FMRP regulates mRNAs encoding distinct functions in the cell body and dendrites of CA1 pyramidal neurons
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The authors performed transcriptomic analyses from compartment-specific, micro-dissected hippocampal region tissue from transgenic mice. One feature that distinguishes this work from previous studies is the use of conditional knock-in tags (GFP or HA) and tissue specific expression of the Cre recombinase to target a population of pyramidal neurons in the CA1 region. The strengths of the paper are the rich data sets and innovative integration of methods that will provide a valuable technical resource for the field.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Neurons rely on translation of synaptic mRNAs in order to generate activity-dependent changes in plasticity. Here, we develop a strategy combining compartment-specific crosslinking immunoprecipitation (CLIP) and translating ribosome affinity purification (TRAP) in conditionally tagged mice to precisely define the ribosome-bound dendritic transcriptome of CA1 pyramidal neurons. We identify CA1 dendritic transcripts with differentially localized mRNA isoforms generated by alternative polyadenylation and alternative splicing, including many that have altered protein-coding capacity. Among dendritic mRNAs, FMRP targets were found to be overrepresented. Cell-type-specific FMRP-CLIP and TRAP in microdissected CA1 neuropil revealed 383 dendritic FMRP targets and suggests that FMRP differentially regulates functionally distinct modules in CA1 dendrites and cell bodies. FMRP regulates ~15–20% of mRNAs encoding synaptic functions and 10% of chromatin modulators, in the dendrite and cell body, respectively. In the absence of FMRP, dendritic FMRP targets had increased ribosome association, consistent with a function for FMRP in synaptic translational repression. Conversely, downregulation of FMRP targets involved in chromatin regulation in cell bodies suggests a role for FMRP in stabilizing mRNAs containing stalled ribosomes in this compartment. Together, the data support a model in which FMRP regulates the translation and expression of synaptic and nuclear proteins within different compartments of a single neuronal cell type.
Article activity feed
-

-

Author Response:
Reviewer #1 (Public Review):
This manuscript integrates conditional mouse models for TRAP, PAPERCLIP and FMRP-CLIP together with compartment specific profiling of mRNA in hippocampal CA1 neurons. Previously, similar approaches have been used to interrogate mRNA localization, differential regulation of 3'UTR isoforms, their local translation, and FMRP-dependent mRNA regulation. This study builds on these previous findings by combining all three approaches, together with analysis of mRNA dysregulation in Fmr1 KO neuron model of FXS. The strengths of the paper are the rich data sets and innovative integration of methods that will provide a valuable technical resource for the field. The weakness of the paper is the limited conceptual advance as well as lack of deeper mechanistic insights on FMRP biology over previous …
Author Response:
Reviewer #1 (Public Review):
This manuscript integrates conditional mouse models for TRAP, PAPERCLIP and FMRP-CLIP together with compartment specific profiling of mRNA in hippocampal CA1 neurons. Previously, similar approaches have been used to interrogate mRNA localization, differential regulation of 3'UTR isoforms, their local translation, and FMRP-dependent mRNA regulation. This study builds on these previous findings by combining all three approaches, together with analysis of mRNA dysregulation in Fmr1 KO neuron model of FXS. The strengths of the paper are the rich data sets and innovative integration of methods that will provide a valuable technical resource for the field. The weakness of the paper is the limited conceptual advance as well as lack of deeper mechanistic insights on FMRP biology over previous studies, although the present study validates and integrates past studies, adding some new information on 3'UTR isoforms.
We appreciate the Reviewer’s recognition that “the present study validates and integrates past studies, adding some new information on 3'UTR isoforms”. We also appreciate the Reviewer’s recognition that “The strengths of the paper are the rich data sets and innovative integration of methods that will provide a valuable technical resource for the field.”
We differ, however, with the concern that the work presents a “limited conceptual advance.” Specifically, we find, for the first time, that FMRP regulates two different biologically coherent sets of mRNAs in CA1 neuronal cell bodies and neurites. This provides a profound new insight into FMRP-RNA regulation, including the fact that these two different sets of mRNA targets (encoding chromatin-associated proteins and synaptic proteins, respectively) are both translationally regulated by FMRP and transcribed from genes implicated in autism.
