Complex pattern of facial remapping in somatosensory cortex following congenital but not acquired hand loss
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This is a remarkably ambitious study that has been skilfully executed on a strong number of control participants, amputees, and one-handers. The complementarity of state-of-the-art uni- and multi-variate analyses are in the service of the research question, and the paper is clearly written. The main contribution of this paper, relative to previous studies, resides in the mapping of multiple face-part all at once in the three groups. The study suggests that the deprived hand cortical territory is not invaded by the facial cortical neighbor, the forehead, but instead by the lips that are more distant but functionally related to the hand as it can be used to compensate hand loss for manipulating objects.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1, Reviewer #2 and Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Cortical remapping after hand loss in the primary somatosensory cortex (S1) is thought to be predominantly dictated by cortical proximity, with adjacent body parts remapping into the deprived area. Traditionally, this remapping has been characterised by changes in the lip representation, which is assumed to be the immediate neighbour of the hand based on electrophysiological research in non-human primates. However, the orientation of facial somatotopy in humans is debated, with contrasting work reporting both an inverted and upright topography. We aimed to fill this gap in the S1 homunculus by investigating the topographic organisation of the face. Using both univariate and multivariate approaches we examined the extent of face-to-hand remapping in individuals with a congenital and acquired missing hand (hereafter one-handers and amputees, respectively), relative to two-handed controls. Participants were asked to move different facial parts (forehead, nose, lips, tongue) during functional MRI (fMRI) scanning. We first confirmed an upright face organisation in all three groups, with the upper-face and not the lips bordering the hand area. We further found little evidence for remapping of both forehead and lips in amputees, with no significant relationship to the chronicity of their phantom limb pain (PLP). In contrast, we found converging evidence for a complex pattern of face remapping in congenital one-handers across multiple facial parts, where relative to controls, the location of the cortical neighbour – the forehead – is shown to shift away from the deprived hand area, which is subsequently more activated by the lips and the tongue. Together, our findings demonstrate that the face representation in humans is highly plastic, but that this plasticity is restricted by the developmental stage of input deprivation, rather than cortical proximity.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
Using fMRI-based univariate and multivariate analyses, Root, Muret, et al. investigated the topography of face representation in the somatosensory cortex of typically developed two-handed individuals and individuals with a congenital and acquired missing hand. They provide clear evidence for an upright face topography in the somatosensory cortex in all three groups. Moreover, they find that one-handers, but not amputees, show shorter distances from lip representations to the hand area, suggesting a remapping of the lips. They also find a shift away of the upper face from the deprived hand area in one-handers, and significantly greater dissimilarity between face part representations in amputees and one-handers. The authors argue that this pattern of remapping is different to that of …
Author Response
Reviewer #1 (Public Review):
Using fMRI-based univariate and multivariate analyses, Root, Muret, et al. investigated the topography of face representation in the somatosensory cortex of typically developed two-handed individuals and individuals with a congenital and acquired missing hand. They provide clear evidence for an upright face topography in the somatosensory cortex in all three groups. Moreover, they find that one-handers, but not amputees, show shorter distances from lip representations to the hand area, suggesting a remapping of the lips. They also find a shift away of the upper face from the deprived hand area in one-handers, and significantly greater dissimilarity between face part representations in amputees and one-handers. The authors argue that this pattern of remapping is different to that of cortical neighborhood theories and points toward a remapping of face parts which have the ability to compensate for hand function, e.g., using the lips/mouth to manipulate an object.
These findings provide interesting insights into the topographic organization of face parts and the principles of cortical (re)organization. The authors use several analytical approaches, including distance measures between hand- and face-part-responsive regions and representational similarity analysis (RSA). Particularly commendable is the rigorous statistical analysis, such as the use of Bayesian comparisons, and careful interpretation of absent group differences.
We thank the reviewer for their positive and constructive feedback.
Reviewer #2 (Public Review):
After amputation, the deafferented limb representation in the somatosensory cortex is activated by stimulation of other body parts. A common belief is that the lower face, including the lips, preferentially "invades" deafferented cortex due to its proximity to cortex. In the present study, this hypothesis is tested by mapping the somatosensory cortex using fMRI as amputees, congenital one-handers, and controls moved their forehead, nose, lips or tongue. First, they found that, unlike its counterpart in monkeys, the representation of the face in the somatosensory cortex is right-side up, with the forehead most medial (and abutting the hand) and the lips most lateral. Second, there was little evidence of "reorganization" of the deafferented cortex in amputees, even when tested with movements across the entire face rather than only the lips. Third, congenital one-handers showed significant reorganization of deafferented cortex, characterized principally by the invasion of the lower face, in contrast to predictions from the hypothesis that proximity was the driving factor. Fourth, there was no relationship between phantom limb pain reports and reorganization.
