Rapid odor processing by layer 2 subcircuits in lateral entorhinal cortex
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Bitzenhofer and colleagues provide unprecedented details of odor response properties of layer 2 cells in LEC. The authors show that firing rates of LEC ensembles conveyed information about odor identify whereas timing of spikes odor intensity and that, on average, fan cells responded earlier than pyramidal neurons, and pyramidal neurons, but not fan cells, changed their peak timing in response to changes in concentrations, providing a basis for temporal coding of odor concentrations. The results provide important information about odor coding in LEC, an understudied area of the brain.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Olfactory information is encoded in lateral entorhinal cortex (LEC) by two classes of layer 2 (L2) principal neurons: fan and pyramidal cells. However, the functional properties of L2 cells and how they contribute to odor coding are unclear. Here, we show in awake mice that L2 cells respond to odors early during single sniffs and that LEC is essential for rapid discrimination of both odor identity and intensity. Population analyses of L2 ensembles reveal that rate coding distinguishes odor identity, but firing rates are only weakly concentration dependent and changes in spike timing can represent odor intensity. L2 principal cells differ in afferent olfactory input and connectivity with inhibitory circuits and the relative timing of pyramidal and fan cell spikes provides a temporal code for odor intensity. Downstream, intensity is encoded purely by spike timing in hippocampal CA1. Together, these results reveal the unique processing of odor information by LEC subcircuits and highlight the importance of temporal coding in higher olfactory areas.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
The lateral entorhinal cortex (LEC) receives direct inputs from the olfactory bulb (OB) but their odor response properties have not been well characterized despite a recent increase in interests in the role of LEC in olfactory behaviors. In this study, Bitzenhofer and colleagues provide unprecedented details of odor response properties of layer 2 cells in LEC. The authors first show that LEC neurons respond to odors with a rapid burst of activity time-locked to inhalation onset, similarly to the piriform cortex (PCx), but distinct from the OB. Firing rates of LEC ensembles conveyed information about odor identify whereas timing of spikes odor intensity. The authors then examined the difference between two major cell types in LEC layer 2 - fan cells and pyramidal neurons, and found that, …
Author Response:
Reviewer #1 (Public Review):
The lateral entorhinal cortex (LEC) receives direct inputs from the olfactory bulb (OB) but their odor response properties have not been well characterized despite a recent increase in interests in the role of LEC in olfactory behaviors. In this study, Bitzenhofer and colleagues provide unprecedented details of odor response properties of layer 2 cells in LEC. The authors first show that LEC neurons respond to odors with a rapid burst of activity time-locked to inhalation onset, similarly to the piriform cortex (PCx), but distinct from the OB. Firing rates of LEC ensembles conveyed information about odor identify whereas timing of spikes odor intensity. The authors then examined the difference between two major cell types in LEC layer 2 - fan cells and pyramidal neurons, and found that, on average, fan cells responded earlier than pyramidal neurons, and pyramidal neurons, but not fan cells, changed their peak timing in response to changes in concentrations, providing a basis for temporal coding of odor concentrations. Additionally, the authors show that inactivation of LEC impairs odor discrimination based on either identify or intensity, and demonstrate different cellular properties of fan cells and pyramidal neurons. Finally, the authors also examined the odor response properties of hippocampal CA1 neurons, and showed that odor identify can be decoded by firing rate responses, while decoding of odor concentration depended on spike timing.
The authors performed a large amount of experiments, and provide an impressive set of data regarding odor response properties of LEC layer 2 neurons in a cell type specific manner. The results reported are very interesting, and will be a point of reference for future studies on odor coding and processing in the LEC. The manuscript is clearly written, and data are well analyzed and presented clearly. I have only relatively minor concerns or suggestions.
- The authors infer the time at which "mice could discriminate odors" from the time at which d-prime becomes significantly different between baseline and odor stimulation conditions (line 111 and line 121). However, the statistical test applied to these data does not guarantee that an observer can accurately discriminate odors. For example, a small p-value can be obtained even when discrimination accuracy is only slightly above chance if there are many trials. The statement such as "mice could discriminate two odors by as early as 225 ms after inhalation onset" (line 111) can be misleading because this might sound as if mice can accurately discriminate odors at this timepoint, while this is not necessarily the case (as indicated by the d-prime value).
