Single-cell profiling reveals periventricular CD56bright NK cell accumulation in multiple sclerosis
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This is a well-written, well-illustrated and well-conducted study of the immune cell landscape of multiple sclerosis (MS) tissue, with a particular focus on the periventricular region (septum) and choroid plexus, using single cell mass cytometry (CyTOF). Overall the work is an impressive analysis of an understudied cell-type in MS, and represents an important resource. It will be important to follow up to establish how representative the findings are given the heterogeneity of the disease and the limited population studied here.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Multiple sclerosis (MS) is a chronic demyelinating disease characterised by immune cell infiltration resulting in lesions that preferentially affect periventricular areas of the brain. Despite research efforts to define the role of various immune cells in MS pathogenesis, the focus has been on a few immune cell populations while full-spectrum analysis, encompassing others such as natural killer (NK) cells, has not been performed. Here, we used single-cell mass cytometry (CyTOF) to profile the immune landscape of brain periventricular areas – septum and choroid plexus – and of the circulation from donors with MS, dementia and controls without neurological disease. Using a 37-marker panel, we revealed the infiltration of T cells and antibody-secreting cells in periventricular brain regions and identified a novel NK cell signature specific to MS. CD56 bright NK cells were accumulated in the septum of MS donors and displayed an activated and migratory phenotype, similar to that of CD56 bright NK cells in the circulation. We validated this signature by multiplex immunohistochemistry and found that the number of NK cells with high expression of granzyme K, typical of the CD56 bright subset, was increased in both periventricular lesions and the choroid plexus of donors with MS. Together, our multi-tissue single-cell data shows that CD56 bright NK cells accumulate in the periventricular brain regions of MS patients, bringing NK cells back to the spotlight of MS pathology.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
Overall the work is an impressive analysis of an understudied cell-type in human MS, and represents an important finding. The paper is well presented and the figures very clear. However, the manuscript is descriptive and, although this is not a problem by itself, the depth and limitations of the Cytof (only 37 markers) leaves the reader without a clear idea of what these cells could be doing.
Some single-cell RNAseq and other ways to interrogate potential mechanisms and function would be particularly helpful here, but is perhaps beyond the scope of the paper.
We thank the reviewer for this nice comment. We fully agree that a next informative step would be the investigation of the function and mechanisms of the NK cell populations in MS pathology. At this moment, that is indeed beyond …
Author Response:
Reviewer #1 (Public Review):
Overall the work is an impressive analysis of an understudied cell-type in human MS, and represents an important finding. The paper is well presented and the figures very clear. However, the manuscript is descriptive and, although this is not a problem by itself, the depth and limitations of the Cytof (only 37 markers) leaves the reader without a clear idea of what these cells could be doing.
Some single-cell RNAseq and other ways to interrogate potential mechanisms and function would be particularly helpful here, but is perhaps beyond the scope of the paper.
We thank the reviewer for this nice comment. We fully agree that a next informative step would be the investigation of the function and mechanisms of the NK cell populations in MS pathology. At this moment, that is indeed beyond the scope of the current manuscript. We do believe that our findings can guide future studies to explore potential mechanisms of NK cells in more depth.
At minimum more immunohistochemical and smFish or in situ hybridization to validate key findings (using the markers identified by CyTOF) and add to the spatial relationships of Nk Cells with other border and brain cells would be informative.