As a non-expert in fMRI, I cannot evaluate the methodology. That being said, I am not convinced that the current consensus is that the representation of the face in humans is flipped compared to that of monkeys. Indeed, the overwhelming majority of somatosensory homunculi I have seen for humans has the face right side up. My sense is that the fMRI studies that found an inverted (monkey-like) face representation contradict the consensus.
Thank you for point this out. As we tried to emphasise in the introduction, very few neuroimaging studies actually investigated face somatotopy in humans, with inconsistent results. We agree the default consensus tends to be dominated by the up-right depiction of Penfield’s homunculus (recently replicated by Roux et al, 2018). However, due to methodological and practical constraints, alignment across subjects in the case of intracortical recordings is usually difficult to achieve, and thus makes it difficult to assess the consistency in topographical organisation. Moreover, previous imaging studies did not manage to convincingly support Penfield’s homunculus. For these two key reasons, the spatial orientation of the human facial homunculus is still debated. A further limiting factor of previous studies in humans is that the vast majority of human studies investigating face (re)mapping in humans focused solely on the lip representation, using the cortical proximity hypothesis to interpret their results. Consequently, as we highlight above in our response to the Editor, there is a wide-spread and false representation in the human literature of the lips neighbouring the hand area.
To account for the reviewer’s critic and convey some of this context, we changed our title from: Reassessing face topography in primary somatosensory cortex and remapping following hand loss; to: Complex pattern of facial remapping in somatosensory cortex following congenital but not acquired hand loss. This was done to de-emphasise the novelty of face topography relative to our other findings.
We also rewrote our introduction (lines 79-94) as follows:
“The research focus on lip cortical remapping in amputees is based on the assumption that the lips neighbour the hand representation. However, this assumption goes against the classical upright orientation of the face in S126–30, as first depicted in Penfield’s Homunculus and in later intracortical recordings and stimulation studies26–29, with the upper-face (i.e., forehead) bordering the hand area. In contrast, neuroimaging studies in humans studying face topography provided contradictory evidence for the past 30 years. While a few neuroimaging studies provided partial evidence in support of the traditional upright face organisation31, other studies supported the inverted (or ‘upside-down’) somatotopic organisation of the face, similar to that of non-human primates32,33. Other studies suggested a segmental organisation34, or even a lack of somatotopic organisation35–37, whereas some studies provided inconclusive or incomplete results38–41. Together, the available evidence does not successfully converge on face topography in humans. In line with the upright organisation originally suggested by Penfield, recent work reported that the shift in the lip representation towards the missing hand in amputees was minimal42,43, and likely to reside within the face area itself. Surprisingly, there is currently no research that considers the representation of other facial parts, in particular the upper-face (e.g., the forehead), in relation to plasticity or PLP.”
We also updated the discussion accordingly (lines 457, 469-477, 490-492).
Similarly, it is not clear to me how the observations (1) of limited reorganization in amputees, (2) of significant reorganization in congenital one-handers, and (3) of the lack of relationship between PLP and reorganization is novel given the previous work by this group. Perhaps the authors could more clearly articulate the novelty of these results compared to their previous findings.
Thank you for giving us the opportunity to clarify on this important point. The novelty of these results can be summarised as follow:
(1) Conceptually, it is crucial for us to understand if deprivation-triggered plasticity is constrained by the local neighbourhood, because this can give us clues regarding the mechanisms driving the remapping. We provide strong topographic evidence about the face orientation in controls, amputees and one-handers.
(2) The vast majority of previous research on brain plasticity following hand loss (both congenital and acquired) in humans has exclusively focused on the lower face, and lips in particular. We provide systematic evidence for stable organisation and remapping of the neighbouring upper face, as well as the lower face. We also study topographic representation of the tongue (and nose) for the first time.
(3) The vast majority of previous research on brain remapping following hand loss (both congenital and acquired, neuroimaging and electrophysiological) was focused on univariate activity measures, such as the spatial spread of units showing a similar feature preference, or the average activity level across individual units. We are going beyond remapping by using RSA, which allows us to ask not only if new information is available in the deprived cortex (as well as the native face area), but also whether this new information is structured consistently across individuals and groups. We show that representational content is enhanced in the deprived cortex one-handers whereas it is stable in amputees relative to controls (and to their intact hand region).