We have added plots of performance accuracy over time under control conditions (LED off) to Figure 2-supplement 1. These plots of fraction of correct responses (binned every 50 ms) show that mice (n = 6) are making choices significantly different from chance within 200 ms of odor inhalation. We changed the wording in the Results to now say: “Moreover, by analyzing lick timing, we determined that the discriminability measure d’ became significantly different under control conditions as early as 225 ms after inhalation onset and performance accuracy increased within 200 ms of inhalation (Fig. 2b, Figure 2-supplement 1).”
- Optogenetic identification can be a little tricky when identifying excitatory neurons as in this study. Please discuss some rational or difficulty regarding how to distinguish those that are activated directly by light from those activated indirectly (i.e. synaptically). Do the results hold if the authors use only those that the authors are more confident about identification?
We only used the cells that were confidently identified using a combination of two criteria. First, tagged cells had to show a significant increase in firing (p_Rate <0.01) during the 5 ms LED illumination period versus 100 randomly selected time windows before LED stimulation. Cells also had to respond with a fixed latency to reduce the chance of including cells recruited by polysynaptic excitation. Further, we used the stimulus associated spike latency test (SALT) as detailed in Kvitsiani et al., 2013. To be judged as tagged, units had to show significantly less spike jitter during the 5 ms LED illumination than 100 randomly selected time windows before LED stimulation (p_SALT<0.01). Only those cells with BOTH p_Rate<0.01 and p_Salt<0.01 were considered as tagged (both methods typically agreed for most cells). Moreover, slice work testing synaptic connections between LEC layer 2 cells found extremely low levels of connectivity between fan and pyramidal cells Nilssen et al., J. Neuroscience, 2018. This makes it unlikely that LED-induced firing of fan or pyramidal cells would recruit indirectly (synaptically) excited cells.
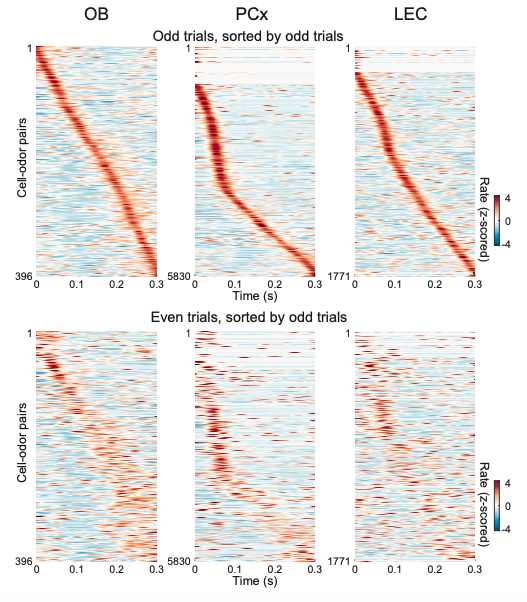
- The authors sort odor response profiles by peak timing, and indicate that odor responses peak at different timing that tiles respiration cycles. However, this analysis does not indicate the reliability of peak timing. Sorting random activity by "peak timing" could generate similar figure. One way to show the reliability or significance of peaks is to cross-validate. For instance, one can use a half of the trials to sort, and plot the rest of the trials. If the peak timing is reliable, the original pattern will be replicated by the other half, and those neurons that are not reliable will lose their peaks. Please use such a method so that we can evaluate the reliability of peaks.
We analyzed the data as suggested by this reviewer as shown below (Author response image 1). Plotting only the odd trials sorted by the odd trials in the dataset (top) looked identical to the data from all trails used in Figure 1g. More importantly, plotting only the even trials sorted by the odd trials (bottom), though noisier due to trial-by-trial variation, showed the same general structure of tiling throughout the respiration cycle for OB cells.
