Sigma oscillations protect or reinstate motor memory depending on their temporal coordination with slow waves
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The authors report a preregistered study which tests the effects of targeted memory reactivation (TMR), which is typically studied in the context of declarative memory, on motor memory consolidation during sleep. In a nap study, the authors use a standard TMR paradigm. Their results suggest a key role of oscillatory activity for motor memory consolidation, where distinct features of the slow oscillation spindle interaction mediate memory formation. Overall, this is a timely interesting. It is scientifically rigorous and transparently reported. The claims are well supported by the data.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Targeted memory reactivation (TMR) during post-learning sleep is known to enhance motor memory consolidation but the underlying neurophysiological processes remain unclear. Here, we confirm the beneficial effect of auditory TMR on motor performance. At the neural level, TMR enhanced slow wave (SW) characteristics. Additionally, greater TMR-related phase-amplitude coupling between slow (0.5–2 Hz) and sigma (12–16 Hz) oscillations after the SW peak was related to higher TMR effect on performance. Importantly, sounds that were not associated to learning strengthened SW-sigma coupling at the SW trough. Moreover, the increase in sigma power nested in the trough of the potential evoked by the unassociated sounds was related to the TMR benefit. Altogether, our data suggest that, depending on their precise temporal coordination during post learning sleep, slow and sigma oscillations play a crucial role in either memory reinstatement or protection against irrelevant information; two processes that critically contribute to motor memory consolidation.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
A clear strength of the present manuscript is its scientific rigor. The authors put a lot of emphasis on transparent reporting and pre-registered their hypotheses. The within-person experimental design is well constructed and deals upfront with several potential confounds. All in all, the experimental design allowed a replication and extension of findings related to evoked neural responses due to auditory presentation during sleep. Nevertheless, the exact neural mechanisms that should drive sleepdependent learning gains due to reactivation remain elusive. In part this is due to analytical choices - especially with regard to the phase-amplitude coupling analyses. For example, it remains to be established that there is a reliable coupling of SOs and SPs before any condition specific analyses …
Author Response
Reviewer #1 (Public Review):
A clear strength of the present manuscript is its scientific rigor. The authors put a lot of emphasis on transparent reporting and pre-registered their hypotheses. The within-person experimental design is well constructed and deals upfront with several potential confounds. All in all, the experimental design allowed a replication and extension of findings related to evoked neural responses due to auditory presentation during sleep. Nevertheless, the exact neural mechanisms that should drive sleepdependent learning gains due to reactivation remain elusive. In part this is due to analytical choices - especially with regard to the phase-amplitude coupling analyses. For example, it remains to be established that there is a reliable coupling of SOs and SPs before any condition specific analyses appear appropriate.
We thank the reviewer for these constructive remarks. We acknowledge that the description of the phase-amplitude coupling analyses lacked details in the initial submission and we therefore clarified the approach in the revised manuscript. Moreover, we followed the suggestion of the reviewer and performed additional analyses to test for coupling within each stimulation condition and at rest separately. Briefly, the results show a reliable coupling between the phase of the slow oscillations and the amplitude of the signal in the sigma band irrespective of the stimulation condition. These results are reported in Supplemental Figure S5 of the revised submission.
Reviewer #2 (Public Review):
The work by Nicolas et al. investigates neurophysiological processes in response to sound cues delivered during sleep. Importantly, the presented sound cues were previously associated with a motor sequence participants had to practice. By presenting the sound cues during sleep, performance in pressing the motor sequence was increased (targeted memory reactivation, TMR). At the neural level, presenting sound cues associated with a motor sequence resulted in a higher amplitude (of the evoked response as well as of spontaneous slow waves) than presenting sound cues without any association. Further, the precise interplay between slow and sigma oscillations correlated with the behavioural TMR benefit.
This finding is of high interest. However, some aspects of the analyses have to be clarified and the interpretation of sigma oscillations protecting motor memory (by being nested in the trough of the slow oscillation peak) has to be more substantiated by further results.
