Proton export drives the Warburg Effect
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This manuscript addresses a phenomenon of great interest to researchers in cell metabolism and cancer biology: namely, why do cancer cells often secrete high levels of lactate, despite the presence of abundant oxygen to power nutrient oxidation (Warburg effect). The authors propose that lactate export and subsequent extracellular acidification provides a selective advantage and the concomitant rise in intracellular pH is sufficient to drive flux through glycolysis, thereby sustaining the Warburg effect. This is an intriguing hypothesis that ties together many published observations, but it would require further support both from the technical and conceptual side.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Aggressive cancers commonly ferment glucose to lactic acid at high rates, even in the presence of oxygen. This is known as aerobic glycolysis, or the “Warburg Effect”. It is widely assumed that this is a consequence of the upregulation of glycolytic enzymes. Oncogenic drivers can increase the expression of most proteins in the glycolytic pathway, including the terminal step of exporting H + equivalents from the cytoplasm. Proton exporters maintain an alkaline cytoplasmic pH, which can enhance all glycolytic enzyme activities, even in the absence of oncogene-related expression changes. Based on this observation, we hypothesized that increased uptake and fermentative metabolism of glucose could be driven by the expulsion of H + equivalents from the cell. To test this hypothesis, we stably transfected lowly-glycolytic MCF-7, U2-OS, and glycolytic HEK293 cells to express proton exporting systems: either PMA1 (yeast H + -ATPase) or CAIX (carbonic anhydrase 9). The expression of either exporter in vitro enhanced aerobic glycolysis as measured by glucose consumption, lactate production, and extracellular acidification rate. This resulted in an increased intracellular pH, and metabolomic analyses indicated that this was associated with an increased flux of all glycolytic enzymes upstream of pyruvate kinase. These cells also demonstrated increased migratory and invasive phenotypes in vitro , and these were recapitulated in vivo by more aggressive behavior, whereby the acid-producing cells formed higher grade tumors with higher rates of metastases. Neutralizing tumor acidity with oral buffers reduced the metastatic burden. Therefore, cancer cells with increased H + export increase intracellular alkalization, even without oncogenic driver mutations, and this is sufficient to alter cancer metabolism towards a Warburg phenotype.
Article activity feed
-

Author Response:
Evaluation Summary:
This manuscript addresses a phenomenon of great interest to researchers in cell metabolism and cancer biology: namely, why do cancer cells often secrete high levels of lactate, despite the presence of abundant oxygen to power nutrient oxidation (Warburg effect). The authors propose that lactate export and subsequent extracellular acidification provides a selective advantage and the concomitant rise in intracellular pH is sufficient to drive flux through glycolysis, thereby sustaining the Warburg effect. This is an intriguing hypothesis that ties together many published observations, but it would require further support both from the technical and conceptual side.
The concept proposed in the evaluation summary is not quite correct, in this paper we have tried to show that it is not lactate export …
Author Response:
Evaluation Summary:
This manuscript addresses a phenomenon of great interest to researchers in cell metabolism and cancer biology: namely, why do cancer cells often secrete high levels of lactate, despite the presence of abundant oxygen to power nutrient oxidation (Warburg effect). The authors propose that lactate export and subsequent extracellular acidification provides a selective advantage and the concomitant rise in intracellular pH is sufficient to drive flux through glycolysis, thereby sustaining the Warburg effect. This is an intriguing hypothesis that ties together many published observations, but it would require further support both from the technical and conceptual side.
The concept proposed in the evaluation summary is not quite correct, in this paper we have tried to show that it is not lactate export that drives extracellular acidification, but that cells which can increase proton export, via over-expression or increased activity of proton exporting proteins, can subsequently drive upregulation of glycolysis and increased lactate production, likely due to increased intracellular pH (pHi) and the ability of glycolytic enzymes to have enhanced activity under slightly higher pHi. As mentioned in the summary, although some of these observations are known, the novelty lies in that they have not been directly proven by inducing acid export prior to a glycolytic phenotype, we believe showing the casual nature of proton export on glycolysis is the novelty of this research.
