The control and training of single motor units in isometric tasks are constrained by a common input signal
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
Bräcklein et al. provide compelling data suggesting that humans cannot learn to independently control multiple motor units innervating a single lower-limb muscle. These results suggest that common drive to motor units, along with the classical size-recruitment order, will impose strong constraints on the use of high-resolution muscle recordings for controlling brain-computer interfaces. Additional analysis related to task construction and motor unit identification/sorting is required to justify this claim.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Recent developments in neural interfaces enable the real-time and non-invasive tracking of motor neuron spiking activity. Such novel interfaces could provide a promising basis for human motor augmentation by extracting potentially high-dimensional control signals directly from the human nervous system. However, it is unclear how flexibly humans can control the activity of individual motor neurons to effectively increase the number of degrees of freedom available to coordinate multiple effectors simultaneously. Here, we provided human subjects (N = 7) with real-time feedback on the discharge patterns of pairs of motor units (MUs) innervating a single muscle (tibialis anterior) and encouraged them to independently control the MUs by tracking targets in a 2D space. Subjects learned control strategies to achieve the target-tracking task for various combinations of MUs. These strategies rarely corresponded to a volitional control of independent input signals to individual MUs during the onset of neural activity. Conversely, MU activation was consistent with a common input to the MU pair, while individual activation of the MUs in the pair was predominantly achieved by alterations in de-recruitment order that could be explained by history-dependent changes in motor neuron excitability. These results suggest that flexible MU recruitment based on independent synaptic inputs to single MUs is unlikely, although de-recruitment might reflect varying inputs or modulations in the neuron’s intrinsic excitability.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
- There was little comment on the strategy/mechanism that enabled subjects to readily attain Target I (MU 1 active alone), and then Target II (MU1 and MU2 active to the same relative degree). To accomplish this, it would seem that the peak firing rate of MU1 during pursuit of Target II could not exceed that during Target I despite an increased neural drive needed to recruit MU2. The most plausible explanation for this absence of additional rate coding in MU1 would be that associated with firing rate saturation (e.g., Fuglevand et al. (2015) Distinguishing intrinsic from extrinsic factors underlying firing rate saturation in human motor units. Journal of Neurophysiology 113, 1310-1322). It would be helpful if the authors might comment on whether firing rate saturation, or other mechanism, …
Author Response:
Reviewer #1 (Public Review):
- There was little comment on the strategy/mechanism that enabled subjects to readily attain Target I (MU 1 active alone), and then Target II (MU1 and MU2 active to the same relative degree). To accomplish this, it would seem that the peak firing rate of MU1 during pursuit of Target II could not exceed that during Target I despite an increased neural drive needed to recruit MU2. The most plausible explanation for this absence of additional rate coding in MU1 would be that associated with firing rate saturation (e.g., Fuglevand et al. (2015) Distinguishing intrinsic from extrinsic factors underlying firing rate saturation in human motor units. Journal of Neurophysiology 113, 1310-1322). It would be helpful if the authors might comment on whether firing rate saturation, or other mechanism, seemed to be at play that allowed subjects to attain both targets I and II.
To place the cursor inside TII, both MU1 and MU2 must discharge action potentials at their corresponding average discharge rate during 10% MVC (± 10% due to the target radius and neglecting the additional gain set manually in each direction). Therefore, subjects could simply exert a force of 10% MVC to reach TII and would successfully place the cursor inside TII. However, to get to TI, MU1 must discharge action potentials at the same rate as during TII hits (i.e. average discharge rate at 10% MVC) while keeping MU2 silent. Based on the performance analysis in Fig 3D, subjects had difficulties moving the cursor towards TI when the difference in recruitment threshold between MU1 and MU2 was small (≤ 1% MVC). In this case, the average discharge rate of MU1 during 10% MVC could not be reached without activating MU2. As could be expected, reaching towards TI became more successful when the difference in recruitment threshold between MU1 and MU2 was relatively large (≥3% MVC). In this case, subjects were able to let MU1 discharge action potentials at its average discharge rate at 10% MVC without triggering activation of MU2 (it seems the discharge rate of MU1 saturated before the onset of MU2). Such behaviour can be observed in Fig. 2A. MUs with a lower recruitment threshold saturate their discharge rate before the force reaches 10% MVC. We adapted the Discussion accordingly to describe this behaviour in more detail.
- Figure 4 (and associated Figure 6) is nice, and the discovery of the strategy used by subjects to attain Target III is very interesting. One mechanism that might partially account for this behavior that was not directly addressed is the role inhibition may have played. The size principle also operates for inhibitory inputs. As such, small, low threshold motor neurons will tend to respond to a given amount of inhibitory synaptic current with a greater hyperpolarization than high threshold units. Consequently, once both units were recruited, subsequent gradual augmentation of synaptic inhibition (concurrent with excitation and broadly distributed) could have led to the situation where the low threshold unit was deactivated (because of the higher magnitude hyperpolarization), leaving MU2 discharging in isolation. This possibility might be discussed.
We agree with the reviewer’s comment that inhibition might have played a critical role in succeeding to reach TIII. Hence, we have added this concept to our discussion.
- In a similar vein as for point 2 (above), the argument that PICs may have been the key mechanism enabling the attainment of target III, while reasonable, also seems a little hand wavy. The problem with the argument is that it depends on differential influences of PICs on motor neurons that are 1) low threshold, and 2) have similar recruitment thresholds. This seems somewhat unlikely given the broad influence of neuromodulatory inputs across populations of motor neurons.
