Coupling of pupil- and neuronal population dynamics reveals diverse influences of arousal on cortical processing
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The study presents novel results on spontaneous fluctuations in pupil dilation in relation to the spectral dynamics in a large sample of human participants. The study is based on MEG recordings allowing for quantifying these relations in time and space. The data provide important new insight into the temporal and spatial characteristics of pupil-linked changes in cortical states which form the basis for incorporating this insight in future clinical and cognitive neuroscience studies.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1, Reviewer #2 and Reviewer #3 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Fluctuations in arousal, controlled by subcortical neuromodulatory systems, continuously shape cortical state, with profound consequences for information processing. Yet, how arousal signals influence cortical population activity in detail has so far only been characterized for a few selected brain regions. Traditional accounts conceptualize arousal as a homogeneous modulator of neural population activity across the cerebral cortex. Recent insights, however, point to a higher specificity of arousal effects on different components of neural activity and across cortical regions. Here, we provide a comprehensive account of the relationships between fluctuations in arousal and neuronal population activity across the human brain. Exploiting the established link between pupil size and central arousal systems, we performed concurrent magnetoencephalographic (MEG) and pupillographic recordings in a large number of participants, pooled across three laboratories. We found a cascade of effects relative to the peak timing of spontaneous pupil dilations: Decreases in low-frequency (2–8 Hz) activity in temporal and lateral frontal cortex, followed by increased high-frequency (>64 Hz) activity in mid-frontal regions, followed by monotonic and inverted U relationships with intermediate frequency-range activity (8–32 Hz) in occipito-parietal regions. Pupil-linked arousal also coincided with widespread changes in the structure of the aperiodic component of cortical population activity, indicative of changes in the excitation-inhibition balance in underlying microcircuits. Our results provide a novel basis for studying the arousal modulation of cognitive computations in cortical circuits.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
[...] Recently, pupil dilation was linked to cholinergic and noradrenergic neuromodulation as well as cortical state dynamics in animal research. This work adds substantially to this growing research field by revealing the temporal and spatial dynamics of pupil-linked changes in cortical state in a large sample of human participants.
The analyses are thorough and well conducted, but some questions remain, especially concerning unbiased ways to account for the temporal lag between neural and pupil changes. Moreover, it should be stressed that the provided evidence is of indirect nature (i.e., resting state pupil dilation as proxy of neuromodulation, with multiple neuromodulatory systems influencing the measure), and the behavioral relevance of the findings cannot be shown in the current …
Author Response
Reviewer #1 (Public Review):
[...] Recently, pupil dilation was linked to cholinergic and noradrenergic neuromodulation as well as cortical state dynamics in animal research. This work adds substantially to this growing research field by revealing the temporal and spatial dynamics of pupil-linked changes in cortical state in a large sample of human participants.
The analyses are thorough and well conducted, but some questions remain, especially concerning unbiased ways to account for the temporal lag between neural and pupil changes. Moreover, it should be stressed that the provided evidence is of indirect nature (i.e., resting state pupil dilation as proxy of neuromodulation, with multiple neuromodulatory systems influencing the measure), and the behavioral relevance of the findings cannot be shown in the current study.
Thank you for your positive feedback and constructive suggestions. We are especially grateful for the numerous pointers to other work relevant to our study.
- Concerning the temporal lag: The authors' uniformly shift pupil data (but not pupil derivative) in time for their source-space analyses (see above). However, the evidence for the chosen temporal lags (930 ms and 0 ms) is not that firm. For instance, in the cited study by Reimer and colleagues [1] , cholinergic activation shows a temporal lag of ~ 0.5 s with regard to pupil dilation - and the authors would like to relate pupil time series primarily to acetylcholine. Moreover, Joshi and colleagues [2] demonstrated that locus coeruleus spikes precede changes in the first derivative of pupil dilation by about 300 ms (and not 0 ms). Finally, in a recent study recording intracranial EEG activity in humans [3], pupil dilation lagged behind neural events with a delay between ~0.5-1.7s. Together, this questions the chosen temporal lags.
More importantly, Figures 3 and S3 demonstrate variable lags for different frequency bands (also evident for the pupil derivative), which are disregarded in the current source-space analyses. This biases the subsequent analyses. For instance, Figure S3 B shows the strongest correlation effect (Z~5), a negative association between pupil and the alpha-beta band. However, this effect is not evident in the corresponding source analyses (Figure S5), presumably due to the chosen zero-time-lag (the negative association peaked at ~900 ms)).