Reviewer #1 (Public Review):
In this manuscript, the authors tackle an interesting puzzle: why do cancer cells secrete most of their glucose as lactate? The authors propose that acid export is sufficient to enhance glycolysis and provide a selective advantage to cancer cells growing in vivo. To this end, the authors show that clonal lines expressing CA-IX or PMA1, each of which will facilitate proton export, have elevated capacity to acidify extracellular medium and can drive increased migration/invasion and tumor growth or metastases. In support of the model that extracellular pH is a key driver of metastases, the effect of CA-IX expression on lung metastases is reversed following bicarbonate treatment. While many of the individual conclusions of the manuscript are not novel-for example, pH has been reported to control glycolysis and it is established that CA-IX expression modulates migration/metastases-providing a comprehensive assessment of the ability of proton export to drive the Warburg effect, and assessing the significance of metabolic rewiring driven by acid export on tumor growth, would represent an important resource for researchers intrigued by the pervasive observation that cancer cells secrete lactate despite potential bioenergetic disadvantages of discarding biomass.
The strength of the manuscript lies therefore in tying these disparate observations together in a coherent model and testing the role of acid export per se on glycolytic flux. The technical weaknesses of the paper prevent such coherent model building. A major concern is that all cell lines appear to be generated by transient transfection followed by clonal selection, giving rise to cells with notable variability and inconsistent phenotypes. More traditional approaches to manipulate enzyme expression will provide more robust model systems to test the proposed model. Similarly, direct measures of glycolytic flux are required to make conclusions about the role of acid export in promoting glycolysis. Another strength is the use of heterologous enzyme systems to alter proton export in cancer cells, but alternative explanations for these results are not fully considered. Ultimately, to what extent acid export per se, as opposed to altered metabolism driven by acid export, drives enhanced tumor metastases is not addressed.
We agree wholly with Reviewer 1 that although individual components of this manuscript have previously been implicated in cancer research, the novelty lies in directly assessing metabolic changes, specifically the Warburg effect, as a result of proton production to determine causality rather than correlation as previous studies have shown. The reviewer makes a valid point about our use of clones and this is something we considered at length. When originally designing these experiments, we had many conversations within our lab and with collaborators and colleagues, and the overall consensus was that bulk populations are more likely to have heterogeneous expression levels unrelated to transfection, which could result in the phenotype generated being noisy and not indicative of what occurs when proton exporters are over-expressed. We chose to isolate single clones, maintaining these in antibiotic selection media, to ensure stable over-expression. After confirming over-expression, cells were grown without antibiotics and screened regularly for maintenance of protein expression. This was also one of the reasons why we utilized over-expression of two different proton exporters in multiple different cell lines to be confident that proton export was changing the metabolic phenotype and not just due to changes in an individual isolated clonal line. We utilized bulk population for the MOCK clones, to ensure we weren’t selecting for a clone which had inherently different metabolic traits from the parental population. As described in the paper, while some of the behaviors of the different clones are indeed divergent, the impact of expression on increased glucose uptake and lactate production is wholly consistent and highly correlated to expression of PMA1 or CA-IX. Although we utilized metabolic profiling, we do not claim to infer flux from these data. Flux was assessed via lactate production and glucose consumption rates. The metabolomic analyses showed that glycolytic intermediates upstream of Pyruvate Kinase (PK) were uniformly increased in transfectants. This was an unequivocal finding and, given the increased flux, we have concluded that transfection results in activating glycolytic enzymes upstream of PK. The pleiotropic nature of these effects have led us to propose that intracellular pH was increasing and likely enhancing glycolytic enzyme activity throughout the glycolytic pathway. We measured the intracellular pH and showed that it was generally elevated in the transfectants. Finally, the reviewer was concerned that we did not address the mechanism by which pH increases metastases. Such a study would be beyond the scope of this paper and, indeed, was the subject of a two-volume special issue of Cancer Mets. Rev. in 2019 (PMC6625888). Hence, in this paper, we were not trying to address the mechanism by which pH affects metastasis, but simply wanted to show additional biological relevance.