We agree with the reviewer’s point and reasoning that a mixture of neuromodulation and inhibition likely introduced the variability in MU activity we observed in this study. This comment is addressed in the answer to comment 3.
Reviewer #2 (Public Review):
[...]
- Some subjects seemed to hit TIII by repeatedly "pumping" the force up and down to increase the excitability of MU2 (this appears to happen in TIII trials 2-6 in Fig. 4 - c.f. p18 l30ff). It would be useful to see single-trial time series plots of MU1, MU2, and force for more example trials and sessions, to get a sense for the diversity of strategies subjects used. The authors might also consider providing additional analyses to test whether multiple "pumps" increased MU2 excitability, and if so, whether this increase was usually larger for MU2 than MU1. For example, they might plot the ratio of MU2 (and MU1) activation to force (or, better, the residual discharge rate after subtracting predicted discharge based on a nonlinear fit to the ramp data) over the course of the trial. Is there a reason to think, based on the data or previous work, that units with comparatively higher thresholds (out of a sample selected in the low range of <10% MVC) would have larger increases in excitability?
We added a supplementary figure (Supplement 4) that visualizes additional trials from different conditions and subjects for TIII-instructed trials and noted this in the text.
MU excitability might indeed be pronounced during repeated activations within a couple of seconds (see, for example, M. Gorassini, J. F. Yang, M. Siu, and D. J. Bennett, “Intrinsic Activation of Human Motoneurons: Reduction of Motor Unit Recruitment Thresholds by Repeated Contractions,” J. Neurophysiol., vol. 87, no. 4, pp. 1859–1866, 2002.). Such an effect, however, seems to be equally distributed to all active MUs. Moreover, we are not aware of any recent studies suggesting that MUs, within the narrow range of 0-10% MVC, may be excited differently by such a mechanism. Supplement 4C and D illustrate trials in which subjects performed multiple “pumps”. Visually, we could not find changes in the excitability specific to any of the two MUs nor that subjects explored repeated activation of MUs as a strategy to reach TIII. It seems subjects instead tried to find the precise force level which would allow them to keep MU2 active after the offset of MU1. We further discussed that PICs act very broadly on all MUs. The observed discharge patterns when successfully reaching TIII may likely be due to an interplay of broadly distributed neuromodulation and locally acting synaptic inhibition.
- I am somewhat surprised that subjects were able to reach TIII at all when the de-recruitment threshold for MU1 was lower than the de-recruitment threshold for MU2. It would be useful to see (A) performance data, as in Fig. 3D or 5A, conditioned on the difference in de-recruitment thresholds, rather than recruitment thresholds, and (B) a scatterplot of the difference in de-recruitment vs the difference in recruitment thresholds for all pairs.
We agree that comparing the difference in de-recruitment threshold with the performance of reaching each target might provide valuable insights into the strategies used to perform the tasks. Hence, we added this comparison to Figure 4E at p. 16, l. 1. A scatterplot of the difference in de-recruitment threshold and the difference in recruitment threshold has been added to Supplement 3A. The Results section was modified in line with the above changes.
- Using MU1 / MU2 rates to directly control cursor position makes sense for testing for independent control over the two MUs. However, one might imagine that there could exist a different decoding scheme (using more than two units, nonlinearities, delay coordinates, or control of velocity instead of position) that would allow subjects to generate smooth trajectories towards all three targets. Because the authors set their study in a BCI context, they may wish to comment on whether more complicated decoding schemes might be able to exploit single-unit EMG for BCI control or, alternatively, to argue that a single degree of freedom in input fundamentally limits the utility of such schemes.
This study aimed to assess whether humans can learn to decorrelate the activity between two MUs coming from the same functional MU pool during constraint isometric conditions. The biofeedback was chosen to encourage subjects to perform this non-intuitive and unnatural task. Transferring biofeedback on single MUs into an application, for example, BCI control, could include more advanced pre-processing steps. Not all subjects were able to navigate the cursor along both axes consistently (always hitting TI and TIII). However, the performance metric (Figure 4C) indicated that subjects became better over time in diverging from the diagonal and thus increased their moving range inside the 2D space for various combinations of MU pairs. Hence, a weighted linear combination of the activity of both MUs (for example, along the two principal components based on the cursor distribution) may enable subjects to navigate a cursor from one axis to another. Similarly, coadaptation methods or different types of biofeedback (auditory or haptic) may help subjects. Furthermore, using only two MUs to drive a cursor inside a 2-D space is prone to interference. Including multiple MUs in the control scheme may improve the performance even in the presence of noise. We have shown that the activation of a single MU pool exposed to a common drive does not necessarily obey rigid control. State-dependent flexible control due to variable intrinsic properties of single MUs may be exploited for specific applications, such as BCI. However, further research is necessary to understand the potentials and limits of such a control scheme.
- The conclusions of the present work contrast somewhat with those of Marshall et al. (ref. 24), who claim (for shoulder and proximal arm muscles in the macaque) that (A) violations of the "common drive" hypothesis were relatively common when force profiles of different frequencies were compared, and that (B) microstimulation of different M1 sites could independently activate either MU in a pair at rest. Here, the authors provide a useful discussion of (A) on p19 l11ff, emphasizing that independent inputs and changes in intrinsic excitability cannot be conclusively distinguished once the MU has been recruited. They may wish to provide additional context for synthesizing their results with Marshall et al., including possible differences between upper / lower limb and proximal / distal muscles, task structure, and species.