We recognize that FMRP was known, by our own work and that of others (as noted by the Reviewer) to regulate specific targets “in bulk” in neuronal cell types, brain and even in CA1 neurons. What is most unexpected here? Among directly bound FMRP mRNAs in brain CA1 neurons, there is subcellular compartmentalization of this regulation. This is new for FMRP, and in fact is new for RNA binding proteins more generally (recognizing of course the extensive work on RNA localization in different compartments previously discovered by others, beginning with Rob Singer’s work on actin localization and up to the present in work on neurons).
We also think it is also important for readers to understand up-front the novelty in “combining approaches” referred to. We use cell-specific (cTag) CLIP to define direct FMRP interactions in subcompartments--dendrites vs cell bodies--of CA1 neurons within mouse brain hippocampus. We also normalize this data to ribosome-bound mRNAs in CA1 neurons, and validate observations by studying WT and FMRP-null brains. This set of complex mouse models and methods is completely new, and its application is what allowed us to make robust conclusions about FMRP translational regulation of different mRNAs in different cellular compartments.
We strongly disagree with the Reviewer’s comment that FMRP directly interacts with functional classes of mRNAs in different cellular compartments “has previously been shown in the field.” Compartment-specific FMRP-CLIP has not been reported that we’re aware of, much less in a cell-type specific manner. Our previous cell-type specific FMRP-CLIP experiments have been on bulk neuronal material (Sawicka et al. 2019; Van Driesche et al., n.d.). Although cell-type specific TRAP-seq has been performed on microdissected CA1 compartments (Ainsley et al. 2014), investigators were unable to isolate significant amounts of RNA from resting neurons, and degradation of the isolated RNAs did not allow the types of 3’UTR and alternative splicing analyses that were performed here. The Schuman group has performed extensive analysis of mRNAs from microdissected CA1 compartments (Cajigas et al. 2012a; Tushev et al. 2018), but have not performed FMRP-CLIP or any experiments using cell-type specific or direct protein-RNA regulatory methods. In vitro systems have been used to analyze mRNA localization in FMRP KO systems (i.e. (Goering et al. 2020)), but in vitro systems are unable to fully recapitulate the complexities of in vivo brain regions, and did not analyze direct RNA-protein interactions. As our work is on in vivo brain slices, is cell-type specific, and integrates TRAP-seq, PAPERCLIP and CLIP-seq datasets, we believe that our work is novel and will be of great interest to the field.
Despite the fact that FMRP targets are overrepresented in the dendritic transcriptome, it does not appear from this study that FMRP plays an active role in the mechanism of dendritic mRNA localization, at least under steady state conditions. One goal of the manuscript is to address a major question in the mRNA localization field, which is how FMRP may differentially modulate "localization" of functional classes of mRNAs such as those encoding transcriptional regulators and synaptic plasticity genes (Line 78-90). The data here indicate that FMRP directly interacts with functional classes of mRNAs in different cellular compartments, which has previously been shown in the field. However, no evidence is provided that mechanistically reveal a role for FMRP to promote subcellular localization of different functional classes of mRNAs. The correlative evidence presented in this manner does not add mechanistic insight.
We do recognize that the question of what localizes FMRP mRNA targets differentially in the dendrite (and cell body) is of great interest, and remains unanswered. We also appreciate that, despite the Reviewer’s comment above, they also recognize “it does not appear from this study that FMRP plays an active role in the mechanism of dendritic mRNA localization, at least under steady state conditions.”
We believe that some of the confusion here lies in the Reviewer’s comment “One goal of the manuscript is to address a major question in the mRNA localization field, which is how FMRP may differentially modulate "localization" of functional classes of mRNAs such as those encoding transcriptional regulators and synaptic plasticity genes (Line 78-90).” While this is a question of interest that has been studied, we think there is a major disconnect here in the Reviewer’s comments and our findings. To be clear, in the original manuscript, we did not find evidence, in WT vs KO CA1 neurons, that FMRP was acting to differentially localize mRNAs, including those mentioned by the Reviewer.