Reviewer #2 (Public Review):
The work by Xu et al proposes that the Warburg effect - the increase of glycolytic metabolism usually displayed by tumor cells, is driven by increased proton excretion rather than by oncogenic dysregulation of glycolytic enzyme levels. As a proof-of-principle, they engineered tumor cells to increase proton excretion. They observed an increase in glycolytic rate, pH, and malignancy in their engineered cells.
- My main issue with this work is that I do not agree with the authors when they say that the "canonical view" is that oncolytic mutations are thought to drive the Warburg effect. What I understand the consensus to be, is that it is fast proliferating cells - rather than malignant cells - the ones who display this form of metabolism. The rationale is that glycolytic metabolism allows keeping biomass by redirecting lactate and from the phosphate pentose pathway. In contrast, the end product of oxidative phosphorylation is CO2 that cannot be further utilized in cell metabolism.
They claim that they Vander Heiden et al., 2009 shows that "fermentation under aerobic conditions is energetically unfavorable and does not confer any clear evolutionary benefits." This is incorrect. While that review states that the Warburg effect has little effect on the ATP/ADP ratio, they do show this form of metabolism has significant benefits for fast proliferating cells. In fact, the whole review is about how the Warburg effect is a necessary metabolic adaptation for fast proliferation rather than a unique feature of malignant cells.
- Their main observation is not surprising. From a biochemical standpoint, protons are final product of glycolysis (from the production of lactic acid). Thus, by mass action, any mechanism to remove protons from the cell will result in accelerated glycolytic rate. Similarly, reducing intracellular pH will necessarily slow down LDHA's activity, which in turn will slow down pyruvate kinase and so on.
- Their experiments are conducted on transformed cells - that by definition - have oncogenic driver mutations. They should test the effect of proton exporter using primary non-transformed cells (fresh MEFs, immune cells, etc). I would expect that they will still see the increase in glycolysis in this case. And yet, I would still have my concerns I expressed in my previous point.
- The fact that they can accelerate the Warburg effect by increasing proton export does not mean is the mechanism used by tumor cells in patients or "the driver" of this effect. As I mentioned, their observation is expected by mass action but tumors that do not overexpress proton transporter may still drive their Warburg effect via oncogenic mutations. The biochemical need here is to increase the sources of biomass and redox potential and evolution will select for more glycolytic phenotypes.
Comment 1: We disagree with the reviewer that the energetic demands of a faster proliferating cell drive glycolysis in order to produce the biomass needed for generation of new cells. Available evidence does not support this hypothesis. As the reviewer mentioned, there is a correlation between proliferation and aerobic glycolysis (i.e. if cells are stimulated to grow they will consume more glucose), and the same can be said for motility (i.e. more motile cells have higher aerobic glycolysis). This is also true for normal cells and tissues that exhibit high levels of aerobic glycolysis. We agree that glycolytic ATP generation is more rapid than oxidative phosphorylation and that this may confer some selective advantage for transporters, as we described in PMC4060846. Nonetheless, it is clear that under conditions of similar proliferation and motility, more aggressive cancer cells ferment glucose at much higher rates. However, correlations between neither proliferation nor motility are the “Warburg Effect” which is a higher rate of aerobic glycolysis in cancers, regardless of proliferation or migration. As we described in PMID 18523064, the prevailing view in the cancer literature is that the Warburg effect is driven by oncogenes (ras, myc), transcription factors (HIF) and tumor suppressors (p53/TIGAR) through increased expression of glycolytic enzymes. This assumes that expression levels drive flux which has not been proved empirically. In biochemical pathways, it is canon that flux is regulated by demand (e.g. ATP) or through some post-transcriptional control (e.g. pH). In Vander Heiden’s paper the steady state levels are reported of ATP/ADP ratios, not flux. The first paragraph of the intro has been modified to accommodate this concern.