Author response image 1
Reviewer #2 (Public Review):
In this study, Bitzenhofer et al recorded odor-evoked activity in the LEC and examined the coding of odor identity and intensity using extracellular recordings in head-fixed mice, and used the standard suite of quantitative tools to interpret these data (decoding analyses, dimensionality reduction, etc). In addition, they performed behavioral experiments to show the necessity of LEC in odor identity and intensity discrimination, and deploy some elegant and straightforward 'circuit-busting' slice physiology experiments to characterize this circuit. Importantly, they performed some of their experiments in Ntng1-cre and Calb-cre mice, which allowed them to differentiate between the two major classes of LEC principal neurons, fan cells and pyramidal cells, respectively. Many of their results are contrasted with what has previously been observed in the piriform cortex (PCx), where odor coding has been studied much more extensively.
Their major conclusions are:
Cells in the LEC respond rapidly to odor stimuli. Within the first 300 ms after inhalation, odor identity is encoded by the ensemble of active neurons, while odor intensity (more specifically, responses to different concentrations) is encoded by the timing of the LEC response; specifically, the synchrony of the response. These coding strategies have been described in the PCx by Bolding & Franks. Bolding also found two populations of responses to different concentrations: one population of responses was rapid and barely changed with concentration and the second population of responses had onset latencies that decreased with increasing concentration. Roland et al also found two populations of responses using calcium imaging in anesthetized mice: one population of responses was concentration-dependent and another population was 'concentration-invariant'. However, neither Bolding nor Roland were able to determine whether these populations of responses emerged from distinct populations of cells. Here, the authors elegantly register these two response types in LEC to different cell types: fan cells respond early and stably, and pyramidal cells response latencies decrease with concentration. This is a novel and important finding. They also showed that, unlike PCx or LEC where concentration primarily affects timing rather than rate/number, odor concentration in CA1 is only reflected in the timing of responses.
Using optogenetic suppression of LEC in a 2AFC task, the authors purport to show that LEC is required for both the discrimination of odor identity and odor intensity. If true, this is an important result, but see below.
In slice experiments, the authors characterize the differential connectivity of fan and pyramidal cells to direct olfactory bulb input, input from PCx, and inhibitory inputs from SOM and PV cells. This work is elegant, novel, and important, although it is a little out of place in this manuscript. As such, their findings are irrelevant/orthogonal to the rest of the results in this study. But fine.
The simultaneous recordings from three different stations along the olfactory pathway are impressive.
Major concern
My major concern with this manuscript regards the behavioral experiments. The authors show that blue light over the LEC in GAD2-Cre/Ai32 mice completely abolishes (i.e. to chance) the mouse's ability to perform a 2AFC task discriminating between either two different odorants or one odorant at different concentrations. Their interpretation is that LEC is required for rapid odor-driven behavior. The sensory component of the task is so easy, and the effect is so striking that I find this result surprising and almost too good to be true. The authors do control for a blue-light distraction effect by repeating the experiments in mice that don't express ChR2, but do not control for the effect of rapidly shutting down a large part of the sensory/limbic system. If they did this experiment in the bulb I would be impressed with how clean the result was but not conceptually surprised by the outcome. I think a different negative control is needed here to convince me that the LEC is necessary for this simple sensory discrimination task. For example, the authors could activate all the interneurons (i.e. use this protocol) in another part of the brain, ideally in the olfactory pathway not immediately upstream of the LEC, and show that the behavior is not affected.
This reviewer suggests a negative control experiment for the effects we observe on behavior when optogenetically silencing LEC. However, we disagree that it would be informative to silence other olfactory pathways in search of those that do not affect behavior. Our strong effects on behavior are also in complete agreement with recent findings that muscimol inactivation of LEC abolishes discrimination of learned odor associations (Extended Data Figure 8, Lee et. al., Nature, 2021).