Nonetheless, to further address the issue of a possible role for FMRP in localizing the transcripts it regulates, we have now performed quantitative analysis of FMRP target mRNA localization in dendrites from WT vs. Fmr1 KO mice. These results are now presented in Supplemental Figures 9 and 10 of the manuscript, and which we present and summarize below.
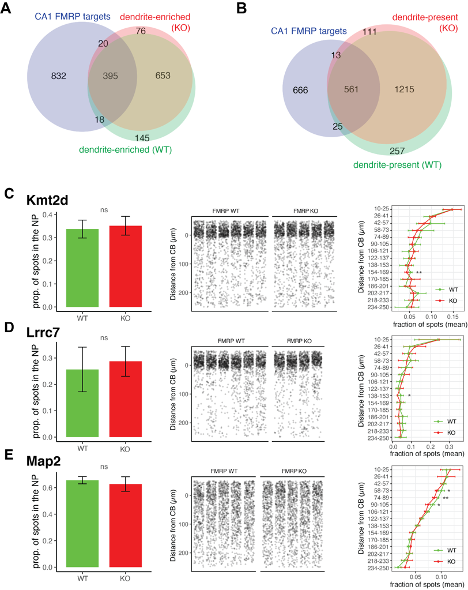
Supplemental Figure 9. FMRP is not required for localization of its targets into the dendrites of CA1 neurons. A) Dendrite-enriched mRNAs were defined in FMRP KO mice (red) in the same manner as in Figure 1 for FMRP WT animals using bulk RNA-seq and TRAP-seq data. Overlap with dendrite-enriched mRNAs in WT (Figure 1, shown here in green) and CA1 FMRP targets (blue) in shown. 95.6% of dendrite-enriched FMRP targets in the WT were also found to be enriched in the dendrites of FMRP KO animals. B) Dendrite-present mRNAs were defined in FMRP KO. Overlap with dendrite-present mRNAs in WT (Figure 1) and CA1 FMRP targets is shown. 95.7% of dendrite-present FMRP targets in WT are also to be found as dendrite-present in KO animals. C-E) FISH was performed to assess FMRP target localization (Kmt2d (C) , Lrrc7 (D) and Map2 (E)) in FMRP KO mouse brain slices. Left panel shows the proportion of detected mRNAs that were detected in the neuropil (> 10 um from the predicted Cell bodies layer) in WT and KO animals. Wilcoxon ranked sum was performed to detect significance. Middle panel shows densitometry of 1000 spots samples from each picture analyzed. Distance from the CB was determined as described in methods and Figure 1. In the right panel, spots were binned into 15 groups according to the distance traveled from the CB, and the fraction of spots in each genotype in this range was analyzed by t-test to determined differences in the fraction of spots at each location in FMRP WT and KO animals (* indicates p-value < .05, ** is < .01).
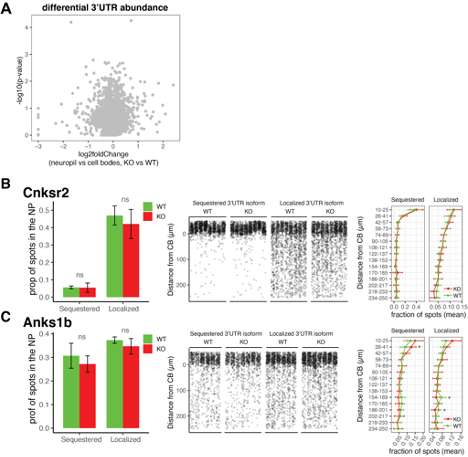
Supplemental Figure 10. FMRP is not required for differential localization of 3’UTR isoforms of its targets. A) Differential 3’UTR usage was analyzed using DEXseq as described in Figure 2 to identify 3’UTRs whose ratio of usage between neuropil and CB in FMRP WT and KO animals were altered. Shown is results from DEXseq analysis showing the log2foldChange (neuropil vs cell bodies, KO vs WT) and -log10(p-value) of each 3’UTR. Gray spots indicate that all 3’UTRs analyzed have an FDR > .05, indicating no significant change in usage between FMRP KO and WT animals. B and C) FISH analysis of localization of 3’UTR isoforms of Cnksr2 (B) and Anks1b (C ) isoforms in FMRP WT and KO animals. These genes were found in Figure 2 to express 3’UTR isoforms that are differentially localized to dendrites. Sequestered isoforms are those that are significantly localized to cell bodies in FMRP WT, and Localized are those that are significantly used in the dendrites of WT CA1 neurons. Left panel, the fraction of spots that are found to be localized to the neuropil (> 10 um from the cell body layer) are shown for each isoform in FMRP WT and KO animals. Differences were assessed by wilcoxon ranked sum tests. Middle panel, densitometry of the distance traveled from the cell bodies for a representative 1000 spots from each picture that was analyzed. Right panel, as described in Supplemental Figure 9, detected mRNAs were binned into 15 bins according to the distance traveled from the cell bodies, and differences in the fractions of spots in each bin in FMRP WT and KO slices were analyzed. Significance indicates results of t-tests (* indicates p-value < .05).