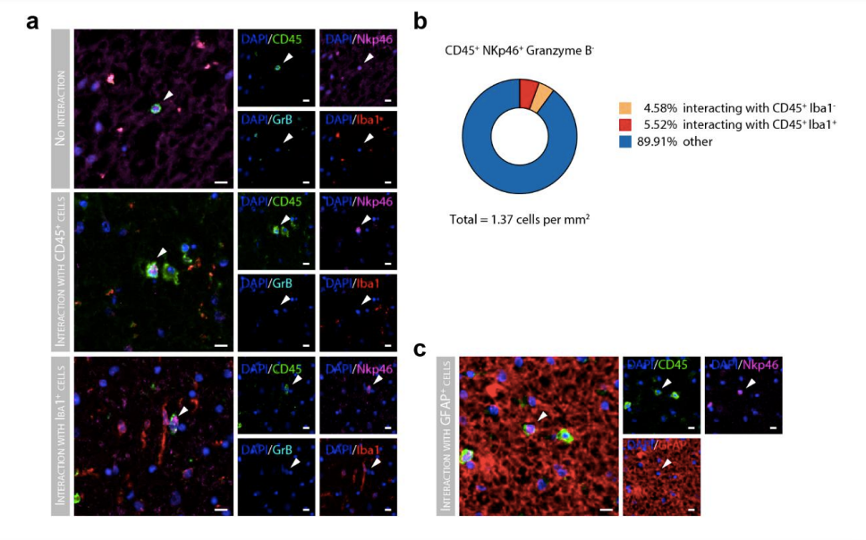
We appreciate this suggestion and have performed different immunohistochemical analysis to study the spatial relationship of NK cells and other immune and brain cells in the MS brain (Essential Revisions Fig. 1). We have stained the same cohort described in the manuscript for CD45, NKp46, GrB and Iba1 as well as CD45, NKp46, GrB and GFAP, to study the interaction of NK cells with microglia/macrophages and astrocytes, respectively, and with CD45+ immune cells in general. In MS lesions, we were able to detect a small but similar percentage of putative CD56bright NK cells (CD45+ NKp46+ GrB- cells) interacting with CD45+ Iba1- cells and with CD45+ Iba1+ cells (Essential Revisions Figure 1a-b). Due to astrogliosis, the processes of astrocytes densely populate the MS lesions and as such, we cannot infer if the interaction between NK cells and astrocytes is functional. Furthermore, the absolute number of NK cells in control brains is low, so we can only obtain reliable data from MS brains. As a result, we are unable to compare the observed interactions in MS lesions with a control condition. Of note, CD56bright NK cells are potent cytokine producers and their potential regulatory functions are not be limited to contact-dependent interactions.
Essential Revisions Fig. 1 cellular interactions of Granzyme B- NK cells (a) Representative immunohistochemical staining of Granzyme B- NK cells stained for CD45 (green), NKp46 (magenta) and negative for Granzyme B (cyan), together with microglia stained with iba1 (red). Scale bar = 10µm. (b) Pie chart displays the percentage of CD45+ NKp46+ Granzyme Bcells interacting with CD45+ Iba1+ and C45+ Iba1- cells in MS lesions. (c) Representative immunohistochemical staining of NK cells stained for CD45 (green), NKp46 (magenta) and negative for Granzyme B (cyan), together with astrocytes stained with GFAP (red). Scale bar = 10µm.
A major weakness of the study is that is is underpowered and thus not clear how robust or representative these findings are in MS given the heterogeneity of the disease and also potential differences in Sex, Age and lack of healthy controls. (AD samples labelled as control.)
We thank the reviewer for their comment. First we would like to comment on the presumed lack of healthy controls. In this study, we included two ‘control’ groups, one of them consisted out of non-neurological controls (“NNC”), free of any neurological disease, and the other consisted of neurological controls (“NC”), including demented and Alzheimer patients. We acknowledge that this terminology leaves the reader confused; as such, we renamed the “NC” group with patients suffering from dementia to “Dementia” and the “NNC” group of donors without neurological disease to “Controls”.
Secondly, while our sample size is rather small, it is comparable to other studies that use fresh post-mortem brain tissue (Böttcher et al, 2020).. The usage of this unique postmortem brain tissue from human donors is severely limited by the number of well-characterized samples available, their demographics and clinical background. To overcome the underpowered design and possible effects of confounders as sex and age, we validated our main finding by multiplex immunohistochemistry in a separate cohort. This included 5 controls (2 females, 3 males, f:m ratio of 0.667) and 7 MS cases (3 females and 4 males, f:m of 0.75), with a similar female/male ratio and matched age (Wilcoxon rank sum test with continuity correction, p-value = 0.41). We now included the characteristics of the validation cohort in the manuscript as well.
“Finally, to confirm that CD56bright NK cells accumulate in periventricular brain regions in MS donors, we used multiplex immunohistochemistry in an independent cohort (Table 1), wherein MS and control groups were age-matched (Wilcoxon rank sum test with continuity correction, p-value = 0.41) and had a similar female:male ratio (0.667 in controls and 0.75 in MS).”
Böttcher C, van der Poel M, Fernández-Zapata C, Schlickeiser S, Leman JKH, Hsiao CC, Mizee MR, Adelia, Vincenten MCJ, Kunkel D, Huitinga I, Hamann J, Priller J (2020) Single-cell mass cytometry reveals complex myeloid cell composition in active lesions of progressive multiple sclerosis. Acta neuropathologica communications, 8(1), 1-18
It is also important to show the NK cells are actually in the parenchyma and interacting with other cells (e.g., microglia) of the lesion. If the authors have this tissue and antibodies to do that, this would add to the study. Moreover, the details on samples and controls should be more clearly communicated in the text and legends as well as the caveats and limitations of the study in the Discussion.
