Enriched dietary saturated fatty acids induce trained immunity via ceramide production that enhances severity of endotoxemia and clearance of infection
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
In this manuscript entitled "Dietary palmitic acid induces innate immune memory via ceramide production that enhances severity of acute septic shock and clearance of infection" Seufert and colleagues have investigated how saturated fatty acids increase susceptibility of the host in a murine model of LPS-mediated septic shock. Pretreatment of macrophages with palmitic acid (PA) was found to reprogram macrophages towards hyper-inflammatory phenotype, which was dependent on ceremide. Importantly, depletion of macrophages intracellular ceremide with oleic acid reversed their hyper-inflammatory phenotype. Interestingly, while PA was harmful in the LPS-acute septic shock model, it was beneficial in clearance of C. albicans in Rag-deficient mice lacking both B and T cells. While this is an exciting study, the presented data don't fully support the central hypothesis and the link with trained immunity is currently weak.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Trained immunity is an innate immune memory response that is induced by a primary inflammatory stimulus that sensitizes monocytes and macrophages to a secondary pathogenic challenge, reprogramming the host response to infection and inflammatory disease. Dietary fatty acids can act as inflammatory stimuli, but it is unknown if they can act as the primary stimuli to induce trained immunity. Here we find mice fed a diet enriched exclusively in saturated fatty acids (ketogenic diet; KD) confer a hyper-inflammatory response to systemic lipopolysaccharide (LPS) and increased mortality, independent of diet-induced microbiome and hyperglycemia. We find KD alters the composition of the hematopoietic stem cell compartment and enhances the response of bone marrow macrophages, monocytes, and splenocytes to secondary LPS challenge. Lipidomics identified enhanced free palmitic acid (PA) and PA-associated lipids in KD-fed mice serum. We found pre-treatment with physiologically relevant concentrations of PA induces a hyper-inflammatory response to LPS in macrophages, and this was dependent on the synthesis of ceramide. In vivo, we found systemic PA confers enhanced inflammation and mortality in response to systemic LPS, and this phenotype was not reversible for up to 7 days post-PA-exposure. Conversely, we find PA exposure enhanced clearance of Candida albicans in Rag1 -/- mice. Lastly, we show that oleic acid, which depletes intracellular ceramide, reverses PA-induced hyper-inflammation in macrophages and enhanced mortality in response to LPS . These implicate enriched dietary SFAs, and specifically PA, in the induction of long-lived innate immune memory and highlight the plasticity of this innate immune reprogramming by dietary constituents.
Article activity feed
-

-

Author Response
Reviewer #1 (Public Review):
The study presented by AL Seufert et al. follows the trajectory of trained immunity research in the context of sterile inflammatory diseases such as gout, cardiovascular disease and obesity. Previous studies in mice have shown that a 4 week Western-type diet is sufficient to induce systemic trained immunity, with gross reorganization of the bone marrow to support a potentiated inflammatory response [PMID: 29328911]. The current study demonstrates that mice on a Western-type diet (WD) and the more extreme Ketogenic diet (KD; where carbohydrates are essentially eliminated from the diet) for 2 weeks results in a state of increased monocyte-driven immune responsiveness when compared to standard chow diets (SC). This increased immune responsiveness after high-fat diet resulted in a deadly …
Author Response
Reviewer #1 (Public Review):
The study presented by AL Seufert et al. follows the trajectory of trained immunity research in the context of sterile inflammatory diseases such as gout, cardiovascular disease and obesity. Previous studies in mice have shown that a 4 week Western-type diet is sufficient to induce systemic trained immunity, with gross reorganization of the bone marrow to support a potentiated inflammatory response [PMID: 29328911]. The current study demonstrates that mice on a Western-type diet (WD) and the more extreme Ketogenic diet (KD; where carbohydrates are essentially eliminated from the diet) for 2 weeks results in a state of increased monocyte-driven immune responsiveness when compared to standard chow diets (SC). This increased immune responsiveness after high-fat diet resulted in a deadly hyper-inflammatory in the mice in response to endotoxin (LPS) challenge in vivo.
These initial findings as displayed in Figure 1 are made difficult to interpret because the authors use a mix of male and female mice coupled with very small sample sizes (n = 5 - 9). Male and female mice are shown to have dimorphic responses to LPS exposure in vivo, with males having elevated cytokine levels (TNF, IL-6, IL1β, and also interesting IL-10) increased rates severe outcomes to LPS challenge [PMID: 27631979]. As a reader it is impossible to discern from their methodological description what the proportion of the sexes were in each group, and therefore cannot determine if their data are skewed or biased due to sexual dimorphic responses to LPS rather than diet. Additionally due to the very small sample sizes, the authors can't perform a stratified analysis based on sex to determine whether the diets are having the greatest effects in accordance with LPS induce inflammation.