In summary, we characterized the dendritic transcriptome in FMRP KO animals, and compared it to the FMRP WT results presented in Figures 1 and 2, as suggested by the Reviewers. We find that the dendritic transcriptome of FMRP KO animals is extremely similar to that of FMRP WT animals, with ~95% of mRNAs found to be dendrite-present or dendrite-enriched in WT also being found in FMRP KO animals (Figure S9). We validated these results with FISH and found no evidence for significant disruption in the localization of FMRP targets Kmt2d (Figure S9C), Lrrc7 (Figure S9D) or Map2 (Figure S9E) to the CA1 neuropil.
To detect FMRP-dependent changes in distribution of 3’UTR isoforms of FMRP targets, we first performed global analysis of 3’UTR usage in TRAP from FMRP KO animals, using the expressed 3’UTR isoforms that were found in Figure 2. DEXseq analysis on 3’UTR expression in CA1 neuropil vs cell bodies TRAP showed no significant instances of altered 3’UTR usage ratios in FMRP KO animals (Figure S10A). We validated these results by performing FISH on the sequestered and localized 3’UTR isoforms of Cnksr2 and Anks1b genes and show no significant changes in the localization of the 3’UTR isoforms in FMRP KO animals (Figure S10B-C). Taken together, this data suggests that FMRP is not significantly involved in localization of its targets in resting CA1 neurons, but rather shows remarkable selection for localized mRNA isoforms. Instead, we find evidence that FMRP regulates the ribosome association of its targets in a compartment-specific manner by showing an increase in ribosome association of a subset of FMRP targets in the dendrites of CA1 neurons (see Figure 7E).
Besides the addition of the figures described above, we have also now made corrections to the text of the manuscript, enumerated below, to address this.
First, we have, as much as possible, reduced our emphasis throughout the manuscript on the “localization” of mRNAs and rather point out that the study seeks to characterize the differences between the regulated transcriptomes in CA1 cell bodies and dendrites. For example, for Figure 4, instead characterizing the log2FoldChange (neuropil vs CA1 cell bodies) as “dendritic localization”, we change the wording to “relative dendritic abundance” to focus on changes in the abundance of these transcripts in the dendrite vs the cell bodies. We also changed the section heading in the results that describes analysis in the FMRP KO animal from “Dysregulation of mRNA localization in FMRP KO animals” to “FMRP regulates the ribosome association of its targets in dendrites”. We believe that these changes will help to clear up this confusion for the reader.
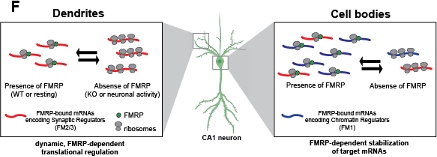
Second, we reformatted the model in Figure 7F. The new version of the model (shown here) emphasizes the point that our study reveals compartment-specific FMRP regulation of a subset of its targets without implying a role for FMRP in the mRNA localization of these transcripts. The text of the manuscript and figure legends have been updated accordingly.