As the conducted cross-correlations provided direct evidence for the lags for each frequency band, using these for subsequent analyses seems less biased.
This is an important point and we gladly take the opportunity to clarify this in detail. In essence, choosing one particular lag over others was a decision we took to address the multi-dimensional issue of presenting our results (spectral, spatial and time dimensions) and fix one parameter for the spatial description (see e.g. Figure 4). It is worth pointing out first that our analyses were all based on spectral decompositions that necessarily have limited temporal resolutions. Therefore, any given lag represents the center of a band that we can reasonably attribute to a time range. In fact, Figure 3C shows how spread out the effects are. It also shows that the peaks (troughs) of low and high frequency ranges align with our chosen lag quite well, while effects in the mid-frequency range are not “optimally” captured.
As picking lags based on maximum effects may be seen as double dipping, we note that we chose 0.93 sec a priori based on the existing literature, and most prominently based on the canonical impulse response of the pupil to arousing stimuli that is known to peak at that latency on average (Hoeks & Levelt, 1993; Wierda et al. 2012; also see Burlingham et al.; 2021). This lag further agrees with the results of reference [3] cited by the reviewer as it falls within that time range, and with Reimer et al.’s finding (cited as [1] above), as well as Breton-Provencher et al. (2019) who report a lag of ~900 ms sec (see their Supplementary Figure S8) between noradrenergic LC activation and pupil dilation. Finally, note that it was not our aim to relate pupil dilations to either ACh or NE in particular as we cannot make this distinction based on our data alone. Instead, we point out and discuss the similarities of our findings with time lags that have been reported for either neurotransmitter before.
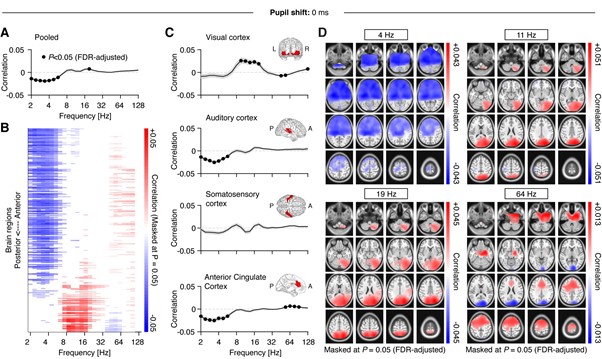
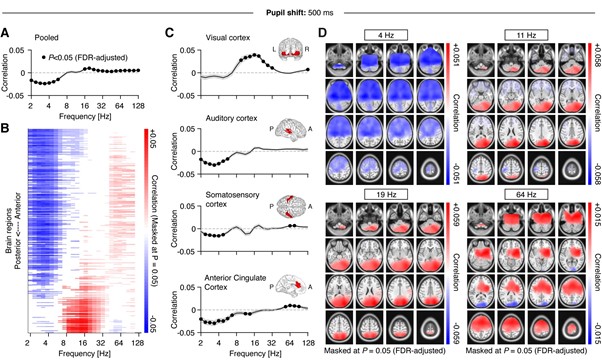
With respect to using different lags, changing the lag to 0 or 500 msec is unlikely to alter the reported effects qualitatively for low- and high frequency ranges (see Figure 3C), as both the pupil time series as well as fluctuations in power are dominated by very slow fluctuations (<< 1 Hz). As a consequence, shifting the signal by 500 msec has very little impact. For comparison, below we provide the reviewer with the results presented in Figure 4 but computed based on zero (Figure R1) and 500-msec (Figure R2) lags. While there are small quantitative differences, qualitatively the results remain mostly identical irrespective of the chosen lag.
Figure R1. Figure equivalent to main Figure 4, but without shifting the pupil.
In sum, choosing one common lag a priori (as we did here) does not necessarily impose more of a bias on the presentation of the results than choosing them post-hoc based on the peaks in the cross-correlograms. However, we have taken this point as a motivation to revise the Results and Methods sections where applicable to strengthen the rationale behind our choice. Most importantly, we changed the first paragraph that mentions and justifies the shift as follows, because original wording may have given the false impression that the cross-correlation results influenced lag choice:
“Based on previous reports (Hoeks & Levelt, 1993; Joshi et al., 2016; Reimer et al., 2016), we shifted the pupil signal 930 ms forward (with respect to the MEG signal). We introduced this shift to compensate for the lag that had previously been observed between external manipulations of arousal (Hoeks & Levelt, 1993) as well as spontaneous noradrenergic activity (Reimer et al., 2016) and changes in pupil diameter. In our data, this shift also aligned with the lags for low- and high-frequency extrema in the cross-correlation analysis (Figure 3B).”