The Reviewer brings up an important point, all studies with endotoxemia in wild-type conventional mice were carried out in 6–8-week female BALB/c mice, as mentioned in the Methods section under “Ethical approval of animal studies” and “endotoxin-induced model of sepsis” sections. This is extremely important to mention more clearly in the results text, because the Reviewer 1 is correct, sexual dimorphism and age differences can have very large effects on LPS treatment outcome. This was not stated clearly enough in the results and now the age, sex, and background of mice have been explicitly stated in each Results and Figure Legend section for each experiment.
When comparing SC to the KD, the authors identify large changes in fatty acid distribution circulating in the blood. The majority of the fatty acids were shown to relate to saturated fatty acids (SFA). Although Lauric, Myristic, and Myristovaccenic acid where the most altered after KD, the authors focus their research on the more thoroughly studied palmitic acid (PA).
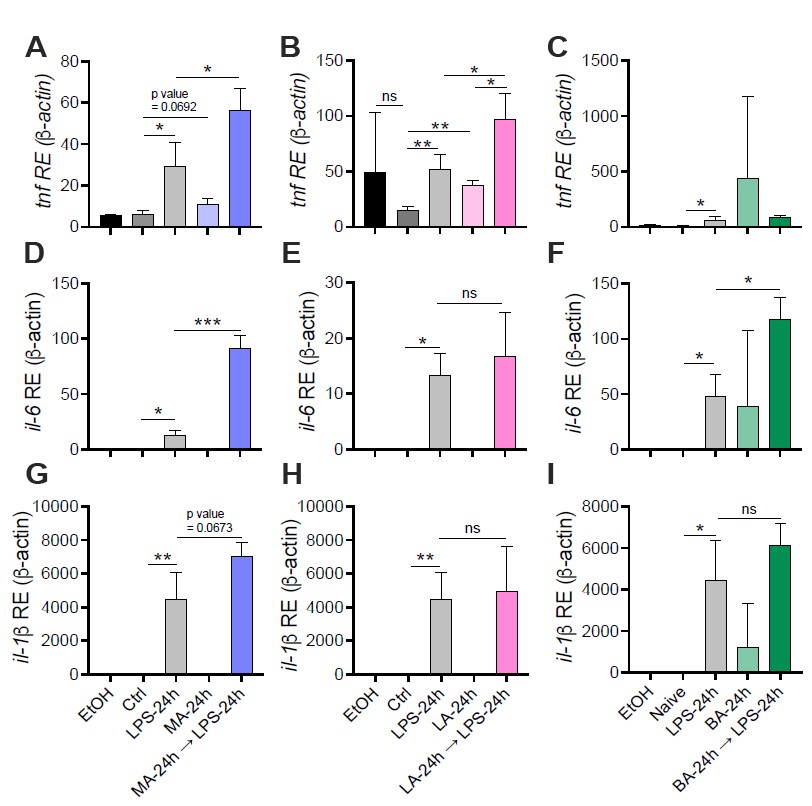
We followed up on multiple saturated fatty acids (SFAs; Myristic, Lauric, and Behenic acid) that were identified in the lipidomic data, and found no robust or repeatable phenotypes in vitro using physiologically relevant concentrations. The inability to reproduce some of the findings with these SFAs may be due to the instability of some of these fats in solution, and plan to troubleshoot these assays in order to understand the complexity of SFA-dependent control of inflammation in macrophages. Please see Fig. R1 in this document for data showing LPS-stimulated BMDMs pre-treated with Myristic (Fig R1 A-C), Lauric (Fig R1 D-F), or Behenic (Fig R1 G-I) fatty acids. The physiological concentrations used in these studies were referenced from Perreault et. al., 2014.
Figure R1. The effect of Myristic Acid, Lauric Acid, and Behenic Acid on the response to LPS in macrophages. Primary bone marrowderived macrophages (BMDMs) were isolated from aged-matched (6-8 wk) C57BL/6 female and male mice. BMDMs were plated at 1x106 cells/mL and treated with either ethanol (EtOH; media with 0.05% or 0.35% ethanol to match MA and LA solutions respectively), media (Ctrl), LPS (10 ng/mL) for 24 h, or myristic or lauric acid (MA, LA stock diluted in 0.05%, or 0.35% EtOH; conjugated to 2% BSA) for 24 h, with and without a secondary challenge with LPS (10 ng/mL). After indicated time points, RNA was isolated and expression of (A, B) tnf, (D, E) il- 6, and (G, H) il-1β was measured via qRT-PCR. RAW 264.7 macrophages were thawed and cultured for 3-5 days, pelleted and resuspended in DMEM containing 5% FBS and 2% BSA, and treated identical to BMDM treatments with behenic acid (BA stock diluted in 1.7% EtOH) used as the primary stimulus. (C) tnf, (F) il-6, and (I) il-1β was measured via qRT-PCR. For all plates, all treatments were performed in triplicate. For all panels, a student’s t-test was used for statistical significance. *p< 0.05; **p < 0.01; ***p< 0.001. Error bars shown mean ± SD.