(4) Based on previous studies, the assumption was that reorganisation in congenital one-handers was relatively unspecific, affecting all tested body parts. Here, we provide evidence for a more complex pattern of remapping, with the forehead representation seemingly moving out of the missing hand region (and the nose representation being tentatively similar to controls). That is, we show not just “invasion” but also a shift of the neighbour away from the hand area which has never been documented (or in fact suggested).
(5) Using Bayesian analyses we provide definitive evidence against a relationship between PLP and forehead remapping, providing first and conclusive evidence against the remapping hypothesis, based on cortical neighbourhood.
Our inclination is not to add a summary paragraph of these points in our discussion, as it feels too promotional. Instead, we have re-written large sections of the introduction and discussion to better emphasise each of these points separately throughout the text, where the context is most appropriate. Given the public review strategy taken by eLife, the novelty summary provided above will be available for any interested reader, as part of the public review process. However, should the reviewer feel that a novelty summary paragraph is required (or an emphasis on any of the points summarised above), we will be happy to revise the manuscript accordingly.
Finally, Jon Kaas and colleagues (notably Niraj Jain) have provided evidence in experiments with monkeys that much of the observed reorganization in the somatosensory cortex is inherited from plasticity in the brain stem. Jain did not find an increased propensity for axons to cross the septum between face and hand representations after (simulated) amputation. From this perspective, the relevant proximity would be that of the cuneate and trigeminal nuclei and it would be critical to map out the somatotopic organization of the trigeminal and cuneate nuclei to test hypotheses about the role of proximity in this remapping.
Thank you for highlighting this very relevant point, which we are well aware of. We fully agree with the reviewer that this is an important goal for future study, but functional imaging of the brainstem in humans is particularly challenging and would require ultra high field imaging (7T) and specialised equipment. We have encountered much local resistance due to hypothetical issues for MRI safety for scanning amputees in this higher field strength, meaning we are unable to carry out this research ourselves. Our former lab member Sanne Kikkert, who is now running her independent research programme in Zurich, has been working towards this goal for the past 4 years. So we can say with confidence that this aim is well beyond the scope of the current study. In response to your comment, we mentioned this potential mechanism in the introduction (lines 98-101), we ensured that we only referred to “cortical proximity” throughout our manuscript, and we circle back to this important point in the discussion.
Lines 539-543: “Moreover, even if the remapping we observed here goes against the theory of cortical proximity, it can still arise from representational proximity at the subcortical level, in particular at the brainstem level44,45. While challenging in humans, mapping both the cuneate and trigeminal nuclei would be critical to provide a more complete picture regarding the role of proximity in remapping.”
Reviewer #3 (Public Review):
In their study, the authors set up to challenge the long-held claim that cortical remapping in the somatosensory cortex in hand deprived cortical territories follows somatotopic proximity (the hand region gets invaded by cortical neighbors) as classically assumed. In contrast to this claim, the authors suggest that remapping may not follow cortical proximity but instead functional rules as to how the effector is used. Their data indeed suggest that the deprived hand area is not invaded by the forefront which is the cortical neighbor but instead by the lips which may compensate for hand loss in manipulating objects. Interestingly the authors suggest this is mostly the case for one-handers but not in amputees for who the reorganization seems more limited in general (but see my comments below on this last point).
This is a remarkably ambitious study that has been skilfully executed on a strong number of participants in each group. The complementarity of state-of-the-art uni- and multi-variate analyses are in the service of the research question, and the paper is clearly written. The main contribution of this paper, relative to previous studies including those of the same group, resides in the mapping of multiple face parts all at once in the three groups.
We are grateful to the reviewer for appreciating the immense effort that this study involved.
In the winner takes all approach, the authors only include 3 face parts but exclude from the analyses the nose and the thumb. I am not fully convinced by the rationale for not including nose in univariate analyses - because it does not trigger reliable activity - while keeping it for representational similarity analyses. I think it would be better to include the nose in all analyses or demonstrate this condition is indeed "noisy" and then remove it from all the analyses. Indeed, if the activity triggered by nose movement is unreliable, it should also affect multivariate.
Following this comment, we re-ran all univariate analyses to include the nose, and updated throughout the main text and supplemental results and related figures. In short, adding the nose did not change the univariate results, apart from a now significant group x hemisphere interaction for the CoG of the tongue when comparing amputees and controls, matching better the trends for greater surface coverage in the deprived hand ROI of amputees. Full details are provided in our response to Reviewer 1 above.
The rationale for not including the hand is maybe more convincing as it seems to induce activity in both controls and amputees but not in one-handers. First, it would be great to visualize this effect, at least as supplemental material to support the decision. Then, this brings the interesting possibility that enhanced invasion of hand territory by lips in one-handers might link to the possibility to observe hand-related activity in the presupposed hand region in this population. Maybe the authors may consider linking these.
