Role of YAP in early ectodermal specification and a Huntington's Disease model of human neurulation
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This manuscript harnesses an organoid model of human neurulation to unravel the role of the Hippo signalling pathway in the specification of the three key ectodermal cell types. The authors then investigate how these mechanisms are dysregulated in an organoid model of Huntington’s disease. The overall conclusions of this work are mostly supported by the data, though the implications of the regulatory relationships studied here to Huntington's Disease in adults would need further elucidation. With some clarifications of data acquisition and experimental logic, this work will be of broad interest to readers interested in the process of neurulation and how dysregulation of developmental mechanisms may lead to disease conditions in adulthood.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The Hippo pathway, a highly conserved signaling cascade that functions as an integrator of molecular signals and biophysical states, ultimately impinges upon the transcription coactivator Yes-associated protein 1 (YAP). Hippo-YAP signaling has been shown to play key roles both at the early embryonic stages of implantation and gastrulation, and later during neurogenesis. To explore YAP’s potential role in neurulation, we used self-organizing neuruloids grown from human embryonic stem cells on micropatterned substrates. We identified YAP activation as a key lineage determinant, first between neuronal ectoderm and nonneuronal ectoderm, and later between epidermis and neural crest, indicating that YAP activity can enhance the effect of BMP4 stimulation and therefore affect ectodermal specification at this developmental stage. Because aberrant Hippo-YAP signaling has been implicated in the pathology of Huntington’s Disease (HD), we used isogenic mutant neuruloids to explore the relationship between signaling and the disease. We found that HD neuruloids demonstrate ectopic activation of gene targets of YAP and that pharmacological reduction of YAP’s transcriptional activity can partially rescue the HD phenotype.
Article activity feed
-

-

Author Response:
Reviewer #1:
The manuscript by Piccolo and colleagues employs an in vitro neuruloid system to investigate the role of Hippo/YAP signaling pathway in early ectodermal fate specification. The authors examine YAP expression in forming neuruloids and test how manipulation of Hippo/Yap signaling affects their cellular composition. They observe that YAP expression is dynamic and enriched in cells occupying periphery of the neuruloid. Overactivation of the YAP activity by the Lats-kinase inhibitor TRULI leads to an expansion of TFAP2A+ cells (NNE) at early stages and of KRT18+ cells (epidermal) at later stages of development. Accordingly, the authors propose that YAP acts as a lineage determinant that (i) promotes a NNE fate during early development and (ii) impacts the fate of NNE cells by promoting an epidermal instead of …
Author Response:
Reviewer #1:
The manuscript by Piccolo and colleagues employs an in vitro neuruloid system to investigate the role of Hippo/YAP signaling pathway in early ectodermal fate specification. The authors examine YAP expression in forming neuruloids and test how manipulation of Hippo/Yap signaling affects their cellular composition. They observe that YAP expression is dynamic and enriched in cells occupying periphery of the neuruloid. Overactivation of the YAP activity by the Lats-kinase inhibitor TRULI leads to an expansion of TFAP2A+ cells (NNE) at early stages and of KRT18+ cells (epidermal) at later stages of development. Accordingly, the authors propose that YAP acts as a lineage determinant that (i) promotes a NNE fate during early development and (ii) impacts the fate of NNE cells by promoting an epidermal instead of a neural crest fate. Finally, the authors report that neuruloids developed with cells harboring mutations characteristics of Huntington's disease display elevated Yap activity.
The study takes advantage of the neuruloid system to examine the role of Hippo-Yap in early development and disease. A strength of the study is the use of the neuruloid as a proxy for the human embryo, which allows the authors to examine the control of spatial patterning in early development (in both wild type and altered cellular states). Yet, this model also presents significant limitations. Some of the results indicate a high degree of variability in YAP activity (and ectodermal patterning) in neuruloids obtained from different inductions. This raises the concern that the neuruloid system may interfere with Hippo/YAP. Furthermore, the model proposed by the authors is not consistent with the functional manipulations with pharmacological agents (e.g., pharmacological activation of YAP results in an increase of both neural and NNE cells; inhibition of YAP does not result in the expected phenotypes).