PA was shown to increase the expression of inflammatory cytokines gene expression and protein production of TNF, IL-6 and IL-1β in bone marrow derived macrophages (BMDMs). The authors tie these effects to ceramide synthesis through a pharmacological blockade as well as the use of oleic acid, which allegedly sequesters ceramide synthesis. The author's claim that oleic acid supplementation reverses the inflammatory signaling induced by PA is invalid, as oleic acid was shown to induce a high level of cytokines in their model. When PA was added along with oleic acid, the cytokine levels returned to the levels produced by BMDM's stimulated with PA alone (see Figure 4 panels D- F).
This was an unfortunate oversight in our revisions of this manuscript, original Figure 5A-C was mislabeled (though colored the correct colors) – OA-12h → LPS-24h should have been switched with PA-12h → LPS-24h. These data were labeled correctly in the source file: Source_data_Fig5 and have since been updated in Figure 5 of the manuscript with correct labels. The corrected graphs have been split up in the resubmission in light of new data collected. Please see Fig 3K-M and Fig 5A-C.
Finally the authors test whether injection of PA into mice can recapitulate the systemic inflammatory response seen by WD and KD feeding followed by LPS exposure. They were able to demonstrate that injecting 1 mM of PA, waiting for 12h, and then exposing the mice to LPS for 24h could similarly result in a hyper-inflammatory state resulting in greater mortality. The reviewer is skeptical that 1 mM of PA truly represents post-prandial PA levels as one would expect to see after a single fatty meal, and whether this injection is generally well tolerated by mice. Looking into the paper cited by Eguchi et al. to inform their methods, it's shown that the earlier study continuously infused an emulsified ethyl palmitate solution (which contained 600 mM) at a rate of 0.2 uL/min. As far as I can read by Eguchi, they only managed to reach a serum PA concentration of 0.5 mM. This is hardly the same thing as a single i.p. injection of 1 mM PA. and reflects a single bolus injection of double the serum concentration of PA achieved by Eguchi et al.
The reviewer brings up an important point, Eguchi et al. did use infusions. From their data (Fig 1A), we calculated that after 600mM of i.v. injection (total = 267uL within 14h; 0.2L/min) there was ~420uM absolute PA within the blood. They were using C57BL/6 mice that were 23g on average. Using these results, we extrapolated that one single 200uL injection of a 750mM PA solution within 6–8-week female BALB/c mice (~15-18g) would equate to ~500-1mM of PA within the blood. Considering obese healthy and unhealthy humans vary widely in total PA concentrations in the blood (0.3-4.1 mM) (1, 2), we moved forward with these calculations. Considering this, we thank the reviewer for this advice, and we agree that we have not definitively shown we are increasing systemic levels of PA. Thus, we ran a lipidomic analysis of serum from SC-fed mice with Veh or PA for 12 h. We show that a 750 mM i.p. injection of ethyl palmitate enhances free PA levels in the serum to 173-425 μM at 2 h post-injection, which is within the reported range for humans on high-fat diets (0.34.1mM). We have added this new data to Fig. S7A of the main manuscript.
Importantly, the concentration in the PA-treated mice is greater than that of the Veh-treated mice, however we believe the value shown is an underestimate of maximum serum PA levels enhanced by i.p. injection, because free PA is known to be packaged into chylomicrons within enterocytes and travel through the circulation with a half-life of less than an hour (3, 4). Thus, serum concentrations of free PA are only transiently enhanced by i.p. injection, and is quickly taken up by adipose tissue, skeletal muscle, heart, and liver tissue. These complex lipid transport processes make it difficult to determine maximum concentrations of free PA in the serum.
While all of the details concerning PA circulation following an i.p. injection are unknown, we suggest that this method of “force-feeding” is similar to dietary intake in that uptake of PA into the circulation occurs within the peritoneal space prior to traveling to the blood via the thoracic duct and right lymphatic duct (5).
PA is known to induce inflammation in monocytes and macrophages, therefore the findings certainly make sense in the context of previously published literature. However the authors have made some poor methodological decisions in their mouse studies, namely haphazardly switching between groups of young and old mice (4-6 weeks, 8-9 weeks, and 14-23 weeks), using different LPS injection protocols (6, 10, and 50 mg/ml of LPS), and including multiple sexes of mice. All of which are drastically alter the interpretation of the data, and preventing solid conclusions from being drawn.