Thank you for this comment. As we explain in our response to Reviewer 1 above, we did not intent the thumb condition in one-handers for analysis, as the task given to one-handers (imagine moving a body part you never had before) is inherently different to that given to the other groups (move - or at least attempt to move - your (phantom) hand). As such, we could not pursuit the analysis suggested by the reviewer here. To reduce the discrepancy and following Reviewer 1’s advice, we decided to remove the hand-face dissimilarity analysis which we included in our original manuscript, and might have sparked some of this interest. Upon reflection we agreed that this specific analysis does not directly relate to the question of remapping (but rather of shared representation), in addition to making the paper unbalanced. We will now feature this analysis in another paper that appears more appropriate in the context of referred sensations in amputees (Amoruso et al, 2022 MedRxiv).
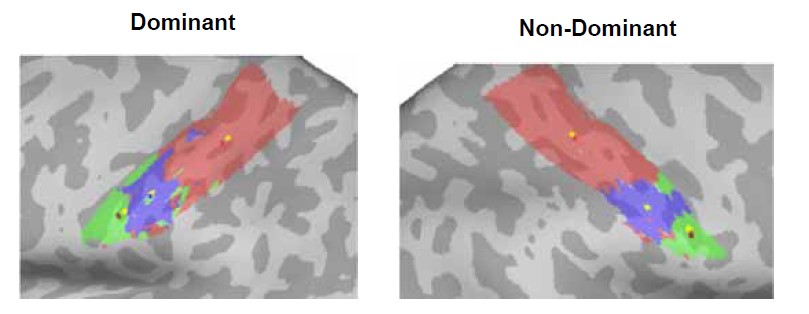
The use of the geodesic distance between the center of gravity in the Winner Take All (WTA) maps between each movement and a predefined cortical anchor is clever. More details about how the Center Of Gravity (COG) was computed on spatially disparate regions might deserve more explanations, however.
We are happy to provide more detail on this analysis, which weights the CoG based on the clusters size (using the workbench command -metric-weighted-stats). Let’s consider the example shown here (Figure 1) for a single control participant, where each CoG is measured either without weighting (yellow vertices) or with cluster weighting (forehead CoG=red, lip CoG=dark blue, tongue CoG=dark red). When the movement produces a single cluster of activity (the lips in the non-dominant hemisphere, shown in blue), the CoG’s location was identical for both weighted (red) and unweighted (yellow) calculations. But other movements, such as the tongue (green), produced one large cluster (at the lateral end), with a few more disparate smaller clusters more medially. In this case, the larger cluster of maximal activity is weighted to a greater extent than the smaller clusters in the CoG calculation, meaning the CoG is slightly skewed towards it (dark red), relative to the smaller clusters.
Figure 1. Centre-of-gravity calculation, weighted and unweighted by cluster size, in an example control participant. Here the winner-takes-all output for each facial movement (forehead=red, lips=blue, tongue=green) was used to calculate the centre-of-gravity (CoG) at the individual-level in both the dominant (left-hand side) and non-dominant (right-hand side) hemisphere, weighted by cluster size (forehead CoG=red, lip CoG=dark blue, tongue CoG=dark red), compared to an unweighted calculation (denoted by yellow dots within each movements’ winner-takes-all output).
This is now explained in the methods (lines 760-765) as follows:
“To assess possible shifts in facial representations towards the hand area, the centre-of-gravity (CoG) of each face-winner map was calculated in each hemisphere. The CoG was weighted by cluster size meaning that in the event of multiple clusters contributing to the calculation of a single CoG for a face-winner map, the voxels in the larger cluster are overweighted relative to those in the smaller clusters. The geodesic cortical distance between each movement’s CoG and a predefined cortical anchor was computed.”
Moreover, imagine that for some reason the forefront region extends both dorsally and ventrally in a specific population (eg amputees), the COG would stay unaffected but the overlap between hand and forefront would increase. The analyses on the surface area within hand ROI for lips and forehead nicely complement the WTA analyses and suggest higher overlap for lips and lower overlap for forehead but none of the maps or graphs presented clearly show those results - maybe the authors could consider adding a figure clearly highlighting that there is indeed more lip activity IN the hand region.
We agree with you on this limitation of the CoG and this is why we interpret all cortical distances analyses in tandem with the laterality indices. The laterality indices correspond to the proportion of surface area in the hand region for a given face part in the winner-maps.