The work by Marshall, Churchland and colleagues shows that when stimulating focally in specific sites in M1 single MUs can be activated, which may suggest a direct pathway from cortical neurons to single motor neurons within a pool. However, it remains to be shown if humans can learn to leverage such potential pathways or if the observations are limited to the artificially induced stimulus. The tibialis anterior receives a strong and direct cortical projection. Thus, we think that this muscle may be well suited to study whether subjects can explore such specific pathways to activate single MUs independently. However, it may very well be that the control of upper limbs show more flexibility than lower ones. However, we are not aware of any study that may provide evidence for a critical mismatch in the control of upper and lower limb MU pools. We have added this discussion to the manuscript.
Reviewer #3 (Public Review):
[...]
Even if the online decomposition of motor units were performed perfectly, the visual display provided to subject smooths the extracted motor unit discharge rates over a very wide time window: 1625 msec. This window is significantly larger than the differences in recruitment times in many of the motor unit pairs being used to control the interface. So while it's clear that the subjects are learning to perform the task successfully, it's not clear to me that subjects could have used the provided visual information to receive feedback about or learn to control motor unit recruitment, even if individuated control of motor unit recruitment by the nervous system is possible. I am therefore not convinced that these experiments were a fair test of subjects' ability to control the recruitment of individual motor units.
Regarding the validating of isolating motor units in the conditions analysed in this study, we have added a full new set of measurements with concomitant surface and intramuscular recordings during recruitment/derecruitment of motor units at variable recruitment speed. This provides a strong validation of the approach and of the accuracy of the online decomposition used in this study. Subjects received visual feedback on the activity of the selected MU pair, i.e. discharge behaviour of both MUs and the resulting cursor movement. This information was not clear from the initial submission and hence, we annotated the current version to clarify the biofeedback modalities. To further clarify the decoding of incoming MU1/MU2 discharge rates into cursor movement, we included Supplement 2. We also included a video that shows that the smoothing window on the cursor position does not affect the immediate cursor movement due to incoming spiking activity. For example, as shown in Supplement 2, for the initial offset of 0ms, the cursor starts moving along the axis corresponding to a sole activation of MU1 and immediately diverges from this axis when MU2 starts to discharge action potentials. We, therefore, think that the biofeedback provided to the subjects does allow exploration of single MU control.
Along similar lines, it seems likely to me that subjects are using some other strategy to learn the task, quite possibly one based on control of over overall force at the ankle and/or voluntary recruitment of other leg/foot muscles. Each of these variables will presumably be correlated with the activity of the recorded motor units and the movement of the cursor on the screen. Moreover, because these variables likely change on a similar (or slower) timescale than differences in motor units recruitment or derecruitment, it seems to me that using such strategies, which do not reflect or require individuated motor unit recruitment, is a highly effective way to successfully complete the task given the particular experimental setup.
In addition to being seated and restricted by an ankle dynamometer, subjects were instructed to only perform dorsiflexion of the ankle. Further, none of the subjects reported compensatory movements as a strategy to reach any of the targets. In addition, to be successfully utilised, such compensatory movements would need to influence various combinations of MUs tested in this study equally, even when they differ in size. Nevertheless, we acknowledge, as pointed out by the reviewer, that our setup has limitations. We only measured force in a single direction (i.e. ankle dorsiflexion) and did not track toe, hip or knee movements. Even though an instructor supervised leg movement throughout the experiment, it may be that very subtle and unknowingly compensatory movements have influenced the activity of the selected MUs. Hence, we updated the limitations section in the Discussion.
To summarize my above two points, it seems like the author's argument is that absence of evidence (subjects do not perform individuated MU recruitment in this particular task) constitutes evidence of absence (i.e. is evidence that individuated recruitment is not possible for the nervous system or for the control of brain-machine interfaces). Therefore given the above-described issues regarding real-time feedback provided to subjects in the paper it is not clear to me that any strong conclusions can be drawn about the nervous system's ability or inability to achieve individuated motor unit recruitment.
We hope that the above changes clarify the biofeedback modalities and their potential to provide subjects with the necessary information for exploring independent MU control. Our experiments aimed to investigate whether subjects can learn under constraint isometric conditions to decorrelate the activity between two MUs coming from the same functional pool. While it seemed that MU activity could be decorrelated, this almost exclusively happened (TIII-instructed trials) within a state-dependent framework, i.e. both MUs must be activated first before the lower threshold one is switched off. We did not observe flexible MU control based exclusively on a selective input to individual MUs (MU2 activated before MU1 during initial recruitment). That does not mean that such control is impossible. However, all successful control strategies that were voluntarily explored by the subjects to achieve flexible control were based on a common input and history-dependent activation of MUs. We have added these concepts to the discussion section.
Second, to support the claims based on their data the authors must explain their online spike-sorting method and provide evidence that it can successfully discriminate distinct motor unit onset/offset times at the low latency that would be required to test their claims. In the current manuscript, authors do not address this at all beyond referring to their recent IEEE paper (ref [25]). However, although that earlier paper is exciting and has many strengths (including simultaneous recordings from intramuscular and surface EMGs), the IEEE paper does not attempt to evaluate the performance metrics that are essential to the current project. For example, the key metric in ref 25 is "rate-of-agreement" (RoA), which measures differences in the total number of motor unit action potentials sorted from, for example, surface and intramuscular EMG. However, there is no evaluation of whether there is agreement in recruitment or de-recruitment times (the key variable in the present study) for motor units measured both from the surface and intramuscularly. This important technical point must be addressed if any conclusions are to be drawn from the present data.