Strengths: The study is elegantly designed (within-subjects design) and allows for testing the proposed hypotheses. The study as a sleep study is well controlled for example by incorporating a habituation nap, by using actigraphy during three nights before the learning nap and by measuring vigilance objectively as well as subjectively.
One of the biggest strengths of the study is its pre-registration. The authors did not just pre-registered the study but moreover highlight and justify any deviation from the pre-registration and state whether an analysis was planned or exploratory. Thus, the whole research process is very transparent and plausible.
We thank the reviewer for these constructive and positive remarks. We acknowledge that some aspects of the analyses lacked details in the initial submission and we therefore clarified the approach in the revised manuscript. Additionally, we have thoroughly considered the reviewer’s suggestions with respect to the analyses and interpretation of the sigma oscillations data (see response to comment #2 below).
Weaknesses: The interpretation of sigma oscillations protecting motor memories (i.e., sigma power towards unassociated sound cues is increased in the trough of an evoked potential) is not very well substantiated by the results.
We thank the reviewer for giving us the opportunity to further examine the role of sigma oscillations (and their coupling with slow oscillations) in the protective processes discussed in the manuscript. Our results indeed suggest that when a control, unknown cue is presented to the sleeping brain, it might trigger protective mechanisms to prevent these “irrelevant” sensory stimuli to be processed and therefore disturb the ongoing consolidation process. Specifically, we speculated that SW-sigma coupling during exposure to unassociated sounds might prevent sound processing which would in turn be reflected by a decrease in the amplitude of the slow electrophysiological responses (i.e., smaller ERP and SWs) during non-associated sound intervals. In order to further examine this possibility, we performed exploratory analyses testing for potential relationships between the eventrelated phase-amplitude coupling (ERPAC) observed on unassociated conditions and slow electrophysiological responses (i.e., ERP and SWs). To do so, we extracted the ERPAC value during unassociated stimulation intervals in the time-frequency window where ERPAC was significantly greater for unassociated as compared to associated and rest conditions (i.e. from -0.5 to 0.5 sec and from 14 to 18 Hz, see Figure 6 in the main text). While the ERPAC during unassociated intervals did not correlate with the amplitude of the unassociated ERPs, it correlated negatively with the properties of the SWs detected during unassociated intervals. Specifically, the higher the ERPAC, the lower SW density (t = 2.9, df = 20, p-value = 0.004) and peak-to-peak amplitude (S = 2460, p-value = 0.037) during unassociated intervals. These analyses, albeit exploratory, provide further support to the protective mechanism discussed in the initial version of the manuscript. These results are now reported in the supplemental information (Supplemental Figure S9) and mentioned in the revised discussion to further substantiate the hypothesized protective mechanism (see p. 13, l. 46 of the revised manuscript).
The motivation for some analysis decisions is not always clear. To highlight one example, it is unclear why the authors average the data across channels. Previous findings demonstrate that slow oscillations and sleep spindles vary across the scalp (Klinzing et al. (2016), Cox et al. (2017)). Thus, averaging across all channels potentially introduces more noise.
We apologize for the lack of justification concerning the averaging procedures in the original manuscript. We now explain in the revised manuscript the motivation for averaging data across channels in our different analyses (see pages 21 and 23). Briefly, as our montage did not allow fine topographical analyses (only 6 EEG channels), we opted to average data across channels in order to decrease the dimensionality of the data. However, we agree with the reviewer that reporting channel level data is important. Therefore, for each analysis presented in the main text, the corresponding channel-level results are reported in the supplements (i.e., ERPs are shown in Supplemental Figure S2 and S4, correlation between targeted memory reactivation index and power modulation is depicted in Supplemental Figure S7, PAC difference at the negative peak of the SW is in Supplemental Figure S6 and PAC/TMR index correlation in Figure S8). Altogether, channel level data revealed that central – and to a lesser extent frontal - electrodes mainly contributed to the pattern of results revealed with averaged data reported in the main text.