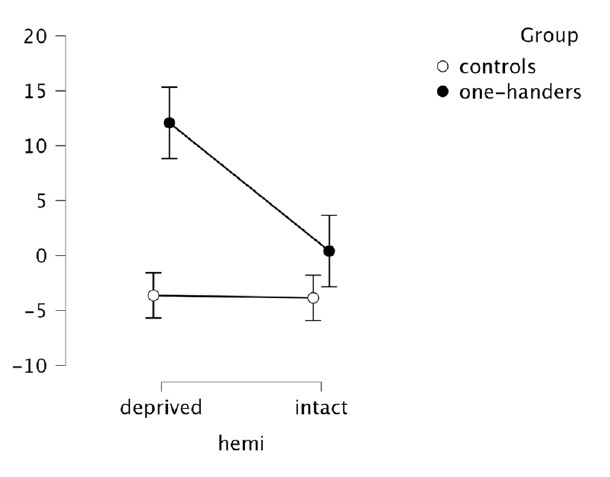
Nevertheless, to further convince the Reviewer, we extracted activity levels (beta values) within the hand region of congenitals and controls, and we ran (as for CoGs) a mixed ANOVA with the factors Hemisphere (deprived x intact) and Group (controls x one-handers).
As expected from the laterality indices obtained for the Lips, we found a significant group x hemisphere interaction (F(1,41)=4.52, p=0.040, n2p=0.099), arising from enhanced activity in the deprived hand region in one-handers compared to the non-dominant hand region in controls (t(41)=-2.674, p=0.011) and to the intact hand region in one-handers (t(41)=-3.028, p=0.004).
Since this kind of analysis was the focus of previous studies (from which we are trying to get away) and since it is redundant with the proportion of face-winner surface coverage in the hand region, we decided not to include it in the paper. But we could add it as a Supplementary result if the Reviewer believes this strengthens our interpretation.
In addition to overlap analyses between hand and other body parts, the authors may also want to consider doing some Jaccard similarity analyses between the maps of the 3 groups to support the idea that amputees are more alike controls than one-handers in their topographic activity, which again does not appear clear from the figures.
We thank the reviewers for this clever suggestion. We now include the Jaccard similarity analysis, which quantified the degree of similarity (0=no overlap between maps; 1=fully overlapping) between winner-takes-all maps (which included the nose; akin to the revised univariate results) across groups. For each face part/amputee, the similarity with the 22 controls and 21 one-handers respectively was averaged. We utilised a linear mixed model which included fixed factors of Group (One-handers x Controls), Movement (Forehead x Nose x Lips x Tongue) and Hemisphere (Intact x Deprived) on Jaccard similarity values (similar to what we used for the RSA analysis). A random effect of participant, as well as covariates of ages, were also included in the model.
Results showed a significant group x hemisphere interaction (F(240.0)=7.70, p=0.006; controlled for age; Fig. 5), indicating that amputees’ maps showed different similarity values to controls’ and one-handers’ depending on the hemisphere. Post-hoc comparisons (corrected alpha=0.025; uncorrected p-values reported) revealed significantly higher similarity to controls’ than to one-handers’ maps in the deprived hemisphere (t(240)=-3.892, p<.001). Amputees’ maps also showed higher similarity to controls’ maps in the deprived relative to the intact hemisphere (t(240)=2.991, p=0.003). Amputees, therefore, displayed greater similarity of facial somatotopy in the deprived hemisphere to controls, suggesting again fewer evidence for cortical remapping in amputees.
We added these results at the end of the univariate analyses (lines 335-351) and in the discussion (lines 464-465 and 497-500).
This brings to another concern I have related to the claim that the change in the cortical organization they observe is mostly observed in one-handers. It seems that most of this conclusion relies on the fact that some effects are observed in one-handers but not in amputees when compared to controls, however, no direct comparisons are done between amputees and one-handers so we may be in an erroneous inference about the interaction when this is actually not tested (Nieuwenhuis, 11). For instance, the shift away from the hand/face border of the forehead is also (mildly) significant in amputees (as observed more strongly in one-handers) so the conclusion (eg from the subtitle of the results section) that it is specific to one-hander might not fully be supported by the data. Similar to the invasion of the hand territory from the lips which is significant in amputees in terms of surface area. All together this calls for toning down the idea that plasticity is restricted to congenital deprivation (eg last sentence of the abstract). Even if numerically stronger, if I am not wrong, there are no stats showing remapping is indeed stronger in one-handers than in amputees and actually, amputees show significant effects when compared to controls along the lines as those shown (even if more strongly) in one-handers.