We have taken this comment in high consideration, and we have performed a validation based on concomitant intramuscular and surface EMG decomposition in the exact experimental conditions of this study, including variations in the speed of recruitment and de-recruitment. This new validation fully supports the accuracy in of the methods used when detecting recruitment and de-recruitment of motor units.
My final concern is that the authors' key conclusion - that the nervous system cannot or does not control motor units in an individuated fashion - is based on the assumption that the robust differences in de-recruitment time that subjects display cannot be due to differences in descending control, and instead must be due to changes in intrinsic motor unit excitability within the spinal cord. The authors simply assert/assume that "[derecruitment] results from the relative intrinsic excitability of the motor neurons which override the sole impact of the receive synaptic input". This may well be true, but the authors do not provide any evidence for this in the present paper, and to me it seems equally plausible that the reverse is true - that de-recrutiment might influenced by descending control. This line of argumentation therefore seems somewhat circular.
When subjects were asked to reach TIII, which required the sole activation of a higher threshold MU, subjects almost exclusively chose to activate both MUs first before switching off the lower threshold MU. It may be that the lower de-recruitment threshold of MU2 was determined by descending inputs changing the excitability of either MU1 or MU2 (for example, see J. Nielsen, C. Crone, T. Sinkjær, E. Toft, and H. Hultborn, “Central control of reciprocal inhibition during fictive dorsiflexion in man,” Exp. brain Res., vol. 104, no. 1, pp. 99–106, Apr. 1995 or E. Jankowska, “Interneuronal relay in spinal pathways from proprioceptors,” Prog. Neurobiol., vol. 38, no. 4, pp. 335–378, Apr. 1992). Even if that is the case, it remains unknown why such a command channel that potentially changes the excitability of a single MU was not voluntarily utilized at the initial recruitment to allow for direct movement towards TIII (as direct movement was preferred for TI and TII). We cannot rule out that de-recruitment was affected by selective descending commands. However, our results match observations made in previous studies on intrinsic changes of MU excitability after MU recruitment. Therefore, even if descending pathways were utilized throughout the experiment to change, for example, MU excitability, subjects were not able to explore such pathways to change initial recruitment and achieve general flexible control over MUs. The updated discussion explains this line of reasoning.
Reviewer #4 (Public Review):
[...]
- Figure 6a nicely demonstrates the strategy used by subjects to hit target TIII. In this example, MU2 was both recruited and de-recruited after MU1 (which is the opposite of what one would expect based on the standard textbook description). The authors state (page 17, line 15-17) that even in the reverse case (when MU2 is de-recruited before MU1) the strategy still leads to successful performance. I am not sure how this would be done. For clarity, the authors could add a panel similar to panel A to this figure but for the case where the MU pairs have the opposite order of de-recruitment.
We have added more examples of successful TIII-instructed trials in Supplement 4. Supplement 4C and D illustrate examples of subjects navigating the cursor inside TIII even when MU2 was de-recruited before MU1. As exemplarily shown, subjects also used the three-stage approach discussed in the manuscript. In contrast to successful trials in which MU2 was de-recruited after MU1 (for example, Supplement 4B), subjects required multiple attempts until finding a precise force level that allowed a continuous firing of MU2 while MU1 remained silent. We have added a possible explanation for such behaviour in the Discussion.
- The authors discuss a possible type of flexible control which is not evident in the recruitment order of MUs (page 19, line 27-28). This reasoning was not entirely clear to me. Specifically, I was not sure which of the results presented here needs to be explained by such mechanism.
We have shown that subjects can decorrelate the discharge activity of MU1 and MU2 once both MUs are active (e.g. reaching TIII). Thus, flexible control of the MU pair was possible after the initial recruitment. Therefore, this kind of control seems strongly linked to a specific activation state of both MUs. We further elaborated on which potential mechanisms may contribute to this state-dependent control.
- The authors argue that using a well-controlled task is necessary for understanding the ability to control the descending input to MUs. They thus applied a dorsi-flexion paradigm and MU recordings from TA muscles. However, it is not clear to what extent the results obtained in this study can be extrapolated to the upper limb. Controlling the MUs of the upper limb could be more flexible and more accessible to voluntary control than the control of lower limb muscles. This point is crucial since the authors compare their results to other studies (Formento et al., bioRxiv 2021 and Marshall et al., bioRxiv 2021) which concluded in favor of the flexible control of MU recruitment. Since both studies used the MUs of upper limb muscles, a fair comparison would involve using a constrained task design but for upper limb muscles.
We agree with the reviewer that our work differs from previous approaches, which also studied flexible MU control. We, therefore, added a paragraph to the limitation section of the Discussion.
- The authors devote a long paragraph in the discussion to account for the variability in the de-recruitment order. They mostly rely on PIC, but there is no clear evidence that this is indeed the case. Is it at all possible that the flexibility in control over MUs was over their recruitment threshold? Was there any change in de-recruitment of the MUs during learning (in a given recording session)?
The de-recruitment threshold did not critically change when compared before and after the experiment on each day (difference in de-recruitment threshold before and after the experiment: -0.16 ± 2.28% MVC, we have now added this result to the Results section). Deviations from the classical recruitment order may be achieved by temporal (short-lived) changes in the intrinsic excitability of single MUs. We, therefore, extended our discussion on potential mechanisms that may explain the observed variability given all MUs receive the same common input.