The description of some methods has to be more precise (for example the detection of slow waves and sleep spindles and specifically the phase coupling).
We thank the reviewer for pointing that out. We have now revised the manuscript to provide the necessary details on the detection algorithms (Vallat & Walker, 2021) as well as on the event-related phase-amplitude coupling method (Voytek et al., 2013, Combrisson et al., 2020). We invite the reviewer to consult the responses to comments #13 and #16 below for detailed responses to these points.
Reviewer #3 (Public Review):
Nicolas et al. performed a nap study in healthy humans to examine the temporal dynamics of sleep oscillations during procedural memory consolidation. To this end, the authors used targeted memory reactivation (TMR) to re-expose participants during a nap to a sound cue previously associated with a finger tapping sequence. As control conditions serve (i) a second encoded sequence with a sound that is not played during sleep, (ii) a novel control cue not heard during prior wakefulness and (iii) so-called rest-periods during which no cueing was performed. Behaviorally, the authors confirm the beneficial effect of TMR as participants perform better (faster) on the reactivated sequence in comparison to the not-reactivated sequence after their nap and even after an additional night spent at home.
Electroencephalography recordings acquired during the nap then revealed that TMR cues evoked stronger responses than control cues hinting a distinct processing of familiar and memory-related cues. This is supported by a general analysis 0.5 to 2 Hz slow waves, one fundamental sleep oscillation linked to memory consolidation, which showed higher densities during intervals of real-cueing. Interestingly, the density of 12-16 Hz sleep spindles was not influenced, however, their frequency decreased and amplitude increased. Finally, the authors assessed the coupling between slow waves and sleep spindles, which rather counter-intuitively showed an increased coupling during intervals cued with control sounds. Moreover, the stronger this coupling the higher the TMR benefit.
Altogether, this data revealed an interesting slow wave-spindle dynamic underlying the processing of familiar and unfamiliar auditory cues and scrutinizes how these brain rhythms mediate memory consolidation
Overall, this is a very well-designed experiment and I salute that it has been pre-registered and how transparent everything has been reported. Moreover, the utilization of a control sound during sleep is currently rarely taken advantage of during TMR study, while they can add important insights. While the analysis pipeline is appropriate and well-rounded, some aspects need to be clarified and extended.
We would like to thank the reviewer for the time devoted to our manuscript and for the constructive comments about our work. We provide below detailed answers to the points raised by the reviewer.
Response to control sounds. It is very surprising that the response to control sounds is, apart from an early evoked component around 100 ms, almost nonexistent. Auditory stimuli are overall known to normally evoke K-complexes and strong spindle responses. Could it be that for some reason control sounds were lower in volume or do they lead to a stronger habituation? Control analysis might help to ensure that there is really no confusion. For example, ERP at the beginning and end of each stimulation interval could be contrasted. Moreover, the authors state that sound cues were balanced across subjects. However, they also state that the volume was adapted for each sound individually. Additional data or statistics on these volumes, randomization and cued slow wave phase might be very helpful.
We thank the reviewer for raising this point and for giving us the opportunity to elaborate on these aspects. The sound volume was indeed adjusted based on the perception level of each sound for each individual. As pointed out by the reviewer, this resulted in different absolute volumes for each sound and individual; however, all sounds were presented at the same percentage of detection thresholds across participants. Moreover, as the sound / condition associations were perfectly balanced in our experiment (each sound was associated to each condition 8 times), differences in sound volume - or frequency – cannot explain our pattern of results.
Further, inspection of the ERP at the individual channel level (cf. Supplemental Figure S2) revealed that unassociated auditory cues can indeed elicit negative peak on some channels (Fz and C3 to a lesser extent). We invite the reviewer to refer to our response to comment #12 of reviewer #2 for a comparison with the relevant literature.