We thank the reviewer for her/his compliments on our work. The reviewer also points to the limitations of our neuruloid models and asks for clarifications.
The authors propose that YAP activation promotes a non-neural ectodermal (NNE) fate in early neuruloids, and subsequently drives NNE to differentiate into epidermis. However, manipulation of Hippo signaling with pharmacological inhibitors does not entirely support this, as treatment of neuruloids with agonist TRULI leads to expansion of both the PAX6 neural population and the NNE Tfap2a population. A prediction of the model is that treatment with verteporfin should neuralize the organoids, which is not the case (Fig 6A). This disconnect between the model presented in Figure 6D and the experimental results should be addressed by the authors.
We would like to thank the reviewer for this request. In our experiments we observed a dual effect of YAP activation (or HD mutation). As noted by the reviewer, ectodermal lineage- specification occurs both early (increased NNE induction) and late (enhanced epidermis differentiation and contraction of NC differentiation). Moreover, we observed a structural consequence of increased YAP activation in neuruloids, failure of the NE domain to fully close. Following the reviewer suggestion, we have now included an additional panel in Figure 6 to illustrate the phenotype alongside the difference in ectodermal lineage specification (panel E). We have also added in the Discussion a paragraph that highlights the architectural aspect of the observed phenotype.
Regarding the interpretation of the effect of pharmacological inhibition of YAP, we believe that the result of verteporfin treatment on WT neuruloids indicates that YAP activity is not required for this specification but can skew the differentiation towards NNE and epidermis. This is now included in the Results, and a new paragraph has been added in the Discussion directly addressing this point.
The study at times conflates YAP expression with activation of the Hippo-YAP pathway. While the images in figures 1,2, and 4 show changes in YAP expression, confirmation of Hippo-YAP pathway activity should include the use of a reporter (e.g., HOP-Flash) or at least high magnification images showing translocation of YAP to the nucleus. Overall, inclusion of better quantification of YAP-activity is crucial to support the manuscript's conclusions (the authors should also state the number of micropatterns used in each quantitative experiment).
Our evidence correlating YAP nuclear localization with activity is based on: (i) Immunoblots (Figures 1D and 2B); (ii) Confocal image analysis (Figures 1E, 2D, and 4B); and (iii) Induction of YAP target-genes expression as demonstrated by our scRNA-seq analysis, occurs in same epidermal (KRT18+) lineage cells that display the highest levels of YAP nuclear accumulation (Figure 2). However, to strengthen this argument and following the reviewer’s advice, we have now added magnified confocal microscope images of YAP/DAPI staining used to measure nuclear YAP localization at D4 (Figure 1—figure supplement 5). We have also added a slowed and magnified videos of the YAP-GFP/H2B-mCherry (and YAP-GFP alone) at D3-D4, which illustrates the dynamic accumulation of YAP in the nucleus of cells upon BMP4 stimulation (Figure 1—video 2, Figure 1—video 3, Figure 1—video 5, Figure 1—video 6, Figure 4—video 2 and Figure 4—video 3). Finally, the number of colonies analyzed for each experiment is now added in the Figure Legends.
A limitation of the study is that it does not investigate the possibility that Hippo/Yap could be affecting cell proliferation in the different lineages, instead of acting as a cell fate determinant. This is particularly important since Hippo is affected by cell density, which varies from the center to the periphery of the neuruloid. Different rates of proliferation over several days could potentially lead to drastic changes in neuruloid cellular composition.