Comment 2: The fact that our results are not surprising is our major argument: i.e. that glycolytic flux can be enhanced by increasing the rate of H+ export. We saw an increase in intracellular pH (pHi), but our metabolomics data do not support a direct effect on LDHA or PK. Instead, we show that clones with higher pHi have a crossover point at PK, due to reduced inhibition of upstream enzymes which is not there in clones at lower pHi.
Comment 3: We agree it would be interesting to study the effects of proton export on immune cells especially given the increase in immunotherapy use in cancer treatment. We did utilize HEK 293 cells shown in supplemental figure S6, to show this was not a cancer cell line specific phenomenon, and we saw increased aerobic glycolysis with over-expression of CA-IX.
Comment 4: We agree that oncogenic mutations can alter glycolytic rate, but we observed that increased expression and activity of proton exporters is sufficient to drive a Warburg effect. Although the reviewer indicates that glycolysis is responsible for generating the biomass needed for these faster proliferating cells, we have shown that proton exporter driven aerobic glycolysis does not increase proliferation rates. The literature, see Vander Heiden’s paper below, suggests that amino acids, mainly glutamine, can support the majority of biomass needs of a proliferating cell. Hence, reliance on aerobic glycolysis remains energetically inefficient and inefficient in that most of the carbons are removed, and thus will not be selected by evolution.
Hosios, A.M., Hecht, V.C., Danai, L.V., Johnson, M.O., Rathmell, J.C., Steinhauser, M.L., Manalis, S.R., & Vander Heiden, M.G. (2016). Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Developmental cell, 36 5, 540-9 .
Reviewer #3 (Public Review):
The authors claim that "proton export drives the Warburg effect". For this, they expressed proton-exporting proteins in cells and measured the intracellular proton concentration and the Warburg effect. Based on their data, however, I do not see elevated Warburg effect in these cells and thus conclude that the claim is not supported.
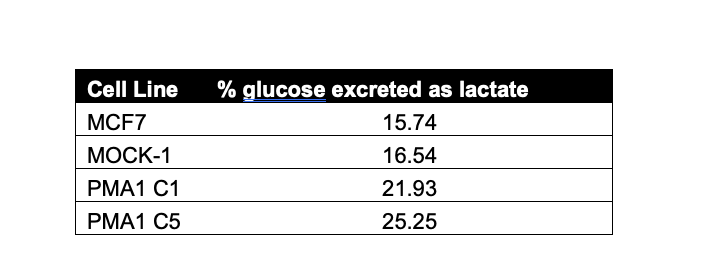
The authors concluded that the CA-IX or PMA1 expressing cells had increased Warburg effect. I don't think this conclusion can be made based on the data presented. For the MCF-7 cells, the glucose consumption is ~18 pmol/cell/24hr (Fig. 5E) and lactate production is ~0.6 pmol/cell/24hr (Fig. 5F), indicating that 0.6/18/2 = 1.7% of the glucose is excreted as lactate. This low percentage remains true for the PMA1 expressing cells. For example, for the PMA1-C5 cells, the percentage of glucose going to lactate is about 1.8/38/2 = 2.4% (Fig. 5EF). While indeed there was an increase of both the glucose and lactate fluxes in the PMA1 expressing cells, the vast majority of the glucose flux ends up elsewhere likely the TCA cycle. This is a very different phenotype from cancer cells that have Warburg effect. The same calculation can be done for the CA-IX cells but the data on the glucose and lactate concentration there are inconsistent and expressed in confusing units (which I will elaborate in the next paragraph). Nevertheless, as there were at most a few folds of increase in lactate production flux in the M1 and M6 cells, the glucose flux going to lactate production is likely also a few percent of the total glucose uptake flux. Again, these cells do not really have Warburg effect.