Thank you for this very important comment. We fully agree – the RSA across-groups comparison is highly informative but insufficient to support our claims. We did not compare the groups directly to avoid multiple comparisons (both for statistical reasons and to manage the size of the results section). But the reviewer’s suggestion to perform a Jaccard similarity analysis complements very nicely the univariate and multivariate results and allows for a direct (and statistically lean) comparison between groups, to assess whether amputees are more similar to controls or to congenital one-handers, taking into account all aspects of their maps (both spatial location/CoG and surface coverage). We added the Jaccard analysis to the main text, at the end of the univariate results (lines 335-385). The Jaccard analysis suggests that amputees’ maps in the deprived hemisphere were more similar to the maps of controls than to the ones of congenital one-handers. This allowed us to obtain significant statistical results to support the claim that remapping is indeed stronger in one-handers than in amputees (lines 346-351). We also compared both amputees and one-handers to the control group. In line with our univariate results, this revealed that the only face part for which controls were more similar to one-handers than to amputees was the tongue (lines 379-381). And that the forehead remapping observed at the univariate level in amputees (surface area), is likely to arise from differences in the intact hemisphere (lines 381-383).
Finally, we also added the post-hoc statistics comparing amputees to congenitals in the RSA analysis (lines 425-427): “While facial information in the deprived hand area was increased in one-handers compared with amputees, this effect did not survive our correction for multiple comparisons (t(70.7)=-2.117, p=0.038).”
Regarding the univariate results mentioned by the reviewer, we would like to emphasise that we had no significant effect for the lips in amputees, though we agree the surface area appears in between controls and one-handers. But this laterality index was not different from zero. This test is now added lines 189-190. Regarding the forehead, we fully agree with the Reviewer, and we adjusted the subtitle accordingly (lines 241-242). For consistency, we also added the t-test vs zero for the forehead surface area (non-significant, lines 251-253).
Also, maybe the authors could explore whether there is actually a link between the number of years without hand and the remapping effects.
To address this question, we explored our data using a correlation analysis. The only body part who showed some suggestive remapping effects was the tongue, and so we explored whether we could find a relationship (Pearson’s correlation) between years since amputation and the laterality index of the Tongue in amputees (r = 0.007, p=0.980, 95% CI [-0.475, 0.475]). We also explored amputees’ global Jaccard similarity values to controls in the deprived hemisphere (r = -0.010, p=0.970, 95% CI [-0.488, 0.473]), and could not find any relationship. Considering there was no strong remapping effect to explain, we find this result too exploratory to include in our manuscript.
One hypothesis generated by the data is that lips remap in the deprived hand area because lips serve compensatory functions. Actually, also in controls, lips and hands can be used to manipulate objects, in contrast to the forehead. One may thus wonder if the preferential presence of lips in the hand region is not latent even in controls as they both link in functions?
We agree with the reviewer’s reasoning, and we think that the distributed representational content we recently found in two-handers (Muret et al, 2022) provides a first hint in this direction. It is worth noting that in that previous publication we did not find differences across face parts in the activity levels obtained in the hand region, except for slightly more negative values for the tongue. But we do think that such latent information is likely to provide a “scaffolding” for remapping. While the design of our face task does not allow to assess information content for each face part (as done for the lips in Muret et al, 2022), this should be further investigated in follow-up studies.
We added a sentence in the discussion to highlight this interesting notion: Lines 556-559: “Together with the recent evidence that lip information content is already significant in the hand area of two-handed participants (Muret et al, 2022), compensatory behaviour since developmental stages might further uncover (and even potentiate) this underlying latent activity.”