Figure R2. Figure equivalent to main Figure 4, but with shifting the pupil with respect to the MEG by 500 ms.
Related to this aspect: For some parts of the analyses, the pupil time series was shifted with regard to the MEG data (e.g., Figure 4). However, for subsequent analyses pupil and MEG data were analyzed in concurrent 2 s time windows (e.g., Figure 5 and 6), without a preceding shift in time. This complicates comparisons of the results across analyses and the reasoning behind this should be discussed.
The signal has been shifted for all analyses that relate to pupil diameter (but not pupil derivative). We have added versions of the following statement in the respective Results and Methods section to clarify (example from Results section ‘Nonlinear relations between pupil-linked arousal and band-limited cortical activity’):
“In keeping with previous analyses, we shifted the pupil time series forward by 930 msec, while applying no shift to the pupil derivative.”
- The authors refer to simultaneous fMRI-pupil studies in their background section. However, throughout the manuscript, they do not mention recent work linking (task-related) changes in pupil dilation and neural oscillations (e.g., [4-6]) which does seem relevant here, too. This seems especially warranted, as these findings in part appear to disagree with the here-reported observations. For instance, these studies consistently show negative pupil-alpha associations (while the authors mostly show positive associations). Moreover, one of these studies tested for links between pupil dilation and aperiodic EEG activity but did not find a reliable association (again conflicting with the here-reported data). Discussing potential differences between studies could strengthen the manuscript.
We have added a discussion of the suggested works to our Discussion section. We point out however that a recent study (Podvalny et al., https://doi.org/10.7554/eLife.68265) corroborates our finding while measuring resting-state pupil and MEG simultaneously in a situation very similar to ours. Also, we note that Whitmarsh et al. (2021) (reference [6]) is actually in line with our findings as we find a similar negative relationship between alpha-range activity in somatomotor cortices and pupil size.
Please also take into account that results from studies of task- or event-related changes in pupil diameter (phasic responses) cannot be straightforwardly compared with the findings reported here (focusing on fluctuations in tonic pupil size) , due to the inverse relationship between tonic (or baseline) and phasic pupil response (e.g. Knapen et al., 2016). This means that on trials with larger baseline pupil diameter, phasic pupil dilation will be smaller and vice versa. Hence, a negative relation between the evoked change in pupil diameter and alpha-band power can very well be consistent with the positive correlation between tonic pupil diameter and alpha-band activity that we report here for visual cortex.
In section ‘Arousal modulates cortical activity across space, time and frequencies’ we have added:
“Seemingly contradicting the present findings, previous work on task-related EEG and MEG dynamics reported a negative relationship between pupil-linked arousal and alpha-range activity in occipito-parietal sensors during visual processing (Meindertsma et al, 2017) and fear conditioning (Dahl et al. 2020).Note however that results from task-related experiments, that focus on evoked changes in pupil diameter rather than fluctuations in tonic pupil size, cannot be directly compared with our findings. Similar to noradrenergic neurons in locus coeruleus (Aston-Jones & Cohen, 2005), phasic pupil responses exhibit an inverse relationship with tonic pupil size (Knapen et al., 2016). This means that on trials with larger baseline pupil diameter (e.g. during a pre-stimulus period), the evoked (phasic) pupil response will be smaller and vice versa. As a consequence, a negative correlation between alpha-band activity in the visual cortex and task-related phasic pupil responses does not preclude a positive correlation with tonic pupil size during baseline or rest as reported here. In line with this, Whitmarsh et al., 2021 found a negative relationship between alpha-activity and pupil size in the somatosensory cortex that agrees with our finding. Although using an event-related design to study attention to tactile stimuli, this relationship occurred in the baseline, i.e. before observing any task-related phasic effects on pupil-linked arousal or cortical activity.”
In section ‘Arousal modulation of cortical excitation-inhibition ratio’ we have added: “The absence of this effect in visual cortices may explain why Kosciessa et al. (2021) found no relationship between pupil-linked arousal and spectral slope when investigating phasic pupil dilation in response to a stimulus during visual task performance. However, this behavioral context, associated with different arousal levels, likely also changes E/I in the visual cortex when compared with the resting state (Pfeffer et al., 2018).”