The glucose and lactate concentration data are key to the study. The data however appear to lack consistency. The lactate concentration data in Fig. 1F shows a ~5-fold increase in the M1 and M6 cells than the controls but the same data in S. Fig. 2 shows a mere ~50% increase. The meaning of the units on these figures is not clear. While "1 ng/ug protein" means 1ng of lactate is produced by 1 ug protein of cells over a 24 hour period, I do not understand what "ng/ul/ug protein" means (Fig. 1F). Also, "g/L/cell" must be a typo (S. Fig. 2). Furthermore, regarding the important glucose consumption flux, it is not clear why the authors did not directly measure it as they did for the PMA1 cells (Fig. 5E). Instead, they showed two indirect measurements which are not consistent with each other (Fig. 1E and S. Fig. 1).
The reviewer pointed out discrepancies in our data and, upon reviewing, we have identified a dilution error leading to miscalculation of glucose consumption in Fig 5E. We have also repeated these experiments which agree with our re-calculation. Originally, it appeared from the data we presented that there was very little lactate flux, we have re-calculated the glucose excreted as lactate (average % using data from Fig. 5E and 5F) and present in a table below. We do believe we observed a Warburg effect in our proton exporting cells consistently. The reviewer points out that we utilized multiple methods to measure glycolysis in these cells leading to inconsistency, however we felt using multiple methods/instruments/kits to assess glucose consumption, lactate production, and glucose induced proton production rates was a strength of our findings as we consistently saw increased glycolysis in our proton exporting clones, irrespective of proton exporter, cell line, or method utilized. We are also not suggesting that glucose is solely being metabolized through glycolysis and do agree that it can metabolized through other metabolic pathways too such as TCA cycle, as the reviewer stated. The units used for these graphs are described in the methods and figure legends, in some assays such as Fig. 1F lactate was graphed as the ng of lactate per ul of cell culture media and then normalized per ug protein, which was determined by calculating the protein concentration of cells per well of the assay. Supplementary figure 2 has been re plotted per 10K cells to match other normalization values in the paper. Fig 1E and Fig. S1 are two different time points, M6 acidified media faster than M1 and this is likely why at 1 hour we are not yet seeing substantial increase in glucose uptake of M1.
-

Evaluation Summary:
This manuscript addresses a phenomenon of great interest to researchers in cell metabolism and cancer biology: namely, why do cancer cells often secrete high levels of lactate, despite the presence of abundant oxygen to power nutrient oxidation (Warburg effect). The authors propose that lactate export and subsequent extracellular acidification provides a selective advantage and the concomitant rise in intracellular pH is sufficient to drive flux through glycolysis, thereby sustaining the Warburg effect. This is an intriguing hypothesis that ties together many published observations, but it would require further support both from the technical and conceptual side.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested …
Evaluation Summary:
This manuscript addresses a phenomenon of great interest to researchers in cell metabolism and cancer biology: namely, why do cancer cells often secrete high levels of lactate, despite the presence of abundant oxygen to power nutrient oxidation (Warburg effect). The authors propose that lactate export and subsequent extracellular acidification provides a selective advantage and the concomitant rise in intracellular pH is sufficient to drive flux through glycolysis, thereby sustaining the Warburg effect. This is an intriguing hypothesis that ties together many published observations, but it would require further support both from the technical and conceptual side.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
In this manuscript, the authors tackle an interesting puzzle: why do cancer cells secrete most of their glucose as lactate? The authors propose that acid export is sufficient to enhance glycolysis and provide a selective advantage to cancer cells growing in vivo. To this end, the authors show that clonal lines expressing CA-IX or PMA1, each of which will facilitate proton export, have elevated capacity to acidify extracellular medium and can drive increased migration/invasion and tumor growth or metastases. In support of the model that extracellular pH is a key driver of metastases, the effect of CA-IX expression on lung metastases is reversed following bicarbonate treatment. While many of the individual conclusions of the manuscript are not novel-for example, pH has been reported to control glycolysis and …
Reviewer #1 (Public Review):
In this manuscript, the authors tackle an interesting puzzle: why do cancer cells secrete most of their glucose as lactate? The authors propose that acid export is sufficient to enhance glycolysis and provide a selective advantage to cancer cells growing in vivo. To this end, the authors show that clonal lines expressing CA-IX or PMA1, each of which will facilitate proton export, have elevated capacity to acidify extracellular medium and can drive increased migration/invasion and tumor growth or metastases. In support of the model that extracellular pH is a key driver of metastases, the effect of CA-IX expression on lung metastases is reversed following bicarbonate treatment. While many of the individual conclusions of the manuscript are not novel-for example, pH has been reported to control glycolysis and it is established that CA-IX expression modulates migration/metastases-providing a comprehensive assessment of the ability of proton export to drive the Warburg effect, and assessing the significance of metabolic rewiring driven by acid export on tumor growth, would represent an important resource for researchers intrigued by the pervasive observation that cancer cells secrete lactate despite potential bioenergetic disadvantages of discarding biomass.