More specifically, both the presentation and the interpretation of the data are confusing. First, there is a lack of detail about the behavioral task. I was not sure exactly when the light comes on and goes off, when the cue was presented, and when the reward was presented. In the manuscript they say (line 108) "…used to suppress activity during odor delivery on a random subset…". There is nothing more about this in the figure legend or Methods. The only clue to this is the dotted line in the 'LED On' example at the bottom of Fig. 2a. The authors also say that (line 660) "Trials were initiated with a 50 ms tone." When exactly was the tone presented? In the absence of any other information, I assume it was presented at odor onset. When was the reward presented? Lines 106-7 say "Mice were free to report their choice (left or right lick) at any time within 2 s of odor onset." Presumably this means the reward was presented to one of the ports for 2 seconds, starting at odor onset.
The LED is applied during odor delivery, the 50 ms tone immediately precedes odor delivery, and water reward is dispensed after the first lick at the correct lick port during the choice period. The choice period begins with the odor onset and odor delivery is terminated by the first lick at either the correct or incorrect port. If there is no lick at either port, odor delivery lasts 1s and is followed by an extended choice period (terminated by correct or incorrect lick) lasting 1s. To clarify the behavior protocol, we have included a schematic of the trial structure in Figure 2-supplement 1.
These details matter because the authors want to claim that "LEC is essential for rapid odor-driven behavior." The data presented in support of this claim are (1) that mice perform this task at chance levels in LED On trials, presumably based on which port the mouse licked first (this is the 'essential' part), and (2) that in control in LED Off trials, d' becomes statistically different from baseline after ~200 ms (this is the 'rapid' part).
To further support the argument that LEC is required for rapid odor-driven behavior, we now show a plot of % correct responses over time from first odor inhalation.
On first reading, these suggested that shutting off LEC makes odor discrimination worse and/or slower. However, the supplementary data clarifies several things. First, the mice never Miss (Fig.2S.2a & c), meaning then they always lick. Second, in LED Off trials (F2S2 & e), the mice make few mistakes, and these only occur immediately after inhalation, presumably meaning the mice occasionally guess, possibly in response to the auditory cue. Thus, the mean time to lick is much shorter for Error trials than Correct trials. To state the obvious, the mice often wait >300 ms before they lick, and when they do wait, they never make mistakes. Now, in the LED On trials, the mice almost always lick within the first 300 ms and perform at chance levels, with the distribution of lick times for Correct and Error trials almost overlapping. In fact, although the authors claim LEC is required for rapid odor discrimination, the mean time to lick on Correct trials appears to decrease in LED On trials. This makes me think that the mice are making ballistic guesses in response to the tone in LED On cases, which doesn't necessarily implicate a dependence on LEC for odor discrimination.
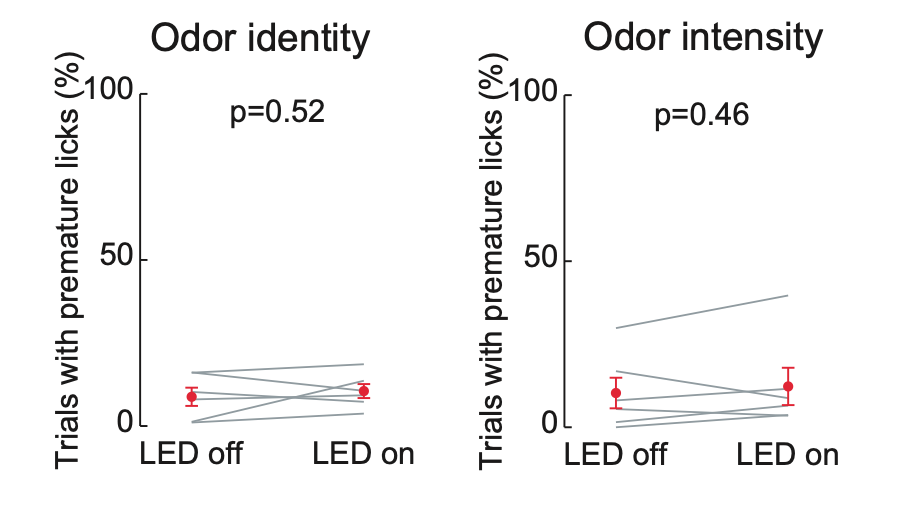
We do not believe that mice are making ballistic guesses in response to the tone for LED on trials. First, although a 50 ms tone immediately precedes odor delivery, all data in Figure 2-supplement 1 shows lick times aligned to the first inhalation of odor. Thus, time 0 ms is not the tone or subsequent odor onset but rather a variable time point coinciding with the first odor inhalation (the delay from odor onset to first inhalation is ~300 ms, the average respiration interval under our conditions). In fact, we excluded trials if mice made premature licks between the time of odor onset and first odor inhalation. We re-analyzed these trials to test the reviewer’s idea that mice were more likely to make fast ballistic guesses when the LEC was silenced. However, we saw no evidence that mice made more premature licks in trials with LED on (Author response image 2).