- The need for a complicated performance measure (define on page 5, line 3-6) is not entirely clear to me. What is the correlation between this parameter and other, more conventional measures such as total-movement time or maximal deviation from the straight trajectory? In addition, the normalization process is difficult to follow. The best performance was measured across subjects. Does this mean that single subject data could be either down or up-regulated based on the relative performance of the specific subject? Why not normalize the single-subject data and then compare these data across subjects?
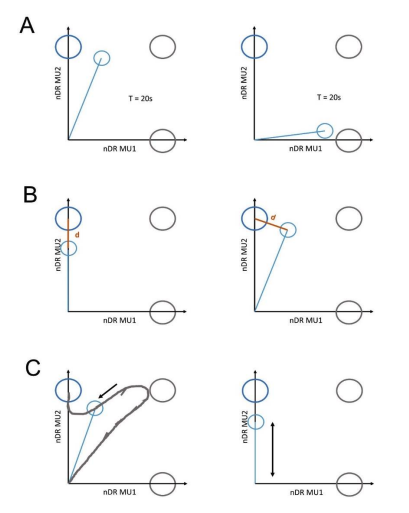
We employed this performance metric to overcome shortcomings of traditional measures such as target hit count, time-to-target or deviation from the straight trajectory. Such problems are described in the illustration below for TIII-instructed trials (blue target). A: the duration of the trial is the same in both examples (left and right); however, on the left, the subject manages to keep the cursor close to the target-of-interest while on the right, the cursor is far away from the target centre of TIII. B: In both images the cursor has the same distance d to the target centre of TIII. However, on the left, the subject manages to switch off MU1 while keeping MU2 active, while on the right, both MUs are active. C: On the left, the subject manages to move the cursor inside the TIII before the maximum trial time was reached, while on the right, the subject moved the cursor up and down, not diverging from the ideal trajectory to the target centre but fails to place the cursor inside TIII within the duration of the trial. In all examples, using only one conventional measure fails to account for a higher performance value in the left scenario than in the right. Our performance metric combines several performance metrics such as time-to-target, distance from the target centre, and the discharge rate ratio between MU1 and MU2 via the angle 𝜑 and thus allows a more detailed analysis of the performance than conventional measures. The normalisation of the performance value was done to allow for a comparison across subjects. The best and worst performance was estimated using synthetic data mimicking ideal movement towards each target (i.e. immediate start from the target origin to the centre of the target, while the normalised discharge rate of the corresponding MU is set to 1). Since the target space is normalised for all subjects in the same manner (mean discharge rate of the corresponding MUs at 10 %MVC) this allows us to compare the performance between subjects, conditions and targets.
- Figure 3C appears to indicate that there was only moderate learning across days for target TI and TII. Even for target TIII there was some improvement but the peak performance in later days was quite poor. The fact that the MUs were different each day may have affected the subjects' ability to learn the task efficiently. It would be interesting to measure the learning obtained on single days.
We have added an analysis that estimated the learning within a session per subject and target (Supplement 3C). In order to evaluate the strength of learning within-session, the Spearman correlation coefficient between target-specific performance and consecutive trials was calculated and averaged across conditions and days. The results suggest that there was little learning within sessions and no significant difference between targets. These results have now been added to the manuscript.
- On page 16 line 12-13, the authors describe the rare cases where subjects moved directly towards TIII. These cases apparently occurred when the recruitment threshold of MU2 was lower. What is the probable source of this lower recruitment level in these specific trials? Was this incidental (i.e., the trial was only successful when the MU threshold randomly decreased) or was there volitional control over the recruitment threshold? Did the authors test how the MU threshold changed (in percentages) over the course of the training day?
We did not track the recruitment threshold throughout the session but only at the beginning and end. We could not identify any critical changes in the recruitment order (see Results section). However, our analysis indicated that during direct movements towards TIII, MU2 (higher threshold MU) was recruited at a lower force level during the initial ramp and thus had a temporary effective recruitment threshold below MU1. It is important to note that these direct movements towards TIII only occurred for pairs of MUs with a similar recruitment threshold (see Figure 6). One possible explanation for this temporal change in recruitment threshold could be altered excitability due to neuromodulatory effects such as PICs (see Discussion). We have added an analysis that shows that direct movements towards TIII occurred in most cases (>90%) after a preceding TII- or TIIIinstructed trial. Both of these targets-of-interest require activation of MU2. Thus, direct movement towards TIII was likely not the result of specific descending control. Instead, this analysis suggests that the PIC effect triggered at the preceding trial was not entirely extinguished when a trial ending in direct movement towards TIII started. Alternatively, the rare scenarios in which direct movements happened could be entirely random. Similar observations were made in previous biofeedback studies [31]. To clarify these points, we altered the manuscript.