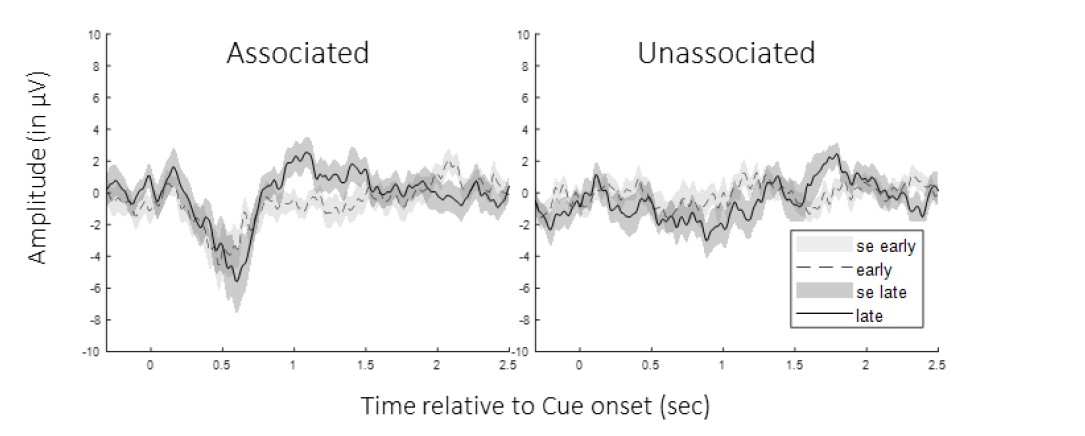
In order to address the comment of the reviewer on potential habituation effects, we performed exploratory analyses on a subset of events. Specifically, we compared the ERPs computed across the 30 first vs. the 30 last cues presented during the nap within each condition (see Figure 1 below). CBP did not reveal any difference between early and late nap ERPs in any conditions (all p-values > 0.2). Importantly, the results observed within the unassociated condition are similar to what is reported in the main text across all trials. Altogether, these analyses suggest that the weaker responses to the unassociated sound are not due to habituation processes.
Figure 1: Event-related Potentials early vs. late nap. Group average (and standard error) of potentials evoked by the 30 first (grey) and the 30 last (black) auditory cues of the nap from cue onset to 2.5 sec post-cue averaged across participants (left: associated cues; right: unassociated cues). CBP did not show any early vs. late differences in ERPs in any conditions.
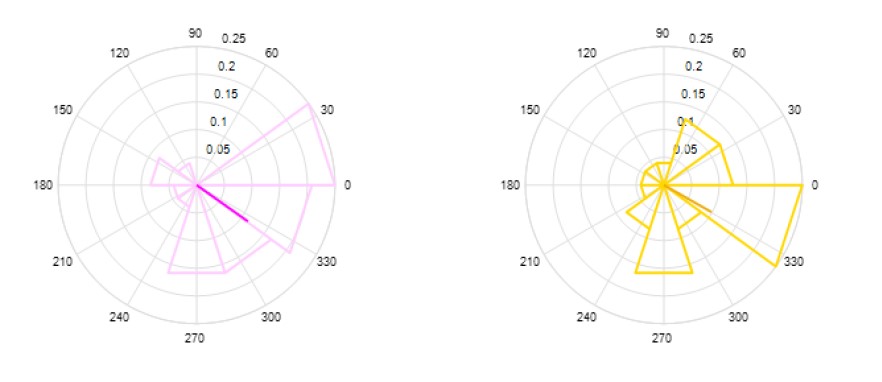
Last, with respect to the point on cued slow wave phase, we extracted the phase of the slow oscillation (0.5-2Hz) at which the auditory cues were sent in each condition separately (see Figure 2 below). We then tested whether the phases differed using Watson-Williams multi-sample test for equal means (Berens, 2009). Results showed no difference between the two conditions (F(1,46)= 0.6, p-value = 0.8), suggesting that the effects reported in the main text were not confounded by this factor.
Figure 2: Phase of slow oscillation at stimulation. Phase in degrees of the SO at the associated (magenta) or unassociated (yellow) auditory cues.