-

Evaluation Summary:
This is a remarkably ambitious study that has been skilfully executed on a strong number of control participants, amputees, and one-handers. The complementarity of state-of-the-art uni- and multi-variate analyses are in the service of the research question, and the paper is clearly written. The main contribution of this paper, relative to previous studies, resides in the mapping of multiple face-part all at once in the three groups. The study suggests that the deprived hand cortical territory is not invaded by the facial cortical neighbor, the forehead, but instead by the lips that are more distant but functionally related to the hand as it can be used to compensate hand loss for manipulating objects.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also …
Evaluation Summary:
This is a remarkably ambitious study that has been skilfully executed on a strong number of control participants, amputees, and one-handers. The complementarity of state-of-the-art uni- and multi-variate analyses are in the service of the research question, and the paper is clearly written. The main contribution of this paper, relative to previous studies, resides in the mapping of multiple face-part all at once in the three groups. The study suggests that the deprived hand cortical territory is not invaded by the facial cortical neighbor, the forehead, but instead by the lips that are more distant but functionally related to the hand as it can be used to compensate hand loss for manipulating objects.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1, Reviewer #2 and Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
Using fMRI-based univariate and multivariate analyses, Root, Muret, et al. investigated the topography of face representation in the somatosensory cortex of typically developed two-handed individuals and individuals with a congenital and acquired missing hand. They provide clear evidence for an upright face topography in the somatosensory cortex in all three groups. Moreover, they find that one-handers, but not amputees, show shorter distances from lip representations to the hand area, suggesting a remapping of the lips. They also find a shift away of the upper face from the deprived hand area in one-handers, and significantly greater dissimilarity between face part representations in amputees and one-handers. The authors argue that this pattern of remapping is different to that of cortical neighborhood …
Reviewer #1 (Public Review):
Using fMRI-based univariate and multivariate analyses, Root, Muret, et al. investigated the topography of face representation in the somatosensory cortex of typically developed two-handed individuals and individuals with a congenital and acquired missing hand. They provide clear evidence for an upright face topography in the somatosensory cortex in all three groups. Moreover, they find that one-handers, but not amputees, show shorter distances from lip representations to the hand area, suggesting a remapping of the lips. They also find a shift away of the upper face from the deprived hand area in one-handers, and significantly greater dissimilarity between face part representations in amputees and one-handers. The authors argue that this pattern of remapping is different to that of cortical neighborhood theories and points toward a remapping of face parts which have the ability to compensate for hand function, e.g., using the lips/mouth to manipulate an object.
These findings provide interesting insights into the topographic organization of face parts and the principles of cortical (re)organization. The authors use several analytical approaches, including distance measures between hand- and face-part-responsive regions and representational similarity analysis (RSA). Particularly commendable is the rigorous statistical analysis, such as the use of Bayesian comparisons, and careful interpretation of absent group differences.
-

Reviewer #2 (Public Review):
After amputation, the deafferented limb representation in the somatosensory cortex is activated by stimulation of other body parts. A common belief is that the lower face, including the lips, preferentially "invades" deafferented cortex due to its proximity to cortex. In the present study, this hypothesis is tested by mapping the somatosensory cortex using fMRI as amputees, congenital one-handers, and controls moved their forehead, nose, lips or tongue. First, they found that, unlike its counterpart in monkeys, the representation of the face in the somatosensory cortex is right-side up, with the forehead most medial (and abutting the hand) and the lips most lateral. Second, there was little evidence of "reorganization" of the deafferented cortex in amputees, even when tested with movements across the entire …
Reviewer #2 (Public Review):
After amputation, the deafferented limb representation in the somatosensory cortex is activated by stimulation of other body parts. A common belief is that the lower face, including the lips, preferentially "invades" deafferented cortex due to its proximity to cortex. In the present study, this hypothesis is tested by mapping the somatosensory cortex using fMRI as amputees, congenital one-handers, and controls moved their forehead, nose, lips or tongue. First, they found that, unlike its counterpart in monkeys, the representation of the face in the somatosensory cortex is right-side up, with the forehead most medial (and abutting the hand) and the lips most lateral. Second, there was little evidence of "reorganization" of the deafferented cortex in amputees, even when tested with movements across the entire face rather than only the lips. Third, congenital one-handers showed significant reorganization of deafferented cortex, characterized principally by the invasion of the lower face, in contrast to predictions from the hypothesis that proximity was the driving factor. Fourth, there was no relationship between phantom limb pain reports and reorganization.
As a non-expert in fMRI, I cannot evaluate the methodology. That being said, I am not convinced that the current consensus is that the representation of the face in humans is flipped compared to that of monkeys. Indeed, the overwhelming majority of somatosensory homunculi I have seen for humans has the face right side up. My sense is that the fMRI studies that found an inverted (monkey-like) face representation contradict the consensus. Similarly, it is not clear to me how the observations (1) of limited reorganization in amputees, (2) of significant reorganization in congenital one-handers, and (3) of the lack of relationship between PLP and reorganization is novel given the previous work by this group. Perhaps the authors could more clearly articulate the novelty of these results compared to their previous findings. Finally, Jon Kaas and colleagues (notably Niraj Jain) have provided evidence in experiments with monkeys that much of the observed reorganization in the somatosensory cortex is inherited from plasticity in the brain stem. Jain did not find an increased propensity for axons to cross the septum between face and hand representations after (simulated) amputation. From this perspective, the relevant proximity would be that of the cuneate and trigeminal nuclei and it would be critical to map out the somatotopic organization of the trigeminal and cuneate nuclei to test hypotheses about the role of proximity in this remapping.