Finally, in the Conclusion we added (note: ‘they’ = the present results): “Further, they largely agree with similar findings of a recent independent report (Podvalny et al., 2021).”
Related to this aspect: The authors frequently relate their findings to recent work in rodents. For this it would be good to consider species differences when comparing frequency bands across rodents and primates (cf. [7,8]).
Throughout our Results section we have mainly remained agnostic with respect to labeling frequency ranges when drawing between-species comparisons, and have only reverted to it as a justification for a dimension reduction for some of the presented analysis. Following your comment however, we have phrased the following section in the Discussion, section ‘Arousal modulates cortical activity across space, time and frequencies’, more carefully:
“The low-frequency regime referred to in rodent work (2—10Hz; e.g., McGinley et al., 2015) includes activity that shares characteristics with human alpha rhythms (3—6Hz; Nestogel and McCormick, 2021; Senzai et al. 2019). The human equivalent however clearly separates from activity in lower frequency bands and,here, showed idiosyncratic relationships with pupil-linked arousal.”
- Figure 1 highlights direct neuromodulatory effects in the cortex. However, seminal [9-11] and more recent work [12,13] demonstrates that noradrenaline and acetylcholine also act in the thalamus which seems relevant concerning the interpretation of low frequency effects observed here. Moreover, neural oscillations also influence neuromodulatory activity, thus the one-headed arrows do not seem warranted (panel C) [3,14].
This is a very good point. First, we would like to note that we have extended on acknowledging thalamic contributions to low-frequency (specifically alpha) effects in response to the Reviewer’s point 11 (‘Recommendations for authors’ section below). Also, we have added a reference to the role of potential top-down (reverse) influences to our Discussion, section ‘Arousal modulates cortical activity across space, time and frequencies’, as follows:
“Further, we note that our analyses and interpretations focus on arousal-related neuromodulatory influences on cortical activity, whereas recent work also supports a reverse “top-down” route, at least for frontal cortex high-frequency activity on LC spiking activity (Totah et al., 2021).”
Ultimately, however, we decided to leave the arrows in Figure 1C uni-directional to keep in line with the rationale of our research that stems mostly from rodent work, which also emphasises the indicated directionality. Also, reference [3] is highly interesting for us because it actually aligns with our data: The authors show that a spontaneous peak of high-frequency band activity (>70 Hz) in insular cortex precedes a pupil dilation peak (or plateau) in two of three participants by ~500msec (which mimics a pattern found for task-evoked activity; see their Figure 5b/c). We find a maximum in our cross-correlation between pupil size and high frequency band activity (>64 Hz) that indicates a similar lag (see our Figure 3B). Importantly, both results do not rule out a common source of neuromodulation for the effects. We have added the following to the end of the section ‘An arousal-triggered cascade of activity in the resting human brain’:
“In fact, Kucyi & Parvizi (2020) found spontaneous peaks of high-frequency band activity (>70 Hz) in the insular cortex of three resting surgically implanted patients that preceded pupil dilation by ~500msec - a time range that is consistent with the lag of our cross-correlation between pupil size and high frequency (>64Hz) activity (see Figure 3B). Importantly, they showed that this sequence mimicked a similar but more pronounced pattern during task performance. Given the purported role of the insula (Menon & Uddin, 2015), this finding lends support to the idea that spontaneous covariations of pupil size and cortical activity signal arousal events related to intermittent 'monitoring sweeps' for behaviourally relevant information.”
- In their discussion, the authors propose a pupil-linked temporal cascade of cognitive processes and accompanying power changes. This argument could be strengthened by showing that earlier events in the cascade can predict subsequent ones (e.g., are the earlier low and high frequency effects predictive of the subsequent alpha-beta synchronization?)-
We added this cascade angle as one possible interpretation of the observed effects. We fully agree that this is an interesting question but would argue that this would ideally be tested in follow-up research specifically designed for that purpose. The suggested analysis would add a post-hoc aspect to our exploratory investigation in the absence of a suitable contrast, while also potentially side-tracking the main aim of the study. We have revised the language in this section and added the following changes (bold) to the last paragraph to emphasise the speculatory aspect, and clarify what we think needs to be done to look into this further and with more explanatory power.