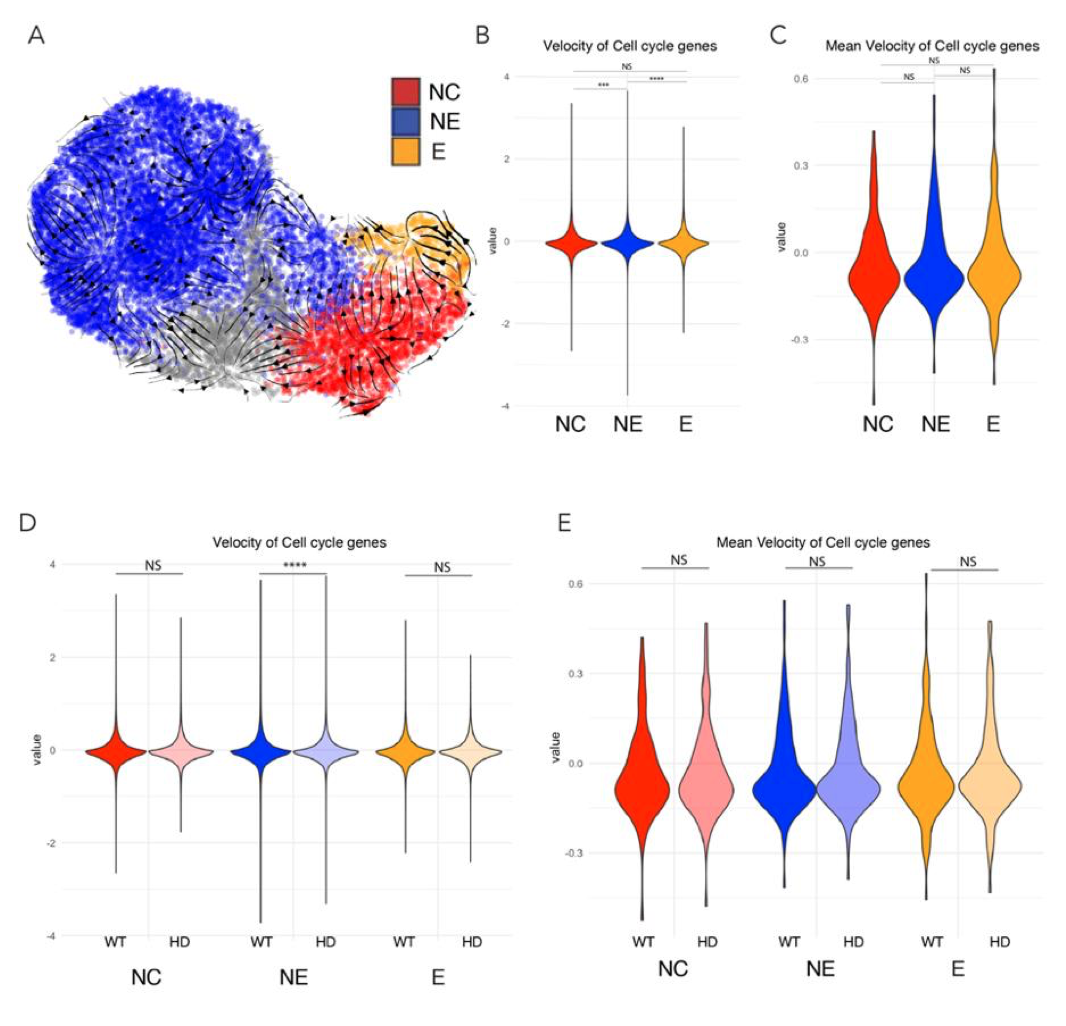
To address the reviewer’s legitimate point, and assess to potential effects of YAP activation in HD-neuruloids, we performed three sets of experiments. First, we performed RNA-velocity analysis to determine the cellular trajectories within each lineage (FigureXA, below), and calculated the velocity of Seurat’s “cell cycle-associated” genes in each cell population in our scRNA-seq dataset at D4. This analysis indicates that the three ectodermal progenitors have a comparable rate of cell division, with NE being slightly faster than the others and epidermis being the slowest (Figure XB). However, these differences are subtle: the mean velocity of these cell-cycle genes within each population are not significantly different across the three ectodermal lineages (FigureXC). Second, comparison of velocity values between WT and HD, highlighted a significant HD-associated increase in the dynamic of cell-cycle associated genes only within the NE population (FigureXD), consistent with the observation that YAP is ectopically active in this lineage. This increase is also not very dramatic, for the mean velocity of these genes is not significantly different in any comparison at this time (Figure XE).
