnight memory consolidation.
Generally, the current study introduces dataset connecting various aspects of 22q11.2DS. It has a great potential for complementing the current state of knowledge not only in the clinical, but also in sleep-science field.
-

Reviewer #2 (Public Review):
This study examines 22q11.2 microdeletion syndrome in 28 individuals and their unaffected siblings. Though the sample size is small, it is on par with many neuroimaging studies of the syndrome. Part of the interest in this disorder arises from the risk this syndrome confers for neuropsychiatric disorders in general and psychosis specifically. The authors examine sleep neurophysiology in 22q11.2DS and their siblings. Principal findings include increase slow wave and spindle amplitudes in deletion carriers as compared to controls.Strengths of this manuscript include:
- The inclusion of siblings as a control group, which minimizes environmental and (other) genetic confounds
- The data analyses of the sleep EEG are appropriate and in-depth
- High-density sleep EEG allows for topographic mappingWeaknesses of …
Reviewer #2 (Public Review):
This study examines 22q11.2 microdeletion syndrome in 28 individuals and their unaffected siblings. Though the sample size is small, it is on par with many neuroimaging studies of the syndrome. Part of the interest in this disorder arises from the risk this syndrome confers for neuropsychiatric disorders in general and psychosis specifically. The authors examine sleep neurophysiology in 22q11.2DS and their siblings. Principal findings include increase slow wave and spindle amplitudes in deletion carriers as compared to controls.Strengths of this manuscript include:
- The inclusion of siblings as a control group, which minimizes environmental and (other) genetic confounds
- The data analyses of the sleep EEG are appropriate and in-depth
- High-density sleep EEG allows for topographic mappingWeaknesses of this manuscript include:
- The manuscript is framed as an investigation of the psychosis and schizophrenia; however, psychotic experiences did not differ between 22q11.2DS and healthy controls. Therefore, the emphasis on schizophrenia and psychosis does not pertain to this sample and the manuscript introduction and discussion should be carefully reframed. The final sentence of the abstract is also not supported by the data: "... out findings may therefore reflect delayed or compromised neurodevelopmental processes which precede, and may be biomarkers for, psychotic disorders".
- What is the rationale for using a mediation model to test for the association between genotype and psychiatric symptoms? Given the modest sample size would a regression to test the association between genotype and psychiatric symptoms be more appropriate?
- From Table 1, which presents means, standard deviations and statistics, it is hard to tell if there is a range of symptoms or if there are some participants with 22q11.2DS who met diagnostic criteria for a the listed disorder while others who have no or sub-threshold symptoms. This is important and informs the statistical analysis. Given the broad range of psychiatric symptoms, I also wonder if a composite score of psychopathology may be more appropriate. What about other psychiatric symptoms such as depression?
- The age range is very broad spanning 6 to 20 years. As there are marked changes in the sleep EEG with age, it is important to understand the influence of age. The small sample size precludes investigating age by group interactions meaningfully, but the presentation of the ages of 22q11.2DS and controls, rather than means, standard deviations and ranges, would be helpful for the reader to understand the sample. Also, a figure showing individual data (e.g., spindle power) as a function of age and group would be informative. The authors should also discuss the possibility that the difference between the groups may vary as a function of age as has been shown for cortical grey matter volume (Bagaiutdinova et al., Molecular Psychiatry, 2021).
- There is a large group difference with regards to full scale IQ. IQ is associated with sleep spindles (e.g., Gruber et al., Int J of Psychphsy, 2013; Geiger et al., SLEEP, 2011). For this reason, the authors should control for IQ in all analyses.
- The authors find greater power in the delta and sigma bands in 22q11.2DS compared to their siblings. Looking at the Figure 2, it appears power is elevated across frequencies. If this were the case, this would likely change the interpretation of the findings, and suggest that the sleep EEG likely reflects changes in cortical thickness between controls and 22q11.2DS participants.