Figure 7F Distinct, compartment-specific FMRP regulation of functionally distinct subsets of mRNAs in CA1 cell bodies and dendrites. In dendrites, the absence of FMRP increases the ribosome association of its targets; this finding is consistent with a model in which FMRP inhibits ribosomal elongation and thereby translation (J. C. Darnell et al. 2011). In resting neurons, the translation of FMRP-bound mRNAs encoding synaptic regulators (FM2 and FM3 mRNAs) is repressed. When FMRP is absent, due to either genetic alteration (FMRP KO or FXS) or neuronal activity-dependent regulation (e.g. FMRP calcium-dependent dephosphorylation (Lee et al. 2011; Bear, Huber, and Warren 2004), ribosome association and translation of targets are increased. In cell bodies, FMRP binds mRNAs that encode for chromatin regulators (the FM1 cluster of FMRP targets), as well as FM2/3 mRNAs (consistent with synapses forming on the cell soma). FM1 targets show patterns of mRNA regulation similar to what our group observed in bulk CA1 neurons: FMRP target abundance is decreased in FMRP KO cells, perhaps due to loss of FMRP-mediated block of degradation of mRNAs with stalled ribosomes (Sawicka et al. 2019; R. B. Darnell 2020).
Third, we have revised the Discussion in order to more completely discuss the model above and also emphasize the finding that FMRP was not found to be involved in the localization of its mRNA targets, but rather in the regulation of the local translation of its targets in a compartment-specific manner. We further speculate on the roles of FMRP in regulation of mRNA abundance and translation in these compartments.
We hope that these changes better reflect the interpretation and novelty of our findings for both the Reviewers and the readers.
Further related to a role of FMRP in mRNA localization, a recent paper in eLife reports that FMRP RGG box promotes mRNA localization of a set of FMRP targets through G-quadruplexes (Goering et al 2020). This relevant paper needs to be cited and discussed.
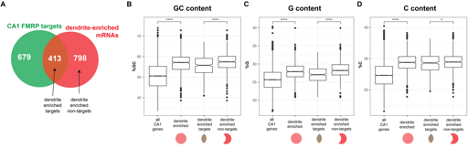
We apologize for this omission, and have now cited and discussed this paper in the Results and Discussion of the manuscript. Importantly, we find that dendrite-enriched mRNAs have high GC content (see figure below, which is now Supplemental Figure 5). This complicates the discovery of potential G-quadruplexes; put another way, G-rich mRNAs will therefore be enriched when compared to not-localized mRNAs, and this is also true for C-rich mRNAs. Dendrite-enriched FMRP directly-bound CA1 neuronal targets (defined by CLIP) are actually G-poor when compared to dendrite-enriched FMRP non-targets (see new Figure S5 and below).
Supplemental Figure 5A-D: Dendrite-enriched are GC rich and dendrite-enriched FMRP targets are GC poor compared to dendrite-enriched non FMRP targets. A) Schematic of the overlap between CA1 FMRP targets and dendrite-enriched mRNAs (defined in Main Figure 1) B) GC content, as defined by percent G + C for all CA1 mRNAs, dendrite enriched mRNAs (1211), dendrite-enriched FMRP targets (413), and dendrite-enriched non-FMRP targets (798, see A). Stars indicate significance in wilcoxon rank sum tests ( is p < .05, **** is p < .0001). C) G content, as defined by percent G, D) C content, as defined by percent C.
In light of these observations, analysis of G- or C- containing motifs needs to be examined in this context. To this end, we performed the experiments suggested here, but did so by searching for the prevalence of G-quadruplexes in dendrite-enriched FMRP targets versus dendrite-enriched FMRP non-targets (Figure S5A). To do this, we used both experimentally-defined G-quadruplexes (described in (Guo and Bartel 2016), Figure S5E), as well as motifs (described in (Goering et al. 2020), Figure S5F). We include the results below, and in a new Figure S5 in the paper.