Figure X. Proliferation rate analysis of D4 neuruloid from scRNAseq dataset. A) transcriptional trajectories were identified in the three ectodermal lineages. B) Velocity of cell cycle associated genes show that NNE lineages (NC and E) are slightly faster than NE. C) However this is not significant the mean population level. D) NE in HD D4 neuruloids display subtle increase in the velocity of cell cycle associated genes. E) Such effect disappears at the mean population level.
Finally, quantification of the number of mitotic nuclei per colony as marked by phospho-histone H3 (Kim et al., 2017) at different time points, demonstrated that YAP activation by TRULI leads to an increase in cell proliferation, especially in late neuruloids. This evidence is now presented in the new Supplemental Figure 4—figure supplement 3. We thank the reviewer for bringing this point to our attention.
It is also important to note that our study does not suggest that YAP is a bona fide cell-fate determinant, but rather that that the global phenotypic signature of YAP activation is influenced by differential regulation of cell-cycle dynamics. Moreover, inasmuch as YAP inhibition with verteporfin does not effect neuruloid formation, we believe that YAP is more of a booster signal operating on top of differentiation programs.
The results of the study contradict a previous reports, and some of these contradictions are not sufficiently addressed. The authors state that the activation of YAP in culture leads to a "complete loss of NC-like SOX10+ colonies"; however, a number of studies in in vivo models support a role for YAP as a positive regulator of neural crest specification.
We thank the reviewer for pointing to the results observed in model systems. We have now included a paragraph in which we acknowledge that YAP has been previously associated with NC specification and survival. However, it should be noted that these conclusions are based on data obtained from non-human model organisms such as Xenopus, or relied on differentiation protocols that are independent of BMP4 stimulation. We believe that the phenotype of unbalanced specification of the NNE depends on an epistatic relationship between BMP4 and Hippo-YAP pathway, which might play a crucial role during human neurulation.
Furthermore, the authors briefly speculate on the finding that Huntington's disease neuruloids have high YAP activity (whereas tissues from patients have low activity), but there is no real clear link to the pathophysiology of the disease.
In our in vitro assay that recapitulates aspects of human neurulation, we observed an early increase (D4) followed by a later decline (D7) in YAP activity associated with HD mutation, which is comparable to a dysregulation of the Hippo pathway that was observed in HD patients. To better clarify this aspect and its potential implication during embryogenesis we have now expanded our Discussion on the possible connection between HD and embryonic development.
Experimental results presented in different figures are often inconsistent throughout the manuscript. This should be examined by the authors since it suggests a lack of reproducibility in the neuruloid protocol. For example, the expression of TFAP2A at D4 neuruloids is a sparse halo at D4 in Fig4D, but robust in Fig1E.
The reviewer is correct in pointing to a certain degree of variability between experiments, especially during the period (D4) when the first NNE lineage begin to emerge (i.e., Supplemental Figure 4—figure supplement 2). Because parallel experimental conditions such as comparison with HD samples or TRULI treatment show consistent trends, however, we believe that our interpretation of these results is fundamentally correct.
The western blot in fig1D shows bands for tYAP and pYAP at D4, while in Fig2B the bands are not present (Fig1D also shows double bands for both markers while fig2B presents single bands).
There are several splicing alternative isoforms of human YAP (Vrbský et al., 2021). Immunoblots for YAP in YAP-GFP biallelically tagged cell lines (Figure 1—figure supplement 1B) show that two isoforms are detectable at pluripotency. During neural induction (D1-D3) both isoforms are downregulated, and upon BMP4 stimulation the larger isoform (top band) is primarily upregulated, so that from D4 onwards only the top band is visible (Figure 2B). To better clarify this point, we now discuss this in the Results and include Supplemental Figures with the quantification of the top and bottom bands (D1-D4, Figure 1D) and only of the top band (D4D7, Figure 2B and Figure 1—figure supplement 4).