Author response image 2
The authors' interpretation of their data would be more solid if, for example, there were a delay between the auditory cue and odor delivery and/or if the reward was only available with some delay after the odor offset. Here, however, it seems just as likely as not that the mice are making ballistic guesses in response to the tone in LED On cases, which doesn't necessarily involve dependence on LEC for odor discrimination. Here, the divergence of d' from baseline in the control (i.e LED Off) condition seems mostly because mice take longer to correctly discriminate under control conditions. While this is not formally contradictory to LEC is essential for rapid odor-driven behavior", it is nevertheless a bit contrived and misleading. An interesting (thought) experiment is what would happen if the authors presented a tone but no odor. I would guess that the mice would continue licking randomly in Light On trials.
While a delay between odor delivery and reward would have been useful for some aspects of interpreting the behavior, we would have lost the ability to examine the role of LEC in response timing. To address this reviewer’s concern, we have added a section to the Discussion mentioning caveats related to the interpretation of experiments using acute optogenetic silencing to understand behavior.
-

Evaluation Summary:
Bitzenhofer and colleagues provide unprecedented details of odor response properties of layer 2 cells in LEC. The authors show that firing rates of LEC ensembles conveyed information about odor identify whereas timing of spikes odor intensity and that, on average, fan cells responded earlier than pyramidal neurons, and pyramidal neurons, but not fan cells, changed their peak timing in response to changes in concentrations, providing a basis for temporal coding of odor concentrations. The results provide important information about odor coding in LEC, an understudied area of the brain.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the …
Evaluation Summary:
Bitzenhofer and colleagues provide unprecedented details of odor response properties of layer 2 cells in LEC. The authors show that firing rates of LEC ensembles conveyed information about odor identify whereas timing of spikes odor intensity and that, on average, fan cells responded earlier than pyramidal neurons, and pyramidal neurons, but not fan cells, changed their peak timing in response to changes in concentrations, providing a basis for temporal coding of odor concentrations. The results provide important information about odor coding in LEC, an understudied area of the brain.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
The lateral entorhinal cortex (LEC) receives direct inputs from the olfactory bulb (OB) but their odor response properties have not been well characterized despite a recent increase in interests in the role of LEC in olfactory behaviors. In this study, Bitzenhofer and colleagues provide unprecedented details of odor response properties of layer 2 cells in LEC. The authors first show that LEC neurons respond to odors with a rapid burst of activity time-locked to inhalation onset, similarly to the piriform cortex (PCx), but distinct from the OB. Firing rates of LEC ensembles conveyed information about odor identify whereas timing of spikes odor intensity. The authors then examined the difference between two major cell types in LEC layer 2 - fan cells and pyramidal neurons, and found that, on average, fan cells …
Reviewer #1 (Public Review):
The lateral entorhinal cortex (LEC) receives direct inputs from the olfactory bulb (OB) but their odor response properties have not been well characterized despite a recent increase in interests in the role of LEC in olfactory behaviors. In this study, Bitzenhofer and colleagues provide unprecedented details of odor response properties of layer 2 cells in LEC. The authors first show that LEC neurons respond to odors with a rapid burst of activity time-locked to inhalation onset, similarly to the piriform cortex (PCx), but distinct from the OB. Firing rates of LEC ensembles conveyed information about odor identify whereas timing of spikes odor intensity. The authors then examined the difference between two major cell types in LEC layer 2 - fan cells and pyramidal neurons, and found that, on average, fan cells responded earlier than pyramidal neurons, and pyramidal neurons, but not fan cells, changed their peak timing in response to changes in concentrations, providing a basis for temporal coding of odor concentrations. Additionally, the authors show that inactivation of LEC impairs odor discrimination based on either identify or intensity, and demonstrate different cellular properties of fan cells and pyramidal neurons. Finally, the authors also examined the odor response properties of hippocampal CA1 neurons, and showed that odor identify can be decoded by firing rate responses, while decoding of odor concentration depended on spike timing.