“The three scenarios described here are not mutually exclusive and may explain one and the same phenomenon from different perspectives. Further, it remains possible that the sequence we observe comprises independent effects with specific timings. A pivotal manipulation to test these assumptions will be to contrast the observed sequence with other potential coupling patterns between pupil-linked arousal and cortical activity during different behavioural states.”
-

Evaluation Summary:
The study presents novel results on spontaneous fluctuations in pupil dilation in relation to the spectral dynamics in a large sample of human participants. The study is based on MEG recordings allowing for quantifying these relations in time and space. The data provide important new insight into the temporal and spatial characteristics of pupil-linked changes in cortical states which form the basis for incorporating this insight in future clinical and cognitive neuroscience studies.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1, Reviewer #2 and Reviewer #3 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
Pfeffer, Keitel et al. collected pupil dilation as a non-invasive proxy of cholinergic and noradrenergic neuromodulation. In a large sample of healthy human participants, they related spontaneous fluctuations in pupil-indexed neuromodulation to concurrently recorded changes in magnetoencephalographic activity.
First, they show that pupil size co-varies with power fluctuations, especially in the alpha-beta band at posterior sensors. Next, in subsequent cross-correlation analyses, they show frequency-specific associations of pupil dilation and band-limited power. Decreases in low (2-4 Hz) as well increases in high (64-128 Hz) frequencies preceded pupil dilations by > 500 ms. For intermediate frequencies (8-16 Hz), both positive and negative associations were found with a closer temporal proximity to peak pupil …
Reviewer #1 (Public Review):
Pfeffer, Keitel et al. collected pupil dilation as a non-invasive proxy of cholinergic and noradrenergic neuromodulation. In a large sample of healthy human participants, they related spontaneous fluctuations in pupil-indexed neuromodulation to concurrently recorded changes in magnetoencephalographic activity.
First, they show that pupil size co-varies with power fluctuations, especially in the alpha-beta band at posterior sensors. Next, in subsequent cross-correlation analyses, they show frequency-specific associations of pupil dilation and band-limited power. Decreases in low (2-4 Hz) as well increases in high (64-128 Hz) frequencies preceded pupil dilations by > 500 ms. For intermediate frequencies (8-16 Hz), both positive and negative associations were found with a closer temporal proximity to peak pupil dilation. Analyses were repeated for the first derivative of pupil dilation, which may be more closely linked to noradrenergic (relative to cholinergic) neuromodulation.
The authors additionally performed pupil-MEG correlations in source-space to reveal the spatial profiles of pupil-linked power fluctuations (findings largely consistent with the sensor-level). For this, they shifted the pupil data in time with respect to the MEG data to account for the sluggishness of pupil response. The temporal lag was determined based on previous research (see below). In a second set of source-space analyses, they additionally tested for quadratic associations of pupil dilation and band-limited cortical activity, which were observed for the alpha-beta band mainly at posterior sites.
Finally, the authors linked pupil dilation to the aperiodic component of the power spectrum. They found that larger pupil dilations were associated with a shallower slope, suggesting a higher excitation to inhibition ratio. Notably, pupil associations with band-limited power remained reliable after removing the aperiodic component (not so for the first derivative).
Recently, pupil dilation was linked to cholinergic and noradrenergic neuromodulation as well as cortical state dynamics in animal research. This work adds substantially to this growing research field by revealing the temporal and spatial dynamics of pupil-linked changes in cortical state in a large sample of human participants.The analyses are thorough and well conducted, but some questions remain, especially concerning unbiased ways to account for the temporal lag between neural and pupil changes. Moreover, it should be stressed that the provided evidence is of indirect nature (i.e., resting state pupil dilation as proxy of neuromodulation, with multiple neuromodulatory systems influencing the measure), and the behavioral relevance of the findings cannot be shown in the current study.
1. Concerning the temporal lag: The authors' uniformly shift pupil data (but not pupil derivative) in time for their source-space analyses (see above). However, the evidence for the chosen temporal lags (930 ms and 0 ms) is not that firm. For instance, in the cited study by Reimer and colleagues [1] , cholinergic activation shows a temporal lag of ~ 0.5 s with regard to pupil dilation - and the authors would like to relate pupil time series primarily to acetylcholine. Moreover, Joshi and colleagues [2] demonstrated that locus coeruleus spikes precede changes in the first derivative of pupil dilation by about 300 ms (and not 0 ms). Finally, in a recent study recording intracranial EEG activity in humans [3], pupil dilation lagged behind neural events with a delay between ~0.5-1.7s. Together, this questions the chosen temporal lags.