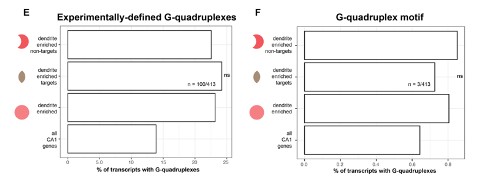
Supplemental Figure 5E-F: mRNAs containing G-quadruplexes are not enriched in dendritic FMRP targets vs dendrite-enriched non-FMRP targets. E) The percent of all CA1 mRNAs, all dendrite-enriched mRNAs, dendrite-enriched FMRP-bound targets (413), and dendrite-enriched non-FMRP targets (798) that contain experimentally-defined G-quadruplexes is plotted. Shown are the results of chi-squared analysis comparing the enrichment of G-quadruplex containing mRNAs in dendrite-enriched FMRP targets vs dendrite-enriched non-FMRP targets. F) As in E, except looking for the presence of mRNAs with G-quadruplex motifs in 3’UTRs as described in (Goering et al. 2020)
Interestingly, we found no difference in the presence of G-quadruplex motifs in the 3’UTRs of these two sets (above and new Supplemental Figure 5). For example, of 413 dendrite-enriched FMRP targets, 100 (24%) had experimentally defined G-quadruplexes in the 3’UTRs, while 159 (22.5%) dendrite-enriched non-FMRP targets had experimentally defined G-quadruplexes. These differences were not significant (by chi-square test).
Searching the 3’UTR sequences of 413 dendrite-enriched FMRP targets above for G-quadruplex motifs (as described in (Goering et al. 2020), which searched for an empirically derived specific motif: GW--G, separated by 7nt), we only found 3 instances in dendrite-enchriched FMRP-bound target mRNAs. Similarly, we found out of 798 non-FMRP targets, only a small subset (6) contained this specific motif in their 3’UTRs. These results were not significant (chi-square test).
In summary, we do not find evidence in our data of G-quadruplexes playing a role in determination of FMRP binding in CA1 dendrites. This data is now included in the results and discussed in the Discussion of the paper.
Reviewer #2 (Public Review):
The authors performed transcriptomic analyses from compartment-specific, micro-dissected hippocampal CA1 region tissue from transgenic mice. One feature that distinguishes this work from previous studies is the use of conditional knock-in of tags (GFP or HA) and tissue specific expression of the Cre recombinase to target a very specific population of pyramidal neurons in the CA1 region--as well as the combined use of TRAPseq, PAPERCLIP and FMRP-CLIP. Also, central to this work are the analysis pipelines that look at large populations of mRNA with the goal of finding features shared by those mRNA that bind FMRP.
First, they established the identity of mRNAs that are dendritically enriched or/and alternatively polyadenylated (APA) by sequencing; followed by validation of a few candidates using smFISH. Next, the APA data was filtered through the rMATS statistical program to identify alternatively spliced (AS) mRNA variants within the APA population. The authors concluded that the majority of splicing events were of the exon-skipping type with NOVA2 as the likely culprit leading to this differential localization of AS isoforms. The authors then proceeded to perform FMRP-CLIP which was analyzed against the TRAP dataset. The (413) mRNAs that were shared by the two experiments (TRAP and FMRP-CLIP) exhibited two notable features: dendrite-enrichment and longer average transcript length. More importantly, They demonstrated that FMRP can preferentially bind to an AS isoform that is enriched in dendrites. Further analyses of FMRP CLIP targets showed that they shared a significant level of genes designated by gene set enrichment analysis (GSEA) as involved in ion transport and receptor signaling and similarly for ASD-related candidate genes.
Strengths: -The combined use of tissue-specific Cre and conditional tags for RPL22, PABPC1 and FMRP help make these pull-downs highly specific and robust. -RNA sequencing approach allows for identification and comparison of populations of ribosome-, PABPC1- and FMRP-associated mRNAs. -Preferential binding of FMRP to AS or APA isoforms in dendrites is an impactful and significant finding.
Weaknesses: -A caution in interpreting comparative or differential RNA-sequencing results as some are correlative.
We appreciate this concern, and agree that RNA-seq analysis alone can be difficult to interpret. However, we feel that our unique approach of combining multiple cell-type specific approaches, including CLIP-seq and PAPERCLIP along with TRAP-seq and RNA-seq result in stronger conclusions that are supported by multiple lines of evidence.