The authors performed a large amount of experiments, and provide an impressive set of data regarding odor response properties of LEC layer 2 neurons in a cell type specific manner. The results reported are very interesting, and will be a point of reference for future studies on odor coding and processing in the LEC. The manuscript is clearly written, and data are well analyzed and presented clearly. I have only relatively minor concerns or suggestions.
1. The authors infer the time at which "mice could discriminate odors" from the time at which d-prime becomes significantly different between baseline and odor stimulation conditions (line 111 and line 121). However, the statistical test applied to these data does not guarantee that an observer can accurately discriminate odors. For example, a small p-value can be obtained even when discrimination accuracy is only slightly above chance if there are many trials. The statement such as "mice could discriminate two odors by as early as 225 ms after inhalation onset" (line 111) can be misleading because this might sound as if mice can accurately discriminate odors at this timepoint, while this is not necessarily the case (as indicated by the d-prime value).
2. Optogenetic identification can be a little tricky when identifying excitatory neurons as in this study. Please discuss some rational or difficulty regarding how to distinguish those that are activated directly by light from those activated indirectly (i.e. synaptically). Do the results hold if the authors use only those that the authors are more confident about identification?
3. The authors sort odor response profiles by peak timing, and indicate that odor responses peak at different timing that tiles respiration cycles. However, this analysis does not indicate the reliability of peak timing. Sorting random activity by "peak timing" could generate similar figure. One way to show the reliability or significance of peaks is to cross-validate. For instance, one can use a half of the trials to sort, and plot the rest of the trials. If the peak timing is reliable, the original pattern will be replicated by the other half, and those neurons that are not reliable will lose their peaks. Please use such a method so that we can evaluate the reliability of peaks.
-

Reviewer #2 (Public Review):
In this study, Bitzenhofer et al recorded odor-evoked activity in the LEC and examined the coding of odor identity and intensity using extracellular recordings in head-fixed mice, and used the standard suite of quantitative tools to interpret these data (decoding analyses, dimensionality reduction, etc). In addition, they performed behavioral experiments to show the necessity of LEC in odor identity and intensity discrimination, and deploy some elegant and straightforward 'circuit-busting' slice physiology experiments to characterize this circuit. Importantly, they performed some of their experiments in Ntng1-cre and Calb-cre mice, which allowed them to differentiate between the two major classes of LEC principal neurons, fan cells and pyramidal cells, respectively. Many of their results are contrasted with …
Reviewer #2 (Public Review):
In this study, Bitzenhofer et al recorded odor-evoked activity in the LEC and examined the coding of odor identity and intensity using extracellular recordings in head-fixed mice, and used the standard suite of quantitative tools to interpret these data (decoding analyses, dimensionality reduction, etc). In addition, they performed behavioral experiments to show the necessity of LEC in odor identity and intensity discrimination, and deploy some elegant and straightforward 'circuit-busting' slice physiology experiments to characterize this circuit. Importantly, they performed some of their experiments in Ntng1-cre and Calb-cre mice, which allowed them to differentiate between the two major classes of LEC principal neurons, fan cells and pyramidal cells, respectively. Many of their results are contrasted with what has previously been observed in the piriform cortex (PCx), where odor coding has been studied much more extensively.