-

Evaluation Summary:
Bräcklein et al. provide compelling data suggesting that humans cannot learn to independently control multiple motor units innervating a single lower-limb muscle. These results suggest that common drive to motor units, along with the classical size-recruitment order, will impose strong constraints on the use of high-resolution muscle recordings for controlling brain-computer interfaces. Additional analysis related to task construction and motor unit identification/sorting is required to justify this claim.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
1. There was little comment on the strategy/mechanism that enabled subjects to readily attain Target I (MU 1 active alone), and then Target II (MU1 and MU2 active to the same relative degree). To accomplish this, it would seem that the peak firing rate of MU1 during pursuit of Target II could not exceed that during Target I despite an increased neural drive needed to recruit MU2. The most plausible explanation for this absence of additional rate coding in MU1 would be that associated with firing rate saturation (e.g., Fuglevand et al. (2015) Distinguishing intrinsic from extrinsic factors underlying firing rate saturation in human motor units. Journal of Neurophysiology 113, 1310-1322). It would be helpful if the authors might comment on whether firing rate saturation, or other mechanism, seemed to be at …
Reviewer #1 (Public Review):
1. There was little comment on the strategy/mechanism that enabled subjects to readily attain Target I (MU 1 active alone), and then Target II (MU1 and MU2 active to the same relative degree). To accomplish this, it would seem that the peak firing rate of MU1 during pursuit of Target II could not exceed that during Target I despite an increased neural drive needed to recruit MU2. The most plausible explanation for this absence of additional rate coding in MU1 would be that associated with firing rate saturation (e.g., Fuglevand et al. (2015) Distinguishing intrinsic from extrinsic factors underlying firing rate saturation in human motor units. Journal of Neurophysiology 113, 1310-1322). It would be helpful if the authors might comment on whether firing rate saturation, or other mechanism, seemed to be at play that allowed subjects to attain both targets I and II.
2. Figure 4 (and associated Figure 6) is nice, and the discovery of the strategy used by subjects to attain Target III is very interesting. One mechanism that might partially account for this behavior that was not directly addressed is the role inhibition may have played. The size principle also operates for inhibitory inputs. As such, small, low threshold motor neurons will tend to respond to a given amount of inhibitory synaptic current with a greater hyperpolarization than high threshold units. Consequently, once both units were recruited, subsequent gradual augmentation of synaptic inhibition (concurrent with excitation and broadly distributed) could have led to the situation where the low threshold unit was deactivated (because of the higher magnitude hyperpolarization), leaving MU2 discharging in isolation. This possibility might be discussed.
3. In a similar vein as for point 2 (above), the argument that PICs may have been the key mechanism enabling the attainment of target III, while reasonable, also seems a little hand wavy. The problem with the argument is that it depends on differential influences of PICs on motor neurons that are 1) low threshold, and 2) have similar recruitment thresholds. This seems somewhat unlikely given the broad influence of neuromodulatory inputs across populations of motor neurons. -

Reviewer #2 (Public Review):
The authors approach the question of whether humans can independently control the activation of multiple motor units (MUs) innervating a single muscle. They performed high-density surface electromyographic (EMG) recordings from the ankle dorsiflexor tibialis anterior, decomposed the signals into multiple single units online, and determined the recruitment order of these units using ramps of isometric force. Next, they selected a lower-threshold unit (MU1) and a higher-threshold unit (MU2) and used the discharge rates to control the position of a cursor in a two-dimensional visual workspace. Subjects were instructed to move this cursor to three targets requiring activation of either MU1 (TI), both units (TII), or MU2 only (TIII). With practice, subjects were able to achieve direct hits on TI by applying a low …
Reviewer #2 (Public Review):
The authors approach the question of whether humans can independently control the activation of multiple motor units (MUs) innervating a single muscle. They performed high-density surface electromyographic (EMG) recordings from the ankle dorsiflexor tibialis anterior, decomposed the signals into multiple single units online, and determined the recruitment order of these units using ramps of isometric force. Next, they selected a lower-threshold unit (MU1) and a higher-threshold unit (MU2) and used the discharge rates to control the position of a cursor in a two-dimensional visual workspace. Subjects were instructed to move this cursor to three targets requiring activation of either MU1 (TI), both units (TII), or MU2 only (TIII). With practice, subjects were able to achieve direct hits on TI by applying a low level of force, and on TII by activating both units at higher force. They were typically unable to move the cursor directly to TIII, however, and instead reached this target using a multi-stage approach by rapidly recruiting MU1 and MU2, lowering the force to inactivate MU1, and slowly increasing the force again to ramp up MU2. These results are broadly consistent with a one-dimensional drive to TA MUs, and suggest that, at least under the experimental conditions used here, humans cannot voluntarily override the size principle and independently control multiple MUs.
The experiments are well-designed, the data and analyses are convincing, the writing is clear, and the conclusions are novel and supported by the data. The manuscript will be of significant interest to researchers in neurophysiology, neuroengineering, and motor control. It has several broad implications. First, as the authors emphasize, EMG-based brain-computer interfaces (BCIs) will be strongly constrained by recruitment order, and the benefits of single-unit EMG for this purpose may be relatively limited. Second, the reachability of any point in a two-dimensional BCI workspace does not imply two controlled degrees of freedom, but may instead reflect hysteresis. Third, while much recent work on supraspinal neural dynamics and control (including intracortical BCIs) has modeled the state of a CNS region as a function of instantaneous firing rates, this study suggests that the neural state space might, in certain settings, need to be augmented with activation history or latent variables such as neuromodulation and persistent currents. I have several suggestions for improving the manuscript, described below.
1. Some subjects seemed to hit TIII by repeatedly "pumping" the force up and down to increase the excitability of MU2 (this appears to happen in TIII trials 2-6 in Fig. 4 - c.f. p18 l30ff). It would be useful to see single-trial time series plots of MU1, MU2, and force for more example trials and sessions, to get a sense for the diversity of strategies subjects used. The authors might also consider providing additional analyses to test whether multiple "pumps" increased MU2 excitability, and if so, whether this increase was usually larger for MU2 than MU1. For example, they might plot the ratio of MU2 (and MU1) activation to force (or, better, the residual discharge rate after subtracting predicted discharge based on a nonlinear fit to the ramp data) over the course of the trial. Is there a reason to think, based on the data or previous work, that units with comparatively higher thresholds (out of a sample selected in the low range of <10% MVC) would have larger increases in excitability?