More importantly, Figures 3 and S3 demonstrate variable lags for different frequency bands (also evident for the pupil derivative), which are disregarded in the current source-space analyses. This biases the subsequent analyses. For instance, Figure S3 B shows the strongest correlation effect (Z~5), a negative association between pupil and the alpha-beta band. However, this effect is not evident in the corresponding source analyses (Figure S5), presumably due to the chosen zero-time-lag (the negative association peaked at ~900 ms)).
As the conducted cross-correlations provided direct evidence for the lags for each frequency band, using these for subsequent analyses seems less biased.
Related to this aspect: For some parts of the analyses, the pupil time series was shifted with regard to the MEG data (e.g., Figure 4). However, for subsequent analyses pupil and MEG data were analyzed in concurrent 2 s time windows (e.g., Figure 5 and 6), without a preceding shift in time. This complicates comparisons of the results across analyses and the reasoning behind this should be discussed.
2. The authors refer to simultaneous fMRI-pupil studies in their background section. However, throughout the manuscript, they do not mention recent work linking (task-related) changes in pupil dilation and neural oscillations (e.g., [4-6]) which does seem relevant here, too. This seems especially warranted, as these findings in part appear to disagree with the here-reported observations. For instance, these studies consistently show negative pupil-alpha associations (while the authors mostly show positive associations). Moreover, one of these studies tested for links between pupil dilation and aperiodic EEG activity but did not find a reliable association (again conflicting with the here-reported data). Discussing potential differences between studies could strengthen the manuscript.
Related to this aspect: The authors frequently relate their findings to recent work in rodents. For this it would be good to consider species differences when comparing frequency bands across rodents and primates (cf. [7,8]).
3. Figure 1 highlights direct neuromodulatory effects in the cortex. However, seminal [9-11] and more recent work [12,13] demonstrates that noradrenaline and acetylcholine also act in the thalamus which seems relevant concerning the interpretation of low frequency effects observed here. Moreover, neural oscillations also influence neuromodulatory activity, thus the one-headed arrows do not seem warranted (panel C) [3,14].
4. In their discussion, the authors propose a pupil-linked temporal cascade of cognitive processes and accompanying power changes. This argument could be strengthened by showing that earlier events in the cascade can predict subsequent ones (e.g., are the earlier low and high frequency effects predictive of the subsequent alpha-beta synchronization?)-
Cited references
1 Reimer, J. et al. (2016) Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 7, 13289
2 Joshi, S. et al. (2016) Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89, 221-234
3 Kucyi, A. and Parvizi, J. (2020) Pupillary dynamics link spontaneous and task-evoked activations recorded directly from human insula. J. Neurosci. 40, 6207-6218
4 Dahl, M.J. et al. (2020) Noradrenergic responsiveness supports selective attention across the adult lifespan. J. Neurosci. 40, 4372-4390
5 Kosciessa, J.Q. et al. (2021) Thalamocortical excitability modulation guides human perception under uncertainty. Nat. Commun. 12, 1-15
6 Whitmarsh, S. et al. (2021) Neuronal correlates of the subjective experience of attention. Eur. J. Neurosci. DOI: 10.1111/ejn.15395
7 Nestvogel, D.B. and Mccormick, D.A. (2021) Visual Thalamocortical Mechanisms of Waking State Dependent Activity and Alpha Oscillations. bioRxiv DOI: 10.1101/2021.04.14.439865
8 Senzai, Y. et al. (2019) Layer-Specific Physiological Features and Interlaminar Interactions in the Primary Visual Cortex of the Mouse. Neuron 101, 500-513.e5
9 Buzsáki, G. et al. (1991) Noradrenergic control of thalamic oscillation: The role of α‐2 receptors. Eur. J. Neurosci. 3, 222-229
10 Buzsáki, G. et al. (1988) Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J. Neurosci. 8, 4007-26
11 McCormick, D.A. (1989) Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 12, 215-221
12 Goard, M. and Dan, Y. (2009) Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci. 12, 1444-1449
13 Rodenkirch, C. et al. (2019) Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat. Neurosci. 22, 120-133
14 Totah, N.K. et al. (2021) Synchronous spiking associated with prefrontal high gamma oscillations evokes a 5 Hz-rhythmic modulation of spiking in locus coeruleus. J. Neurophysiol. DOI: 10.1152/jn.00677.2020 -

Reviewer #2 (Public Review):
This work examines the impact of biological arousal (pupil diameter) throughout the cortex with high spatio-temporal resolution. The analysis of neuromagnetic signals, extending beyond the state-of-the art, supports the overall conclusion that power fluctuations of neuronal oscillations are closely linked to arousal. Independent from stimulus input, it is the peak timing of pupil dilation that coincides and initiates power modulation of low and high frequency activity throughout the cortex.