- Along the same lines as the above comment, it would be interesting to examine REM sleep and test how specific to sleep spindles and slow waves these findings are. -

Reviewer #3 (Public Review):
In this study, Donnelly and colleagues quantified sleep oscillations and their coordination in in young people with 22q11.2 Deletion Syndrome and their siblings. They demonstrate that 22q11.2DS was associated with enhanced power the in slow wave and sleep spindle range, elevated slow-wave and spindle amplitudes and altered coupling between spindles and slow-waves. In addition, spindle and slow-wave amplitudes in 22q11.2DS correlated negatively with the outcomes of a memory test. Overall, the topic and the results of the present study are interesting and timely. The authors employed many thoughtful analyses, making sense out of complicated data. However, some features of the manuscript need further clarification.
Several interesting results of the manuscript are related to altered sleep spindle …
Reviewer #3 (Public Review):
In this study, Donnelly and colleagues quantified sleep oscillations and their coordination in in young people with 22q11.2 Deletion Syndrome and their siblings. They demonstrate that 22q11.2DS was associated with enhanced power the in slow wave and sleep spindle range, elevated slow-wave and spindle amplitudes and altered coupling between spindles and slow-waves. In addition, spindle and slow-wave amplitudes in 22q11.2DS correlated negatively with the outcomes of a memory test. Overall, the topic and the results of the present study are interesting and timely. The authors employed many thoughtful analyses, making sense out of complicated data. However, some features of the manuscript need further clarification.
Several interesting results of the manuscript are related to altered sleep spindle characteristics in 22q11.2DS (increased power, increased amplitudes and altered coupling with slow waves). On top of that the authors report, that the spindle frequency was correlated with age. I was wondering whether the authors might want to take these individual (age-related) differences into account in their analyses. The authors could detect the peak spindle frequency per participant and inform their spindle detection procedure accordingly. This procedure might lead to an even more clear cut picture concerning altered spindle activity in 22q11.2DS.
The authors state in the methods section that EEG data was re-referenced to a common average during pre-processing. Did the authors take into account that this reference scheme will lead to a polarity inversion of the signal, potentially over parietal/occipital areas? This inversion will not affect spindle related analyses, but might misguide the detection of slow waves and hence confound related analyses and results.
I have some issues understanding the reported slow wave - spindle coupling results. Figure 5A indicates that ~100 degrees correspond to the down-state of the slow wave. Figure 5E shows that spindles preferentially clustered at fronto-central electrodes between 0 and 90 degrees, hence they seem to peak towards the slow wave downstate. This finding is rather puzzling given the prototypical grouping of sleep spindles by slow wave up-states (Staresina, 2015; Helfrich, 2018; Hahn, 2020). Could it be that the majority of detected spindles represent slow spindles (9-12 Hz; Mölle, 2011)? Slow spindles are known to peak rather at the up- to down-state transition (which would fit the reported results) and show a frontal distribution (which again would fit to the spindle amplitude topographies in Fig 3E). If that was the case, it would make sense to specifically look at fast spindles (12-16 Hz) as well, given their presumed role in memory consolidation (Klinzing, 2019). In addition, is it possible that the rather strong phase shift from fronto-central to occipital sites is driven by a polarity inversion due to using a common reference (see comment 2)?
Apart from that I would suggest to statistically evaluate non-uniformity using e.g. the Rayleigh test (both within and across participants).Somewhat related to the point raised above. The authors state that in the methods that slow wave spindle events were defined as time-windows were the peaks of spindles overlapped with slow waves. How was the duration of slow waves defined in this scenario? If it was up- to up-state the authors might miss spindles which lock briefly after the post down-state upstate, thereby overrepresenting spindles that lock to early phases of slow waves. Why not just defining a clear slow wave related time-window, such as slow wave down-state {plus minus} 1.5 seconds?
The authors correlated the NREM sleep features with the outcomes of a post-sleep memory test (both encoding and an initial memory test took place pre-sleep). If the authors want to show a clear association between sleep-related oscillations and the behavioural expressions of memory consolidation, taking just the post sleep memory task is probably not the best choice. The post-sleep test will, as the pre-sleep test, in isolation rather reflect general memory related abilities. To uncover the distinct behavioural effects of consolidation the authors should assess the relative difference between the pre- and post-sleep memory performance and correlate this metric with their EEG outcomes.
-