Discrete slow wave analysis. It is reported that the offline detection of slow waves yielded identical numbers across conditions, but this contradicts the later reported differences in densities. If this is true, it implies that the total time during which real cues and control cues were presented as well as the cueing paused (i.e., the rest intervals) differs within subjects. It needs to be ensured that effective stimulation times are comparable between subjects and are not confounded by unfair comparisons.
There might be a misunderstanding on this point, as we did not compare the number of SWs between conditions but only SW density and amplitude. We assume that the reviewer is referring to the number of auditory cues sent during NREM that were indeed not different across conditions.
Statistical results. Consistently across all cluster-based statistics, significant clusters somehow do not reflect the underlying colormaps. One would expect that significances are driven by clusters of greatest difference (Figure 6B and C). That something might be amiss, is reflected in the statement that a contrast of TFRs for real and control cues revealed no significant cluster, although this contrast shown in Figure 7a clear depicts two cluster with strong power differences (before 500 ms around 8 Hz, and after 500 ms around 20 Hz).
Moreover, follow-up analysis revolving around sleep spindles are based on inconsistent frequency ranges. For one analysis a prior significant cluster is used (Figure 8) while for the other it is limited to 12- 16 Hz and a much shorter time window than the overall cluster (Figure 7), even in the pre-registered 1216 Hz window. Overall, these analyses should be checked and streamlined.
We agree with the reviewer that time-frequency representations (TFR) of results can somehow be misleading as inter-subject variability is not represented. As such, clusters showing e.g. a high difference in PAC between conditions but also high inter-subject variability would be represented with warm colors in the TFR but would not be highlighted by the CBP statistics (as seen for example in Figure 6B and C). Instead, what is highlighted by CBP are effects that are consistent across participants and these effects can indeed be of lower amplitude in some cases.
Concerning Figure 7, the initial time-frequency plot presented the power difference between conditions that was subsequently correlated with the TMR index while the statistical cluster showed the results of the correlation. As this was indeed confusing (see also our response to comment #10 below and to comments #26 and #27 of reviewer #2), we now show the rho values issued from the correlation between the power difference and the TMR index. We thank the reviewer for pointing this out, as the new representation improved the readability of the figure.
Last, we want to thank the reviewer for pointing out the discrepancy regarding the procedure used to extract the data for the scatter plots shown in panel B of Figures 7 and 8 (referred to as “follow-up analyses” by the reviewer). We now extract the values in the significant clusters included in the preregistered frequency band (12-16 Hz) for both analyses presented in Figures 7 and 8. It is worth nothing though that this procedure was only used for illustration purposes and was therefore not a formal follow-up analysis. We acknowledge that the p-values displayed on the panel B plots of the original figures might be misleading with that regard, thus they were removed in the revised manuscript.
-

Evaluation Summary:
The authors report a preregistered study which tests the effects of targeted memory reactivation (TMR), which is typically studied in the context of declarative memory, on motor memory consolidation during sleep. In a nap study, the authors use a standard TMR paradigm. Their results suggest a key role of oscillatory activity for motor memory consolidation, where distinct features of the slow oscillation spindle interaction mediate memory formation. Overall, this is a timely interesting. It is scientifically rigorous and transparently reported. The claims are well supported by the data.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the …
Evaluation Summary:
The authors report a preregistered study which tests the effects of targeted memory reactivation (TMR), which is typically studied in the context of declarative memory, on motor memory consolidation during sleep. In a nap study, the authors use a standard TMR paradigm. Their results suggest a key role of oscillatory activity for motor memory consolidation, where distinct features of the slow oscillation spindle interaction mediate memory formation. Overall, this is a timely interesting. It is scientifically rigorous and transparently reported. The claims are well supported by the data.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
A clear strength of the present manuscript is its scientific rigor. The authors put a lot of emphasis on transparent reporting and pre-registered their hypotheses. The within-person experimental design is well constructed and deals upfront with several potential confounds. All in all, the experimental design allowed a replication and extension of findings related to evoked neural responses due to auditory presentation during sleep.