As Hippo responds very quickly to cell density, mechanical forces, etc., these inconsistencies could affect the proposed analyses.
As previously mentioned, we have assessed the effect on proliferation rate due to YAP activation by TRULI or HD mutation in neuruloids by scRNA-seq analysis and by counting the number of mitotic cells at different times. Our manuscript leaves open the relationship between HTT mutation and YAP hyperactivation, which likely is mediated in part by these factors, but we do address possible connections in the discussion.
Reviewer #2:
This manuscript by Piccolo et al identifies YAP signalling as key player in lineage determination during development of early human ectoderm. Additionally, the authors show that neuroloids generated using cells engineered to express penetrant levels of CAG repeats in the HTT gene display aberrant YAP signalling during ectodermal specification and that this phenotype can be partially rescued by inhibition of this pathway. This is interesting study and the similarity of the YAP-activated neuroloids and the HD neuroloids is striking. The value of this work would be increased by providing experiments to definitively demonstrate the role of YAP signalling in NNE specification and in HD neuroloids.
We also thank this reviewer for her/his compliments on our work. The reviewer also expresses specific recommendations listed below:
Specific comment: The authors describe the emergence of non-neuronal ectoderm (NNE) at the edges of the printed island cell colony and neuronal ectoderm (NE) within this circular colony. However, they do not show images of any lineage markers confirming that these regions are, in fact, NNE and NE.
We show in Figure 1E that the edges of the neuruloids are positive for TFAP2A, a marker for the NNE lineage. In Figure 4D we also show TFAP2A at the edge (NNE) and PAX6 at the center (NE). Additionally, the spatial identity of the various ectodermal lineages was full characterized in our previous study (Haremaki et al., 2018).
They also don't show that this YAP-GFP cell line recapitulates endogenous fix-and-stains of YAP in these colonies.
Figure 1E shows YAP expression at D4 by immunolabeling for YAP/DAPI acquired by confocal microscopy, which recapitulates that of immunofluorescence detection of nuclear YAP , shown in Figure 4B , and the results obtained by live fluorescence (YAP-GFP/H2B, Figure 4A).
-

Evaluation Summary:
This manuscript harnesses an organoid model of human neurulation to unravel the role of the Hippo signalling pathway in the specification of the three key ectodermal cell types. The authors then investigate how these mechanisms are dysregulated in an organoid model of Huntington’s disease. The overall conclusions of this work are mostly supported by the data, though the implications of the regulatory relationships studied here to Huntington's Disease in adults would need further elucidation. With some clarifications of data acquisition and experimental logic, this work will be of broad interest to readers interested in the process of neurulation and how dysregulation of developmental mechanisms may lead to disease conditions in adulthood.