-Validation of FMRP interaction with AS or APA isoforms or ASD candidates by smFISH-IF is lacking.
We find that smFISH-IF in the CA1 neuropil is difficult to interpret in mouse brain slices due to dense networks of processes in addition to contaminating cell types, making IF signals dense, noisy and difficult to quantitate. Although we could theoretically attempt these experiments using an in vitro cell culture model, we believe that the novelty of our work is in a) the cell-type specific nature of our analyses and in b) the fact that our analysis and validation is all performed in vivo. We do not feel confident that in vitro systems are similar enough to our in vivo system to be relevant for this work. This is due not only to differences in their transcriptomes, but also due to the limited number of synapses in vitro cells make with other neurons when compared to CA1 neurons in the brain. Instead, we validate the interactions between FMRP and AS and APA isoforms by isolating junction reads among FMRP-CLIP tags isolated in a cell-type specific manner from intact mouse brains (Figure 5). In this manner, we find direct evidence of FMRP selectively binding to dendritic mRNA isoforms in vivo.
-Although hippocampal CA1 region is an excellent site to study FMRP-RNA interactome, are there other projection systems where altered FMRP-RNA interaction may lead to greater dysfunction?
We appreciate this point and now include this in the revised Discussion.
-

Evaluation Summary:
The authors performed transcriptomic analyses from compartment-specific, micro-dissected hippocampal region tissue from transgenic mice. One feature that distinguishes this work from previous studies is the use of conditional knock-in tags (GFP or HA) and tissue specific expression of the Cre recombinase to target a population of pyramidal neurons in the CA1 region. The strengths of the paper are the rich data sets and innovative integration of methods that will provide a valuable technical resource for the field.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This manuscript integrates conditional mouse models for TRAP, PAPERCLIP and FMRP-CLIP together with compartment specific profiling of mRNA in hippocampal CA1 neurons. Previously, similar approaches have been used to interrogate mRNA localization, differential regulation of 3'UTR isoforms, their local translation, and FMRP-dependent mRNA regulation. This study builds on these previous findings by combining all three approaches, together with analysis of mRNA dysregulation in Fmr1 KO neuron model of FXS. The strengths of the paper are the rich data sets and innovative integration of methods that will provide a valuable technical resource for the field. The weakness of the paper is the limited conceptual advance as well as lack of deeper mechanistic insights on FMRP biology over previous studies, although the …
Reviewer #1 (Public Review):
This manuscript integrates conditional mouse models for TRAP, PAPERCLIP and FMRP-CLIP together with compartment specific profiling of mRNA in hippocampal CA1 neurons. Previously, similar approaches have been used to interrogate mRNA localization, differential regulation of 3'UTR isoforms, their local translation, and FMRP-dependent mRNA regulation. This study builds on these previous findings by combining all three approaches, together with analysis of mRNA dysregulation in Fmr1 KO neuron model of FXS. The strengths of the paper are the rich data sets and innovative integration of methods that will provide a valuable technical resource for the field. The weakness of the paper is the limited conceptual advance as well as lack of deeper mechanistic insights on FMRP biology over previous studies, although the present study validates and integrates past studies, adding some new information on 3'UTR isoforms.
Despite the fact that FMRP targets are overrepresented in the dendritic transcriptome, it does not appear from this study that FMRP plays an active role in the mechanism of dendritic mRNA localization, at least under steady state conditions. One goal of the manuscript is to address a major question in the mRNA localization field, which is how FMRP may differentially modulate "localization" of functional classes of mRNAs such as those encoding transcriptional regulators and synaptic plasticity genes (Line 78-90). The data here indicate that FMRP directly interacts with functional classes of mRNAs in different cellular compartments, which has previously been shown in the field. However, no evidence is provided that mechanistically reveal a role for FMRP to promote subcellular localization of different functional classes of mRNAs. The correlative evidence presented in this manner does not add mechanistic insight.