Their major conclusions are:
Cells in the LEC respond rapidly to odor stimuli. Within the first 300 ms after inhalation, odor identity is encoded by the ensemble of active neurons, while odor intensity (more specifically, responses to different concentrations) is encoded by the timing of the LEC response; specifically, the synchrony of the response. These coding strategies have been described in the PCx by Bolding & Franks. Bolding also found two populations of responses to different concentrations: one population of responses was rapid and barely changed with concentration and the second population of responses had onset latencies that decreased with increasing concentration. Roland et al also found two populations of responses using calcium imaging in anesthetized mice: one population of responses was concentration-dependent and another population was 'concentration-invariant'. However, neither Bolding nor Roland were able to determine whether these populations of responses emerged from distinct populations of cells. Here, the authors elegantly register these two response types in LEC to different cell types: fan cells respond early and stably, and pyramidal cells response latencies decrease with concentration. This is a novel and important finding. They also showed that, unlike PCx or LEC where concentration primarily affects timing rather than rate/number, odor concentration in CA1 is only reflected in the timing of responses.
Using optogenetic suppression of LEC in a 2AFC task, the authors purport to show that LEC is required for both the discrimination of odor identity and odor intensity. If true, this is an important result, but see below.
In slice experiments, the authors characterize the differential connectivity of fan and pyramidal cells to direct olfactory bulb input, input from PCx, and inhibitory inputs from SOM and PV cells. This work is elegant, novel, and important, although it is a little out of place in this manuscript. As such, their findings are irrelevant/orthogonal to the rest of the results in this study. But fine.
The simultaneous recordings from three different stations along the olfactory pathway are impressive.
Major concern
My major concern with this manuscript regards the behavioral experiments. The authors show that blue light over the LEC in GAD2-Cre/Ai32 mice completely abolishes (i.e. to chance) the mouse's ability to perform a 2AFC task discriminating between either two different odorants or one odorant at different concentrations. Their interpretation is that LEC is required for rapid odor-driven behavior. The sensory component of the task is so easy, and the effect is so striking that I find this result surprising and almost too good to be true. The authors do control for a blue-light distraction effect by repeating the experiments in mice that don't express ChR2, but do not control for the effect of rapidly shutting down a large part of the sensory/limbic system. If they did this experiment in the bulb I would be impressed with how clean the result was but not conceptually surprised by the outcome. I think a different negative control is needed here to convince me that the LEC is necessary for this simple sensory discrimination task. For example, the authors could activate all the interneurons (i.e. use this protocol) in another part of the brain, ideally in the olfactory pathway not immediately upstream of the LEC, and show that the behavior is not affected.More specifically, both the presentation and the interpretation of the data are confusing. First, there is a lack of detail about the behavioral task. I was not sure exactly when the light comes on and goes off, when the cue was presented, and when the reward was presented. In the manuscript they say (line 108) "...used to suppress activity during odor delivery on a random subset...". There is nothing more about this in the figure legend or Methods. The only clue to this is the dotted line in the 'LED On' example at the bottom of Fig. 2a. The authors also say that (line 660) "Trials were initiated with a 50 ms tone." When exactly was the tone presented? In the absence of any other information, I assume it was presented at odor onset. When was the reward presented? Lines 106-7 say "Mice were free to report their choice (left or right lick) at any time within 2 s of odor onset." Presumably this means the reward was presented to one of the ports for 2 seconds, starting at odor onset.
These details matter because the authors want to claim that "LEC is essential for rapid odor-driven behavior." The data presented in support of this claim are (1) that mice perform this task at chance levels in LED On trials, presumably based on which port the mouse licked first (this is the 'essential' part), and (2) that in control in LED Off trials, d' becomes statistically different from baseline after ~200 ms (this is the 'rapid' part).