2. I am somewhat surprised that subjects were able to reach TIII at all when the de-recruitment threshold for MU1 was lower than the de-recruitment threshold for MU2. It would be useful to see (A) performance data, as in Fig. 3D or 5A, conditioned on the difference in de-recruitment thresholds, rather than recruitment thresholds, and (B) a scatterplot of the difference in de-recruitment vs the difference in recruitment thresholds for all pairs.
3. Using MU1 / MU2 rates to directly control cursor position makes sense for testing for independent control over the two MUs. However, one might imagine that there could exist a different decoding scheme (using more than two units, nonlinearities, delay coordinates, or control of velocity instead of position) that would allow subjects to generate smooth trajectories towards all three targets. Because the authors set their study in a BCI context, they may wish to comment on whether more complicated decoding schemes might be able to exploit single-unit EMG for BCI control or, alternatively, to argue that a single degree of freedom in input fundamentally limits the utility of such schemes.
4. The conclusions of the present work contrast somewhat with those of Marshall et al. (ref. 24), who claim (for shoulder and proximal arm muscles in the macaque) that (A) violations of the "common drive" hypothesis were relatively common when force profiles of different frequencies were compared, and that (B) microstimulation of different M1 sites could independently activate either MU in a pair at rest. Here, the authors provide a useful discussion of (A) on p19 l11ff, emphasizing that independent inputs and changes in intrinsic excitability cannot be conclusively distinguished once the MU has been recruited. They may wish to provide additional context for synthesizing their results with Marshall et al., including possible differences between upper / lower limb and proximal / distal muscles, task structure, and species.
-

Reviewer #3 (Public Review):
This manuscript investigates whether humans can learn to independently control individual motor units (MUs) during voluntary movements. To do so, the authors devised a behavioral paradigm in which subjects received real-time feedback about the discharge rate of individual motor units, which are extracted via an online motor unit deconvolution algorithm. Subjects were tasked with activating an ankle muscle so that they hit experimenter-defined "targets" in the space of (simultaneously-recorded) motor unit activities. The authors argue that humans are able to successfully perform the task not by varying the order in which motor units are initially recruited, but rather in the order that units are de-recruited near the end of each trial. Based on these findings, the authors assert the performance of this task …
Reviewer #3 (Public Review):
This manuscript investigates whether humans can learn to independently control individual motor units (MUs) during voluntary movements. To do so, the authors devised a behavioral paradigm in which subjects received real-time feedback about the discharge rate of individual motor units, which are extracted via an online motor unit deconvolution algorithm. Subjects were tasked with activating an ankle muscle so that they hit experimenter-defined "targets" in the space of (simultaneously-recorded) motor unit activities. The authors argue that humans are able to successfully perform the task not by varying the order in which motor units are initially recruited, but rather in the order that units are de-recruited near the end of each trial. Based on these findings, the authors assert the performance of this task does NOT rely on independent control of the inputs to individual motor neurons, but rather on history-dependent changes in motor unit excitability that affect de-recruitment.
Major comments:
As outlined below I have significant concerns about multiple aspects of this study.
Even if the online decomposition of motor units were performed perfectly, the visual display provided to subject smooths the extracted motor unit discharge rates over a very wide time window: 1625 msec. This window is significantly larger than the differences in recruitment times in many of the motor unit pairs being used to control the interface. So while it's clear that the subjects are learning to perform the task successfully, it's not clear to me that subjects could have used the provided visual information to receive feedback about or learn to control motor unit recruitment, even if individuated control of motor unit recruitment by the nervous system is possible. I am therefore not convinced that these experiments were a fair test of subjects' ability to control the recruitment of individual motor units.
Along similar lines, it seems likely to me that subjects are using some other strategy to learn the task, quite possibly one based on control of over overall force at the ankle and/or voluntary recruitment of other leg/foot muscles. Each of these variables will presumably be correlated with the activity of the recorded motor units and the movement of the cursor on the screen. Moreover, because these variables likely change on a similar (or slower) timescale than differences in motor units recruitment or derecruitment, it seems to me that using such strategies, which do not reflect or require individuated motor unit recruitment, is a highly effective way to successfully complete the task given the particular experimental setup.
To summarize my above two points, it seems like the author's argument is that absence of evidence (subjects do not perform individuated MU recruitment in this particular task) constitutes evidence of absence (i.e. is evidence that individuated recruitment is not possible for the nervous system or for the control of brain-machine interfaces). Therefore given the above-described issues regarding real-time feedback provided to subjects in the paper it is not clear to me that any strong conclusions can be drawn about the nervous system's ability or inability to achieve individuated motor unit recruitment.
Second, to support the claims based on their data the authors must explain their online spike-sorting method and provide evidence that it can successfully discriminate distinct motor unit onset/offset times at the low latency that would be required to test their claims. In the current manuscript, authors do not address this at all beyond referring to their recent IEEE paper (ref [25]). However, although that earlier paper is exciting and has many strengths (including simultaneous recordings from intramuscular and surface EMGs), the IEEE paper does not attempt to evaluate the performance metrics that are essential to the current project. For example, the key metric in ref 25 is "rate-of-agreement" (RoA), which measures differences in the total number of motor unit action potentials sorted from, for example, surface and intramuscular EMG. However, there is no evaluation of whether there is agreement in recruitment or de-recruitment times (the key variable in the present study) for motor units measured both from the surface and intramuscularly. This important technical point must be addressed if any conclusions are to be drawn from the present data.