Further related to a role of FMRP in mRNA localization, a recent paper in eLife reports that FMRP RGG box promotes mRNA localization of a set of FMRP targets through G-quadruplexes (Goering et al 2020). This relevant paper needs to be cited and discussed. This relates to earlier work from the Darnell lab that identified a substantial pool of FMRP targets mRNAs having G-quadruplexes (Darnell et al., Cell 2001). It will be interesting if G-quadruplexes are enriched in their dendritic transcriptome datasets.
Some additional discussion and evaluation of relevant literature would be helpful to explain what aspects fit or do not fit with the proposed model. FMRP biology is more complex and this study should tie together and integrate different mechanisms on translational control (both negative and positive regulation) and mRNA stability.
-

Reviewer #2 (Public Review):
The authors performed transcriptomic analyses from compartment-specific, micro-dissected hippocampal CA1 region tissue from transgenic mice. One feature that distinguishes this work from previous studies is the use of conditional knock-in of tags (GFP or HA) and tissue specific expression of the Cre recombinase to target a very specific population of pyramidal neurons in the CA1 region--as well as the combined use of TRAPseq, PAPERCLIP and FMRP-CLIP. Also, central to this work are the analysis pipelines that look at large populations of mRNA with the goal of finding features shared by those mRNA that bind FMRP.
First, they established the identity of mRNAs that are dendritically enriched or/and alternatively polyadenylated (APA) by sequencing; followed by validation of a few candidates using smFISH. Next, …
Reviewer #2 (Public Review):
The authors performed transcriptomic analyses from compartment-specific, micro-dissected hippocampal CA1 region tissue from transgenic mice. One feature that distinguishes this work from previous studies is the use of conditional knock-in of tags (GFP or HA) and tissue specific expression of the Cre recombinase to target a very specific population of pyramidal neurons in the CA1 region--as well as the combined use of TRAPseq, PAPERCLIP and FMRP-CLIP. Also, central to this work are the analysis pipelines that look at large populations of mRNA with the goal of finding features shared by those mRNA that bind FMRP.
First, they established the identity of mRNAs that are dendritically enriched or/and alternatively polyadenylated (APA) by sequencing; followed by validation of a few candidates using smFISH. Next, the APA data was filtered through the rMATS statistical program to identify alternatively spliced (AS) mRNA variants within the APA population. The authors concluded that the majority of splicing events were of the exon-skipping type with NOVA2 as the likely culprit leading to this differential localization of AS isoforms. The authors then proceeded to perform FMRP-CLIP which was analyzed against the TRAP dataset. The (413) mRNAs that were shared by the two experiments (TRAP and FMRP-CLIP) exhibited two notable features: dendrite-enrichment and longer average transcript length. More importantly, They demonstrated that FMRP can preferentially bind to an AS isoform that is enriched in dendrites. Further analyses of FMRP CLIP targets showed that they shared a significant level of genes designated by gene set enrichment analysis (GSEA) as involved in ion transport and receptor signaling and similarly for ASD-related candidate genes.
Strengths:
-The combined use of tissue-specific Cre and conditional tags for RPL22, PABPC1 and FMRP help make these pull-downs highly specific and robust.
-RNA sequencing approach allows for identification and comparison of populations of ribosome-, PABPC1- and FMRP-associated mRNAs.
-Preferential binding of FMRP to AS or APA isoforms in dendrites is an impactful and significant finding.Weaknesses:
-A caution in interpreting comparative or differential RNA-sequencing results as some are correlative.
-Validation of FMRP interaction with AS or APA isoforms or ASD candidates by smFISH-IF is lacking.
-Although hippocampal CA1 region is an excellent site to study FMRP-RNA interactome, are there other projection systems where altered FMRP-RNA interaction may lead to greater dysfunction?Taken together, the authors were largely successful in demonstrating that FMRP can preferentially associate with its target mRNAs. Previous observations of a large number of FMRP targets suggested that perhaps FMRP could promiscuously bind to many mRNAs. The results presented in this manuscript argues strongly against this idea and show that within a subset of FMRP targets, FMRP can selectively bind in an isoform-specific manner. This work will be of broad interest to the community and serve as a resource and a template for those studying RNA-binding protein(s) and its mRNA targets.
-