On first reading, these suggested that shutting off LEC makes odor discrimination worse and/or slower. However, the supplementary data clarifies several things. First, the mice never Miss (Fig.2S.2a & c), meaning then they always lick. Second, in LED Off trials (F2S2 & e), the mice make few mistakes, and these only occur immediately after inhalation, presumably meaning the mice occasionally guess, possibly in response to the auditory cue. Thus, the mean time to lick is much shorter for Error trials than Correct trials. To state the obvious, the mice often wait >300 ms before they lick, and when they do wait, they never make mistakes. Now, in the LED On trials, the mice almost always lick within the first 300 ms and perform at chance levels, with the distribution of lick times for Correct and Error trials almost overlapping. In fact, although the authors claim LEC is required for rapid odor discrimination, the mean time to lick on Correct trials appears to decrease in LED On trials. This makes me think that the mice are making ballistic guesses in response to the tone in LED On cases, which doesn't necessarily implicate a dependence on LEC for odor discrimination.
The authors' interpretation of their data would be more solid if, for example, there were a delay between the auditory cue and odor delivery and/or if the reward was only available with some delay after the odor offset. Here, however, it seems just as likely as not that the mice are making ballistic guesses in response to the tone in LED On cases, which doesn't necessarily involve dependence on LEC for odor discrimination. Here, the divergence of d' from baseline in the control (i.e LED Off) condition seems mostly because mice take longer to correctly discriminate under control conditions. While this is not formally contradictory to LEC is essential for rapid odor-driven behavior", it is nevertheless a bit contrived and misleading. An interesting (thought) experiment is what would happen if the authors presented a tone but no odor. I would guess that the mice would continue licking randomly in Light On trials.
-

Reviewer #3 (Public Review):
In this manuscript, Bitzenhofer and colleagues report multiple findings regarding processing of odors in the lateral entorhinal cortex (LEC). Specifically, they show:
1. L2 neurons preferentially respond to odors in phase with sniffing and do so relatively early relative to sniff onset.
2. They use optogenetic manipulations during and odor-guided 2AFC task to investigate the impact of silencing LEC on odor discrimination, finding that this silencing reduces the ability of mice to discriminate odor identify (one set of experiments) and odor intensity (a second set of experiments) to chance level.
3. They perform fine-grained analysis on odor coding in LEC, investigating spike rate vs. sniff phase timing and find that while rate conveys information regarding odor identity, timing represents odor intensity.
4. …
Reviewer #3 (Public Review):
In this manuscript, Bitzenhofer and colleagues report multiple findings regarding processing of odors in the lateral entorhinal cortex (LEC). Specifically, they show:
1. L2 neurons preferentially respond to odors in phase with sniffing and do so relatively early relative to sniff onset.
2. They use optogenetic manipulations during and odor-guided 2AFC task to investigate the impact of silencing LEC on odor discrimination, finding that this silencing reduces the ability of mice to discriminate odor identify (one set of experiments) and odor intensity (a second set of experiments) to chance level.
3. They perform fine-grained analysis on odor coding in LEC, investigating spike rate vs. sniff phase timing and find that while rate conveys information regarding odor identity, timing represents odor intensity.
4. They suggest a physiologically plausible mechanism for temporal coding of odor input in the LEC supported by relative spike timing between cell types.
5. Finally, they compare odor coding in the LEC to that in the CA1 region of the hippocampus and find that odor intensity in CA1 is only encoded by spike timing rather than spike rate.
Strengths:
The neural population recordings and encoding analyses are informative and will allow direct comparison to other work done in the olfactory bulb and cortex of awake mice. The behavior has been conducted with appropriate controls for off-target optogenetic effects. The authors' effort to connect the encoding they demonstrate with plausible mechanisms through both recording of cell populations in awake mice as well as in vitro examination of LEC circuitry enhance the impact of this work.
Weaknesses:
Some additional information could be included to further enable direct comparison to previous work. The optogenetic behavioral studies, although conducted with appropriate controls, suffer from the usual issues with interpretation, since this method suddenly disrupts major cortical circuits during behavior, which can often have non-specific effects and could significantly decrease mouse performance on the task independent of its impact on odor encoding in the LEC.
Impact:
The olfactory system, as the most "shallow" of the sensory systems, conveys information broadly to numerous cortical structures directly from the first olfactory relay in the brain, the olfactory bulb. This work will substantially contribute to an understanding of the broad range of encoding that occurs across these cortical structures by adding significant information regarding odor encoding in the LEC.
-