Nevertheless, the exact neural mechanisms that should drive sleep-dependent learning gains due to reactivation remain elusive. In part this is due to analytical choices - especially with regard to the phase-amplitude coupling analyses. For example, it remains to be established that there is a reliable coupling of SOs and SPs before any condition specific analyses appear appropriate.
Reviewer #1 (Public Review):
A clear strength of the present manuscript is its scientific rigor. The authors put a lot of emphasis on transparent reporting and pre-registered their hypotheses. The within-person experimental design is well constructed and deals upfront with several potential confounds. All in all, the experimental design allowed a replication and extension of findings related to evoked neural responses due to auditory presentation during sleep.
Nevertheless, the exact neural mechanisms that should drive sleep-dependent learning gains due to reactivation remain elusive. In part this is due to analytical choices - especially with regard to the phase-amplitude coupling analyses. For example, it remains to be established that there is a reliable coupling of SOs and SPs before any condition specific analyses appear appropriate.
-

Reviewer #2 (Public Review):
The work by Nicolas et al. investigates neurophysiological processes in response to sound cues delivered during sleep. Importantly, the presented sound cues were previously associated with a motor sequence participants had to practice. By presenting the sound cues during sleep, performance in pressing the motor sequence was increased (targeted memory reactivation, TMR). At the neural level, presenting sound cues associated with a motor sequence resulted in a higher amplitude (of the evoked response as well as of spontaneous slow waves) than presenting sound cues without any association. Further, the precise interplay between slow and sigma oscillations correlated with the behavioural TMR benefit.
This finding is of high interest. However, some aspects of the analyses have to be clarified and the …
Reviewer #2 (Public Review):
The work by Nicolas et al. investigates neurophysiological processes in response to sound cues delivered during sleep. Importantly, the presented sound cues were previously associated with a motor sequence participants had to practice. By presenting the sound cues during sleep, performance in pressing the motor sequence was increased (targeted memory reactivation, TMR). At the neural level, presenting sound cues associated with a motor sequence resulted in a higher amplitude (of the evoked response as well as of spontaneous slow waves) than presenting sound cues without any association. Further, the precise interplay between slow and sigma oscillations correlated with the behavioural TMR benefit.
This finding is of high interest. However, some aspects of the analyses have to be clarified and the interpretation of sigma oscillations protecting motor memory (by being nested in the trough of the slow oscillation peak) has to be more substantiated by further results.
Strengths:
The study is elegantly designed (within-subjects design) and allows for testing the proposed hypotheses. The study as a sleep study is well controlled for example by incorporating a habituation nap, by using actigraphy during three nights before the learning nap and by measuring vigilance objectively as well as subjectively.
One of the biggest strengths of the study is its pre-registration. The authors did not just pre-registered the study but moreover highlight and justify any deviation from the pre-registration and state whether an analysis was planned or exploratory. Thus, the whole research process is very transparent and plausible.
Weaknesses:
(1) The interpretation of sigma oscillations protecting motor memories (i.e., sigma power towards unassociated sound cues is increased in the trough of an evoked potential) is not very well substantiated by the results.
(2) The motivation for some analysis decisions is not always clear. To highlight one example, it is unclear why the authors average the data across channels. Previous findings demonstrate that slow oscillations and sleep spindles vary across the scalp (Klinzing et al. (2016), Cox et al. (2017)). Thus, averaging across all channels potentially introduces more noise.
(3) The description of some methods has to be more precise (for example the detection of slow waves and sleep spindles and specifically the phase coupling).