The strength of the manuscript lies therefore in tying these disparate observations together in a coherent model and testing the role of acid export per se on glycolytic flux. The technical weaknesses of the paper prevent such coherent model building. A major concern is that all cell lines appear to be generated by transient transfection followed by clonal selection, giving rise to cells with notable variability and inconsistent phenotypes. More traditional approaches to manipulate enzyme expression will provide more robust model systems to test the proposed model. Similarly, direct measures of glycolytic flux are required to make conclusions about the role of acid export in promoting glycolysis. Another strength is the use of heterologous enzyme systems to alter proton export in cancer cells, but alternative explanations for these results are not fully considered. Ultimately, to what extent acid export per se, as opposed to altered metabolism driven by acid export, drives enhanced tumor metastases is not addressed.
-

Reviewer #2 (Public Review):
The work by Xu et al proposes that the Warburg effect - the increase of glycolytic metabolism usually displayed by tumor cells, is driven by increased proton excretion rather than by oncogenic dysregulation of glycolytic enzyme levels. As a proof-of-principle, they engineered tumor cells to increase proton excretion. They observed an increase in glycolytic rate, pH, and malignancy in their engineered cells.
1. My main issue with this work is that I do not agree with the authors when they say that the "canonical view" is that oncolytic mutations are thought to drive the Warburg effect. What I understand the consensus to be, is that it is fast proliferating cells - rather than malignant cells - the ones who display this form of metabolism. The rationale is that glycolytic metabolism allows keeping biomass by …
Reviewer #2 (Public Review):
The work by Xu et al proposes that the Warburg effect - the increase of glycolytic metabolism usually displayed by tumor cells, is driven by increased proton excretion rather than by oncogenic dysregulation of glycolytic enzyme levels. As a proof-of-principle, they engineered tumor cells to increase proton excretion. They observed an increase in glycolytic rate, pH, and malignancy in their engineered cells.
1. My main issue with this work is that I do not agree with the authors when they say that the "canonical view" is that oncolytic mutations are thought to drive the Warburg effect. What I understand the consensus to be, is that it is fast proliferating cells - rather than malignant cells - the ones who display this form of metabolism. The rationale is that glycolytic metabolism allows keeping biomass by redirecting lactate and from the phosphate pentose pathway. In contrast, the end product of oxidative phosphorylation is CO2 that cannot be further utilized in cell metabolism.
They claim that they Vander Heiden et al., 2009 shows that "fermentation under aerobic conditions is energetically unfavorable and does not confer any clear evolutionary benefits." This is incorrect. While that review states that the Warburg effect has little effect on the ATP/ADP ratio, they do show this form of metabolism has significant benefits for fast proliferating cells. In fact, the whole review is about how the Warburg effect is a necessary metabolic adaptation for fast proliferation rather than a unique feature of malignant cells.
2. Their main observation is not surprising. From a biochemical standpoint, protons are final product of glycolysis (from the production of lactic acid). Thus, by mass action, any mechanism to remove protons from the cell will result in accelerated glycolytic rate. Similarly, reducing intracellular pH will necessarily slow down LDHA's activity, which in turn will slow down pyruvate kinase and so on.