(This preprint has been reviewed by eLife. We include the public reviews from …
Evaluation Summary:
This manuscript harnesses an organoid model of human neurulation to unravel the role of the Hippo signalling pathway in the specification of the three key ectodermal cell types. The authors then investigate how these mechanisms are dysregulated in an organoid model of Huntington’s disease. The overall conclusions of this work are mostly supported by the data, though the implications of the regulatory relationships studied here to Huntington's Disease in adults would need further elucidation. With some clarifications of data acquisition and experimental logic, this work will be of broad interest to readers interested in the process of neurulation and how dysregulation of developmental mechanisms may lead to disease conditions in adulthood.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
The manuscript by Piccolo and colleagues employs an in vitro neuruloid system to investigate the role of Hippo/YAP signaling pathway in early ectodermal fate specification. The authors examine YAP expression in forming neuruloids and test how manipulation of Hippo/Yap signaling affects their cellular composition. They observe that YAP expression is dynamic and enriched in cells occupying periphery of the neuruloid. Overactivation of the YAP activity by the Lats-kinase inhibitor TRULI leads to an expansion of TFAP2A+ cells (NNE) at early stages and of KRT18+ cells (epidermal) at later stages of development. Accordingly, the authors propose that YAP acts as a lineage determinant that (i) promotes a NNE fate during early development and (ii) impacts the fate of NNE cells by promoting an epidermal instead of a …
Reviewer #1 (Public Review):
The manuscript by Piccolo and colleagues employs an in vitro neuruloid system to investigate the role of Hippo/YAP signaling pathway in early ectodermal fate specification. The authors examine YAP expression in forming neuruloids and test how manipulation of Hippo/Yap signaling affects their cellular composition. They observe that YAP expression is dynamic and enriched in cells occupying periphery of the neuruloid. Overactivation of the YAP activity by the Lats-kinase inhibitor TRULI leads to an expansion of TFAP2A+ cells (NNE) at early stages and of KRT18+ cells (epidermal) at later stages of development. Accordingly, the authors propose that YAP acts as a lineage determinant that (i) promotes a NNE fate during early development and (ii) impacts the fate of NNE cells by promoting an epidermal instead of a neural crest fate. Finally, the authors report that neuruloids developed with cells harboring mutations characteristics of Huntington's disease display elevated Yap activity.
The study takes advantage of the neuruloid system to examine the role of Hippo-Yap in early development and disease. A strength of the study is the use of the neuruloid as a proxy for the human embryo, which allows the authors to examine the control of spatial patterning in early development (in both wild type and altered cellular states). Yet, this model also presents significant limitations. Some of the results indicate a high degree of variability in YAP activity (and ectodermal patterning) in neuruloids obtained from different inductions. This raises the concern that the neuruloid system may interfere with Hippo/YAP. Furthermore, the model proposed by the authors is not consistent with the functional manipulations with pharmacological agents (e.g., pharmacological activation of YAP results in an increase of both neural and NNE cells; inhibition of YAP does not result in the expected phenotypes).
Comments:
The authors propose that YAP activation promotes a non-neural ectodermal (NNE) fate in early neuruloids, and subsequently drives NNE to differentiate into epidermis. However, manipulation of Hippo signaling with pharmacological inhibitors does not entirely support this, as treatment of neuruloids with agonist TRULI leads to expansion of both the PAX6 neural population and the NNE Tfap2a population. A prediction of the model is that treatment with verteporfin should neuralize the organoids, which is not the case (Fig 6A). This disconnect between the model presented in Figure 6D and the experimental results should be addressed by the authors.
The study at times conflates YAP expression with activation of the Hippo-YAP pathway. While the images in figures 1,2, and 4 show changes in YAP expression, confirmation of Hippo-YAP pathway activity should include the use of a reporter (e.g., HOP-Flash) or at least high magnification images showing translocation of YAP to the nucleus. Overall, inclusion of better quantification of YAP-activity is crucial to support the manuscript's conclusions (the authors should also state the number of micropatterns used in each quantitative experiment).
A limitation of the study is that it does not investigate the possibility that Hippo/Yap could be affecting cell proliferation in the different lineages, instead of acting as a cell fate determinant. This is particularly important since Hippo is affected by cell density, which varies from the center to the periphery of the neuruloid. Different rates of proliferation over several days could potentially lead to drastic changes in neuruloid cellular composition.
The results of the study contradict a previous reports, and some of these contradictions are not sufficiently addressed. The authors state that the activation of YAP in culture leads to a "complete loss of NC-like SOX10+ colonies"; however, a number of studies in in vivo models support a role for YAP as a positive regulator of neural crest specification. Furthermore, the authors briefly speculate on the finding that Huntington's disease neuruloids have high YAP activity (whereas tissues from patients have low activity), but there is no real clear link to the pathophysiology of the disease.