The location of NK cells within the brain parenchyma is an important determinant of their function within the CNS. Thus, we included a basement membrane marker (collagen IV) in our multiplex IHC panel in order to exclude the cells within the vessel lumen. As this has not been clearly communicated, we have adjusted the sentence from the subsection Multiplex immunohistochemistry in the Methods (from “Cells within the lumen of vessels from the choroid plexus sections were excluded manually” to “Cells within the lumen of vessels were excluded manually with the aid of collagen IV staining.”). We have addressed in Essential Revisions Fig. 1 the additional IHC experiments performed to explore the interactions of NK cells with other brainresident cells. We thank the reviewer for warning us on the difficulty of our nomenclature. We have thus adjusted the labels of the three main groups throughout the manuscript as follows: Control (previously, NNC), Dementia (previously, NC) and MS (same as before). We also have expanded the limitations of this study in the Discussion.
“Our study has two main limitations, first scarcity of fresh human tissue prevented having sex and age-matched groups with large sample sizes for the CyTOF analysis. To overcome the underpowered design and possible effects of confounders, we have validated our main finding by multiplex immunohistochemistry in a separate cohort with a similar age and female/male ratio. Secondly, there is a strong contribution of blood-derived immune cells in the choroid plexus, which precluded a clear distinction between circulating and stromal immune cells. This may have prevented the detection of choroid-plexus specific changes in the stroma, such as an accumulation of CD8+ T cells in the choroid plexus from MS donors, previously described by our group using immunohistochemistry [47]. In addition, the high proportion of granulocytes in the CP as detected by our CyTOF analysis likely originates from the circulation [47,63]. Contrariwise, the scarcity of B cells, despite the high vascularisation, is in line with previous reports [47,63]; and the detection of rare ASCs in the choroid plexus but not in the blood reassures their tissue specificity [63].”
Reviewer #2 (Public Review):
The data are extensive, valuable, convincing, and entirely descriptive (as studies using human post-mortem material must be, of necessity). What emerges is a detailed account of NK cells in specific regions of the MS brain (although here the authors slightly overplay how little is known about NK cells in MS). The study provides a very comprehensive resource. The authors speculate on what their data might mean in terms of disease dynamics is a reasonable and informed way, but much of what is concluded is inference not backed up by experiment studies that would allow this to be more than a resource paper.
We thank the reviewer for his/her compliments and agree that in this manuscript we can only speculate on the role of NK cells and their way of migration or proliferation, to and within the brain. Only future research can solve these speculations. We have addressed these concerns accordingly in the discussion and have removed any concluding or far-fetched speculations which is not backed-up by our own data.
Reviewer #3 (Public Review):
The authors introduce their work in the context of the prevailing uncertainties about the pathogenesis of multiple sclerosis (MS) and, in particular, seem to reference the initiation of immune lesions in early MS. However, the work itself addresses end-stage MS situations, which is quite possibly an entirely different landscape altogether, and may not be informative about MS initiation.
We want to thank the reviewer for pointing out this misleading part of the text. We agree that our study does not provide any information on the initial stages of MS, and have therefore adjusted this part of the introduction to avoid confusion. “Brain regions around the ventricles are hotspots for MS lesions [8,21,39,52], but underlying mechanisms are poorly understood [41]. Since the majority of periventricular MS lesions occur around a central vessel [1,57], it has been suggested that vascular topography may influence MS pathology [33].”
As a textual point, the manuscript makes far too many speculations about possible cell trafficking between compartments than is justified by a cross-section study.
We appreciate this concern and we have therefore tuned down our speculations in the results and discussion sections.
That said, the work itself is a carefully done descriptive characterisation of the leucocyte landscape found in the periventricular septum, choroid plexus (and peripheral blood) post-mortem from cases of multiple sclerosis (MS), non-MS neurological disease (dementia), and non-neurological controls (8-12 each). The material is rare, the post-mortem delays are quite short, the cell lineage characterisation is fairly extensive and some of the data are well supported by immunohistochemistry.
We thank the reviewer for these compliments.
-

Evaluation Summary:
This is a well-written, well-illustrated and well-conducted study of the immune cell landscape of multiple sclerosis (MS) tissue, with a particular focus on the periventricular region (septum) and choroid plexus, using single cell mass cytometry (CyTOF). Overall the work is an impressive analysis of an understudied cell-type in MS, and represents an important resource. It will be important to follow up to establish how representative the findings are given the heterogeneity of the disease and the limited population studied here.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. The reviewers remained anonymous to the authors.)