-

Reviewer #3 (Public Review):
Nicolas et al. performed a nap study in healthy humans to examine the temporal dynamics of sleep oscillations during procedural memory consolidation. To this end, the authors used targeted memory reactivation (TMR) to re-expose participants during a nap to a sound cue previously associated with a finger tapping sequence. As control conditions serve (i) a second encoded sequence with a sound that is not played during sleep, (ii) a novel control cue not heard during prior wakefulness and (iii) so-called rest-periods during which no cueing was performed. Behaviorally, the authors confirm the beneficial effect of TMR as participants perform better (faster) on the reactivated sequence in comparison to the not-reactivated sequence after their nap and even after an additional night spent at home.
Electroencephalogra…
Reviewer #3 (Public Review):
Nicolas et al. performed a nap study in healthy humans to examine the temporal dynamics of sleep oscillations during procedural memory consolidation. To this end, the authors used targeted memory reactivation (TMR) to re-expose participants during a nap to a sound cue previously associated with a finger tapping sequence. As control conditions serve (i) a second encoded sequence with a sound that is not played during sleep, (ii) a novel control cue not heard during prior wakefulness and (iii) so-called rest-periods during which no cueing was performed. Behaviorally, the authors confirm the beneficial effect of TMR as participants perform better (faster) on the reactivated sequence in comparison to the not-reactivated sequence after their nap and even after an additional night spent at home.
Electroencephalography recordings acquired during the nap then revealed that TMR cues evoked stronger responses than control cues hinting a distinct processing of familiar and memory-related cues. This is supported by a general analysis 0.5 to 2 Hz slow waves, one fundamental sleep oscillation linked to memory consolidation, which showed higher densities during intervals of real-cueing. Interestingly, the density of 12-16 Hz sleep spindles was not influenced, however, their frequency decreased and amplitude increased. Finally, the authors assessed the coupling between slow waves and sleep spindles, which rather counter-intuitively showed an increased coupling during intervals cued with control sounds. Moreover, the stronger this coupling the higher the TMR benefit.
Altogether, this data revealed an interesting slow wave-spindle dynamic underlying the processing of familiar and unfamiliar auditory cues and scrutinizes how these brain rhythms mediate memory consolidation
Overall, this is a very well-designed experiment and I salute that it has been pre-registered and how transparent everything has been reported. Moreover, the utilization of a control sound during sleep is currently rarely taken advantage of during TMR study, while they can add important insights. While the analysis pipeline is appropriate and well-rounded, some aspects need to be clarified and extended.
Response to control sounds:
It is very surprising that the response to control sounds is, apart from an early evoked component around 100 ms, almost nonexistent. Auditory stimuli are overall known to normally evoke K-complexes and strong spindle responses. Could it be that for some reason control sounds were lower in volume or do they lead to a stronger habituation? Control analysis might help to ensure that there is really no confusion. For example, ERP at the beginning and end of each stimulation interval could be contrasted. Moreover, the authors state that sound cues were balanced across subjects. However, they also state that the volume was adapted for each sound individually. Additional data or statistics on these volume, randomization and cued slow wave phase might be very helpful.
Discrete slow wave analysis:
It is reported that the offline detection of slow waves yielded identical numbers across conditions, but this contradicts the later reported differences in densities. If this is true, it implies that the total time during which real cues and control cues were presented as well as the cueing paused (i.e., the rest intervals) differs within subjects. It needs to be ensured that effective stimulation times are comparable between subjects and are not confounded by unfair comparisons
Statistical results:
Consistently across all cluster-based statistics, significant clusters somehow do not reflect the underlying colormaps. One would expect that significances are driven by clusters of greatest difference (Figure 6B and C). That something might be amiss, is reflected in the statement that a contrast of TFRs for real and control cues revealed no significant cluster, although this contrast shown in Figure 7a clear depicts two cluster with strong power differences (before 500 ms around 8 Hz, and after 500 ms around 20 Hz).
Moreover, follow-up analysis revolving around sleep spindles are based on inconsistent frequency ranges. For one analysis a prior significant cluster is used (Figure 8) while for the other it is limited to 12- 16 Hz and a much shorter time window than the overall cluster (Figure 7), even in the pre-registered 12-16 Hz window. Overall, these analyses should be checked and streamlined.
-