The central aim of the work, the non-invasive characterization of the link between fluctuations of arousal and cortex wide neuronal population activity, is achieved by analyzing a large dateset across 3 different laboratories, combining eye-tracking and magnetoencephalographic data.Both, the analytic approach and the …
Reviewer #2 (Public Review):
This work examines the impact of biological arousal (pupil diameter) throughout the cortex with high spatio-temporal resolution. The analysis of neuromagnetic signals, extending beyond the state-of-the art, supports the overall conclusion that power fluctuations of neuronal oscillations are closely linked to arousal. Independent from stimulus input, it is the peak timing of pupil dilation that coincides and initiates power modulation of low and high frequency activity throughout the cortex.
The central aim of the work, the non-invasive characterization of the link between fluctuations of arousal and cortex wide neuronal population activity, is achieved by analyzing a large dateset across 3 different laboratories, combining eye-tracking and magnetoencephalographic data.Both, the analytic approach and the conclusions, are substantial new advance and of broad interest bridging across basic and clinical neuroscience. The access to deep brain structures via combination of eye-tracking and biomagnetism sets a new benchmark and a challenge to the often cortico-centric view of contemporary neuroscience.
-

Reviewer #3 (Public Review):
This is a well-written and clearly presented paper exploring an important effect that is somewhat overlooked by the literature. Links between neuronal signals and peripheral body parts such as the pupils a growing and critical area of modern cognitive neuroscience. This manuscript represents an exploratory investigation which will be of interest to researchers interested in brain-body interactions, attention, arousal and neuronal oscillations.
The analyses are of a generally of a high standard and the conclusions are justified by the results. The authors acknowledge and handle points where conclusions are unclear very well. Particular strengths are in sample size, the consideration of a wide range of possible interactions, strong links to literature and balanced discussion. I have three specific concerns in …
Reviewer #3 (Public Review):
This is a well-written and clearly presented paper exploring an important effect that is somewhat overlooked by the literature. Links between neuronal signals and peripheral body parts such as the pupils a growing and critical area of modern cognitive neuroscience. This manuscript represents an exploratory investigation which will be of interest to researchers interested in brain-body interactions, attention, arousal and neuronal oscillations.
The analyses are of a generally of a high standard and the conclusions are justified by the results. The authors acknowledge and handle points where conclusions are unclear very well. Particular strengths are in sample size, the consideration of a wide range of possible interactions, strong links to literature and balanced discussion. I have three specific concerns in which the strength of analyses could be improved.
1 - Two approaches for relating pupil dynamics to time-frequency MEG data appear to be used (a Morlet wavelet and a sliding window Welch's periodogram), it is not clear why multiple methods are used and why these could not be matched?
Further, I have some concerns with the choice of a Morlet wavelet analysis in this application. Each individual frequency band will have a different temporal resolution and all (or at least most) will have a very different time resolution to the relatively slow pupil data. In other words the autocorrelation between successive time-points will dramatically vary between frequencies and are all likely to be fast compared to any pupil dynamics. This may introduce between frequency differences in noise or sensitivity which are tricky to account for. Moreover, conclusions are draw about the relative timings of different frequency bands though each of these bands have different temporal resolutions in the wavelet transform.
2 The spatial maps in figures 4, 5 and 6 are well described but it is not clear whether these represent distinct effects or simply follow the topography of power in those frequency bands. Specifically, a quadratic effect in 10Hz power might be more detectable in a brain region exhibiting high 10Hz power - to what extent are the interesting correlation maps distinct from the simpler power distributions? If these are strongly linearly associated, then the conclusions about of spatial specificity in the pupil-brain interactions are challenging.
3 The present results use a different 3-40Hz range for assessing spectral slope, citing that Gao et al 2017 to link this parameter to E:I balance. However Gao et al specifically conclude that the 30-70Hz range reflects E:I balance and make no claims about other frequencies. The reason for this difference is unclear and undermines the otherwise compelling discussion of E:I balance and arousal.
-