Experimental results presented in different figures are often inconsistent throughout the manuscript. This should be examined by the authors since it suggests a lack of reproducibility in the neuruloid protocol. For example, the expression of TFAP2A at D4 neuruloids is a sparse halo at D4 in Fig4D, but robust in Fig1E. The western blot in fig1D shows bands for tYAP and pYAP at D4, while in Fig2B the bands are not present (Fig1D also shows double bands for both markers while fig2B presents single bands). As Hippo responds very quickly to cell density, mechanical forces, etc., these inconsistencies could affect the proposed analyses.
-

Reviewer #2 (Public Review):
This manuscript by Piccolo et al identifies YAP signalling as key player in lineage determination during development of early human ectoderm. Additionally, the authors show that neuroloids generated using cells engineered to express penetrant levels of CAG repeats in the HTT gene display aberrant YAP signalling during ectodermal specification and that this phenotype can be partially rescued by inhibition of this pathway. This is interesting study and the similarity of the YAP-activated neuroloids and the HD neuroloids is striking. The value of this work would be increased by providing experiments to definitively demonstrate the role of YAP signalling in NNE specification and in HD neuroloids.
Specific comment: The authors describe the emergence of non-neuronal ectoderm (NNE) at the edges of the printed …
Reviewer #2 (Public Review):
This manuscript by Piccolo et al identifies YAP signalling as key player in lineage determination during development of early human ectoderm. Additionally, the authors show that neuroloids generated using cells engineered to express penetrant levels of CAG repeats in the HTT gene display aberrant YAP signalling during ectodermal specification and that this phenotype can be partially rescued by inhibition of this pathway. This is interesting study and the similarity of the YAP-activated neuroloids and the HD neuroloids is striking. The value of this work would be increased by providing experiments to definitively demonstrate the role of YAP signalling in NNE specification and in HD neuroloids.
Specific comment: The authors describe the emergence of non-neuronal ectoderm (NNE) at the edges of the printed island cell colony and neuronal ectoderm (NE) within this circular colony. However, they do not show images of any lineage markers confirming that these regions are, in fact, NNE and NE. They also don't show that this YAP-GFP cell line recapitulates endogenous fix-and-stains of YAP in these colonies.
-

Reviewer #3 (Public Review):
This study presents a human neuruloid model that has been engineered to report for expression and localisation of the transcription factor YAP, which is the downstream target of the Hippo pathway. The authors then use this model to investigate the role of the Hippo signalling cascade in the specification of neural and non-neural cell fates during human neurulation. The main technique used to manipulate YAP activity is the chemical inhibitor TRULI, which supresses YAP phosphorylation and therefore leads to its exclusion from the nucleus. This leads to the conclusion that YAP expression and dynamics are dynamically regulated during neurulation to progressively specify different cell fates. The authors also demonstrate that inhibition of YAP phosphorylation in WT neuruloids causes errors in neurulation that are …
Reviewer #3 (Public Review):
This study presents a human neuruloid model that has been engineered to report for expression and localisation of the transcription factor YAP, which is the downstream target of the Hippo pathway. The authors then use this model to investigate the role of the Hippo signalling cascade in the specification of neural and non-neural cell fates during human neurulation. The main technique used to manipulate YAP activity is the chemical inhibitor TRULI, which supresses YAP phosphorylation and therefore leads to its exclusion from the nucleus. This leads to the conclusion that YAP expression and dynamics are dynamically regulated during neurulation to progressively specify different cell fates. The authors also demonstrate that inhibition of YAP phosphorylation in WT neuruloids causes errors in neurulation that are similar to a Huntington's Disease neuruloid model.
A key strength of this work is the use of the YAP reporter neuruloid model, which has enabled the authors to dissect an otherwise complicated regulatory relationship. They also combine their approach of inhibiting YAP phosphorylation with single cell genomics, therefore uncovering a global view of the effects on the transcriptional landscape.
Overall, the conclusions of this work are supported by the data presented, but some aspects of the data acquisition and experimental logic will need to be clarified to fully support the conclusions.
-