My final concern is that the authors' key conclusion - that the nervous system cannot or does not control motor units in an individuated fashion - is based on the assumption that the robust differences in de-recruitment time that subjects display cannot be due to differences in descending control, and instead must be due to changes in intrinsic motor unit excitability within the spinal cord. The authors simply assert/assume that "[derecruitment] results from the relative intrinsic excitability of the motor neurons which override the sole impact of the receive synaptic input". This may well be true, but the authors do not provide any evidence for this in the present paper, and to me it seems equally plausible that the reverse is true - that de-recrutiment might influenced by descending control. This line of argumentation therefore seems somewhat circular.
For the above reasons, I am not convinced that this study provides significant insight into the important problems it investigates. However, as always I am a big fan of the eLife review/discussion process - I'm very interested to read and discuss the other Reviewers' assessments of this study's strengths and weaknesses.
-

Reviewer #4 (Public Review):
The authors used a smart, elegant paradigm to examine the question of the voluntary control in recruiting MUs. They used a state-of-the-art system to extract large numbers of MUs in a non-invasive manner. They show that recruiting MUs almost exclusively follows the classical recruitment order, so that the flexibility of voluntary control is limited. This is evident in the finding that subjects could not easily recruit a higher threshold of MU2 while suppressing the activity of MU1. Instead, subjects used an alternative strategy which relies on different thresholds for the de-recruitment of these units. However, the design of the study and the specific use of lower limb MUs may impede the comparison to other systems.
In my mind, the deviation of de-recruitment order from the expected order (as depicted in …
Reviewer #4 (Public Review):
The authors used a smart, elegant paradigm to examine the question of the voluntary control in recruiting MUs. They used a state-of-the-art system to extract large numbers of MUs in a non-invasive manner. They show that recruiting MUs almost exclusively follows the classical recruitment order, so that the flexibility of voluntary control is limited. This is evident in the finding that subjects could not easily recruit a higher threshold of MU2 while suppressing the activity of MU1. Instead, subjects used an alternative strategy which relies on different thresholds for the de-recruitment of these units. However, the design of the study and the specific use of lower limb MUs may impede the comparison to other systems.
In my mind, the deviation of de-recruitment order from the expected order (as depicted in figure 2A) surprising, and requires a more thorough investigation. The authors suggest that this deviation is an outcome of persistent inward currents (PIC), but provide no direct evidence for this supposition. In addition, in this study the authors tested lower-limb muscles. It would be interesting to use the same paradigm on MUs of upper limb muscles, where the capacity for volitional control could be more extensive.
Specific comments
- Figure 6a nicely demonstrates the strategy used by subjects to hit target TIII. In this example, MU2 was both recruited and de-recruited after MU1 (which is the opposite of what one would expect based on the standard textbook description). The authors state (page 17, line 15-17) that even in the reverse case (when MU2 is de-recruited before MU1) the strategy still leads to successful performance. I am not sure how this would be done. For clarity, the authors could add a panel similar to panel A to this figure but for the case where the MU pairs have the opposite order of de-recruitment.
- The authors discuss a possible type of flexible control which is not evident in the recruitment order of MUs (page 19, line 27-28). This reasoning was not entirely clear to me. Specifically, I was not sure which of the results presented here needs to be explained by such mechanism.
- The authors argue that using a well-controlled task is necessary for understanding the ability to control the descending input to MUs. They thus applied a dorsi-flexion paradigm and MU recordings from TA muscles. However, it is not clear to what extent the results obtained in this study can be extrapolated to the upper limb. Controlling the MUs of the upper limb could be more flexible and more accessible to voluntary control than the control of lower limb muscles. This point is crucial since the authors compare their results to other studies (Formento et al., bioRxiv 2021 and Marshall et al., bioRxiv 2021) which concluded in favor of the flexible control of MU recruitment. Since both studies used the MUs of upper limb muscles, a fair comparison would involve using a constrained task design but for upper limb muscles.
- The authors devote a long paragraph in the discussion to account for the variability in the de-recruitment order. They mostly rely on PIC, but there is no clear evidence that this is indeed the case. Is it at all possible that the flexibility in control over MUs was over their recruitment threshold? Was there any change in de-recruitment of the MUs during learning (in a given recording session)?
- The need for a complicated performance measure (define on page 5, line 3-6) is not entirely clear to me. What is the correlation between this parameter and other, more conventional measures such as total-movement time or maximal deviation from the straight trajectory? In addition, the normalization process is difficult to follow. The best performance was measured across subjects. Does this mean that single subject data could be either down or up-regulated based on the relative performance of the specific subject? Why not normalize the single-subject data and then compare these data across subjects?
- Figure 3C appears to indicate that there was only moderate learning across days for target TI and TII. Even for target TIII there was some improvement but the peak performance in later days was quite poor. The fact that the MUs were different each day may have affected the subjects' ability to learn the task efficiently. It would be interesting to measure the learning obtained on single days.
- On page 16 line 12-13, the authors describe the rare cases where subjects moved directly towards TIII. These cases apparently occurred when the recruitment threshold of MU2 was lower. What is the probable source of this lower recruitment level in these specific trials? Was this incidental (i.e., the trial was only successful when the MU threshold randomly decreased) or was there volitional control over the recruitment threshold? Did the authors test how the MU threshold changed (in percentages) over the course of the training day?
-

-

-

-

-

-

-

-