3. Their experiments are conducted on transformed cells - that by definition - have oncogenic driver mutations. They should test the effect of proton exporter using primary non-transformed cells (fresh MEFs, immune cells, etc). I would expect that they will still see the increase in glycolysis in this case. And yet, I would still have my concerns I expressed in my previous point.
4. The fact that they can accelerate the Warburg effect by increasing proton export does not mean is the mechanism used by tumor cells in patients or "the driver" of this effect. As I mentioned, their observation is expected by mass action but tumors that do not overexpress proton transporter may still drive their Warburg effect via oncogenic mutations. The biochemical need here is to increase the sources of biomass and redox potential and evolution will select for more glycolytic phenotypes.
-

Reviewer #3 (Public Review):
The authors claim that "proton export drives the Warburg effect". For this, they expressed proton-exporting proteins in cells and measured the intracellular proton concentration and the Warburg effect. Based on their data, however, I do not see elevated Warburg effect in these cells and thus conclude that the claim is not supported.
The authors concluded that the CA-IX or PMA1 expressing cells had increased Warburg effect. I don't think this conclusion can be made based on the data presented. For the MCF-7 cells, the glucose consumption is ~18 pmol/cell/24hr (Fig. 5E) and lactate production is ~0.6 pmol/cell/24hr (Fig. 5F), indicating that 0.6/18/2 = 1.7% of the glucose is excreted as lactate. This low percentage remains true for the PMA1 expressing cells. For example, for the PMA1-C5 cells, the percentage …
Reviewer #3 (Public Review):
The authors claim that "proton export drives the Warburg effect". For this, they expressed proton-exporting proteins in cells and measured the intracellular proton concentration and the Warburg effect. Based on their data, however, I do not see elevated Warburg effect in these cells and thus conclude that the claim is not supported.
The authors concluded that the CA-IX or PMA1 expressing cells had increased Warburg effect. I don't think this conclusion can be made based on the data presented. For the MCF-7 cells, the glucose consumption is ~18 pmol/cell/24hr (Fig. 5E) and lactate production is ~0.6 pmol/cell/24hr (Fig. 5F), indicating that 0.6/18/2 = 1.7% of the glucose is excreted as lactate. This low percentage remains true for the PMA1 expressing cells. For example, for the PMA1-C5 cells, the percentage of glucose going to lactate is about 1.8/38/2 = 2.4% (Fig. 5EF). While indeed there was an increase of both the glucose and lactate fluxes in the PMA1 expressing cells, the vast majority of the glucose flux ends up elsewhere likely the TCA cycle. This is a very different phenotype from cancer cells that have Warburg effect. The same calculation can be done for the CA-IX cells but the data on the glucose and lactate concentration there are inconsistent and expressed in confusing units (which I will elaborate in the next paragraph). Nevertheless, as there were at most a few folds of increase in lactate production flux in the M1 and M6 cells, the glucose flux going to lactate production is likely also a few percent of the total glucose uptake flux. Again, these cells do not really have Warburg effect.
The glucose and lactate concentration data are key to the study. The data however appear to lack consistency. The lactate concentration data in Fig. 1F shows a ~5-fold increase in the M1 and M6 cells than the controls but the same data in S. Fig. 2 shows a mere ~50% increase. The meaning of the units on these figures is not clear. While "1 ng/ug protein" means 1ng of lactate is produced by 1 ug protein of cells over a 24 hour period, I do not understand what "ng/ul/ug protein" means (Fig. 1F). Also, "g/L/cell" must be a typo (S. Fig. 2). Furthermore, regarding the important glucose consumption flux, it is not clear why the authors did not directly measure it as they did for the PMA1 cells (Fig. 5E). Instead, they showed two indirect measurements which are not consistent with each other (Fig. 1E and S. Fig. 1).
-