-

Reviewer #3 (Public Review):
In their study, the authors set up to challenge the long-held claim that cortical remapping in the somatosensory cortex in hand deprived cortical territories follows somatotopic proximity (the hand region gets invaded by cortical neighbors) as classically assumed. In contrast to this claim, the authors suggest that remapping may not follow cortical proximity but instead functional rules as to how the effector is used. Their data indeed suggest that the deprived hand area is not invaded by the forefront which is the cortical neighbor but instead by the lips which may compensate for hand loss in manipulating objects. Interestingly the authors suggest this is mostly the case for one-handers but not in amputees for who the reorganization seems more limited in general (but see my comments below on this last point).
Reviewer #3 (Public Review):
In their study, the authors set up to challenge the long-held claim that cortical remapping in the somatosensory cortex in hand deprived cortical territories follows somatotopic proximity (the hand region gets invaded by cortical neighbors) as classically assumed. In contrast to this claim, the authors suggest that remapping may not follow cortical proximity but instead functional rules as to how the effector is used. Their data indeed suggest that the deprived hand area is not invaded by the forefront which is the cortical neighbor but instead by the lips which may compensate for hand loss in manipulating objects. Interestingly the authors suggest this is mostly the case for one-handers but not in amputees for who the reorganization seems more limited in general (but see my comments below on this last point).
This is a remarkably ambitious study that has been skilfully executed on a strong number of participants in each group. The complementarity of state-of-the-art uni- and multi-variate analyses are in the service of the research question, and the paper is clearly written. The main contribution of this paper, relative to previous studies including those of the same group, resides in the mapping of multiple face parts all at once in the three groups.
In the winner takes all approach, the authors only include 3 face parts but exclude from the analyses the nose and the thumb. I am not fully convinced by the rationale for not including nose in univariate analyses - because it does not trigger reliable activity - while keeping it for representational similarity analyses. I think it would be better to include the nose in all analyses or demonstrate this condition is indeed "noisy" and then remove it from all the analyses. Indeed, if the activity triggered by nose movement is unreliable, it should also affect multivariate.
The rationale for not including the hand is maybe more convincing as it seems to induce activity in both controls and amputees but not in one-handers. First, it would be great to visualize this effect, at least as supplemental material to support the decision. Then, this brings the interesting possibility that enhanced invasion of hand territory by lips in one-handers might link to the possibility to observe hand-related activity in the presupposed hand region in this population. Maybe the authors may consider linking these.
The use of the geodesic distance between the center of gravity in the Winner Take All (WTA) maps between each movement and a predefined cortical anchor is clever. More details about how the Center Of Gravity (COG) was computed on spatially disparate regions might deserve more explanations, however. Moreover, imagine that for some reason the forefront region extends both dorsally and ventrally in a specific population (eg amputees), the COG would stay unaffected but the overlap between hand and forefront would increase. The analyses on the surface area within hand ROI for lips and forehead nicely complement the WTA analyses and suggest higher overlap for lips and lower overlap for forehead but none of the maps or graphs presented clearly show those results - maybe the authors could consider adding a figure clearly highlighting that there is indeed more lip activity IN the hand region.
In addition to overlap analyses between hand and other body parts, the authors may also want to consider doing some Jaccard similarity analyses between the maps of the 3 groups to support the idea that amputees are more alike controls than one-handers in their topographic activity, which again does not appear clear from the figures.This brings to another concern I have related to the claim that the change in the cortical organization they observe is mostly observed in one-handers. It seems that most of this conclusion relies on the fact that some effects are observed in one-handers but not in amputees when compared to controls, however, no direct comparisons are done between amputees and one-handers so we may be in an erroneous inference about the interaction when this is actually not tested (Nieuwenhuis, 11). For instance, the shift away from the hand/face border of the forehead is also (mildly) significant in amputees (as observed more strongly in one-handers) so the conclusion (eg from the subtitle of the results section) that it is specific to one-hander might not fully be supported by the data. Similar to the invasion of the hand territory from the lips which is significant in amputees in terms of surface area. All together this calls for toning down the idea that plasticity is restricted to congenital deprivation (eg last sentence of the abstract). Even if numerically stronger, if I am not wrong, there are no stats showing remapping is indeed stronger in one-handers than in amputees and actually, amputees show significant effects when compared to controls along the lines as those shown (even if more strongly) in one-handers. Also, maybe the authors could explore whether there is actually a link between the number of years without hand and the remapping effects.
One hypothesis generated by the data is that lips remap in the deprived hand area because lips serve compensatory functions. Actually, also in controls, lips and hands can be used to manipulate objects, in contrast to the forehead. One may thus wonder if the preferential presence of lips in the hand region is not latent even in controls as they both link in functions?
-