-

Reviewer #1 (Public Review):
Rodríguez-Lorenzo et al combine multidimensional CyTOF analysis with multiplexed IHC to determine the presence of immune cells in the septum and choroid plexus of control and MS patients. The authors collect a cohort of human brains samples and perform extensive characterization with a 37-antibody panel. Interestingly, they found increased CD8 T-cells, CD4 T-cells and increased NK cells. The NK cells are of special interest as these cells have not been well characterized in MS and previous reports have shown a decline in the periphery. The authors further refine the T-cell and NK cell populations in both the choroid and septum, and the role of B-cells in the blood. Finally the authors use multiplex IHC to localize specific NK subsets to the lesion and border of MS lesions.
Overall the work is an impressive …
Reviewer #1 (Public Review):
Rodríguez-Lorenzo et al combine multidimensional CyTOF analysis with multiplexed IHC to determine the presence of immune cells in the septum and choroid plexus of control and MS patients. The authors collect a cohort of human brains samples and perform extensive characterization with a 37-antibody panel. Interestingly, they found increased CD8 T-cells, CD4 T-cells and increased NK cells. The NK cells are of special interest as these cells have not been well characterized in MS and previous reports have shown a decline in the periphery. The authors further refine the T-cell and NK cell populations in both the choroid and septum, and the role of B-cells in the blood. Finally the authors use multiplex IHC to localize specific NK subsets to the lesion and border of MS lesions.
Overall the work is an impressive analysis of an understudied cell-type in human MS, and represents an important finding. The paper is well presented and the figures very clear. However, the manuscript is descriptive and, although this is not a problem by itself, the depth and limitations of the Cytof (only 37 markers) leaves the reader without a clear idea of what these cells could be doing.
Some single-cell RNAseq and other ways to interrogate potential mechanisms and function would be particularly helpful here, but is perhaps beyond the scope of the paper. At minimum more immunohistochemical and smFish or in situ hybridization to validate key findings (using the markers identified by CyTOF) and add to the spatial relationships of Nk Cells with other border and brain cells would be informative.
A major weakness of the study is that is is underpowered and thus not clear how robust or representative these findings are in MS given the heterogeneity of the disease and also potential differences in Sex, Age and lack of healthy controls. (AD samples labelled as control.)
It is also important to show the NK cells are actually in the parenchyma and interacting with other cells (e.g., microglia) of the lesion. If the authors have this tissue and antibodies to do that, this would add to the study. Moreover, the details on samples and controls should be more clearly communicated in the text and legends as well as the caveats and limitations of the study in the Discussion.
-

Reviewer #2 (Public Review):
The data are extensive, valuable, convincing, and entirely descriptive (as studies using human post-mortem material must be, of necessity). What emerges is a detailed account of NK cells in specific regions of the MS brain (although here the authors slightly overplay how little is known about NK cells in MS). The study provides a very comprehensive resource. The authors speculate on what their data might mean in terms of disease dynamics is a reasonable and informed way, but much of what is concluded is inference not backed up by experiment studies that would allow this to be more than a resource paper.
-

Reviewer #3 (Public Review):
The authors introduce their work in the context of the prevailing uncertainties about the pathogenesis of multiple sclerosis (MS) and, in particular, seem to reference the initiation of immune lesions in early MS. However, the work itself addresses end-stage MS situations, which is quite possibly an entirely different landscape altogether, and may not be informative about MS initiation.
As a textual point, the manuscript makes far too many speculations about possible cell trafficking between compartments than is justified by a cross-section study.
That said, the work itself is a carefully done descriptive characterisation of the leucocyte landscape found in the periventricular septum, choroid plexus (and peripheral blood) post-mortem from cases of multiple sclerosis (MS), non-MS neurological disease …
Reviewer #3 (Public Review):
The authors introduce their work in the context of the prevailing uncertainties about the pathogenesis of multiple sclerosis (MS) and, in particular, seem to reference the initiation of immune lesions in early MS. However, the work itself addresses end-stage MS situations, which is quite possibly an entirely different landscape altogether, and may not be informative about MS initiation.
As a textual point, the manuscript makes far too many speculations about possible cell trafficking between compartments than is justified by a cross-section study.
That said, the work itself is a carefully done descriptive characterisation of the leucocyte landscape found in the periventricular septum, choroid plexus (and peripheral blood) post-mortem from cases of multiple sclerosis (MS), non-MS neurological disease (dementia), and non-neurological controls (8-12 each). The material is rare, the post-mortem delays are quite short, the cell lineage characterisation is fairly extensive and some of the data are well supported by immunohistochemistry.
-