We appreciate this review and suggest that:
- For the LPS models, mice were all female and aged matched between 6-8 weeks. We are aware of sex differences in the endotoxemia model, which is why we specifically use female mice in our studies (6, 7). This is mentioned twice in the methods under the sections “Endotoxin-induced model of sepsis” and “Ethical approval of animal studies”. We have added these specifics of our model to all Results and Figure Legend sections for clarification.
- For Germ-free models, it is notoriously difficult to breed C57BL/6 germ-free mice. It was inherently difficult to obtain enough mice within the same sex and age to carry out these experiments, however since we have published in this model before with mixed sex and age we were aware that our WD phenotype is robust enough in these backgrounds (7). Further, we believe that seeing our robust phenotype independent of age or sex within germ-free mice provides more evidence of the strength of this phenotype. It is important to note that we induce endotoxemia within Germ-free mice with 50mg/kg, instead of 6mg/kg which is used in conventional mice, because this is our reported LD50 for mixed sex Germ-free C57BL/6, as we have published previously in detail (7). This difference is due to the presence of the microbiota (8, 9) and also germ-free mice have an immature immune system that correlates with a hyporesponsiveness to microbial products (10-12). We agree with the reviewer that the ages of the C57BL/6 germ-free mice are significantly older than our conventional 6-8 week mice, thus we confirmed that WD- and KD-fed conventional C57BL/6 female mice aged 20 – 21 weeks old still show enhanced disease severity and mortality in an LPS-induced endotoxemia model, compared to mice fed SC (Fig. S1G-H).
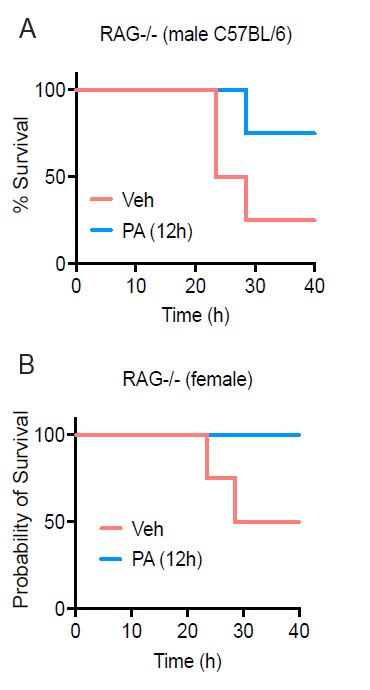
Figure R2. PA treatment enhances survival in both female and male RAG-/- mice. Age-matched (8-9 wk) RAG-/- mice were injected i.v. with ethyl palmitate (PA, 750mM) or vehicle (Veh) solutions 12 h before C. albicans infection. Survival was monitored for 40h post-infection.
- In our preliminary results, we stratified survival during C. albicans infection between male and female C57BL/6 and found no notable difference in survival at 40h post IP infection with Candida albicans (Fig R2 A-B). However, the data presented in the manuscript on CFU is female kidney burden and we do not have data on fungal burden within male mice. This is an important piece of data that we would like to collect for understanding sex differences in the PA-dependent enhanced resistance to systemic C. albicans. We are currently addressing this question within the lab as well as elucidating the cell type and mechanism of PA-dependent enhanced fungal resistance.
-

Evaluation Summary:
In this manuscript entitled "Dietary palmitic acid induces innate immune memory via ceramide production that enhances severity of acute septic shock and clearance of infection" Seufert and colleagues have investigated how saturated fatty acids increase susceptibility of the host in a murine model of LPS-mediated septic shock. Pretreatment of macrophages with palmitic acid (PA) was found to reprogram macrophages towards hyper-inflammatory phenotype, which was dependent on ceremide. Importantly, depletion of macrophages intracellular ceremide with oleic acid reversed their hyper-inflammatory phenotype. Interestingly, while PA was harmful in the LPS-acute septic shock model, it was beneficial in clearance of C. albicans in Rag-deficient mice lacking both B and T cells. While this is an exciting study, the presented data …
Evaluation Summary:
In this manuscript entitled "Dietary palmitic acid induces innate immune memory via ceramide production that enhances severity of acute septic shock and clearance of infection" Seufert and colleagues have investigated how saturated fatty acids increase susceptibility of the host in a murine model of LPS-mediated septic shock. Pretreatment of macrophages with palmitic acid (PA) was found to reprogram macrophages towards hyper-inflammatory phenotype, which was dependent on ceremide. Importantly, depletion of macrophages intracellular ceremide with oleic acid reversed their hyper-inflammatory phenotype. Interestingly, while PA was harmful in the LPS-acute septic shock model, it was beneficial in clearance of C. albicans in Rag-deficient mice lacking both B and T cells. While this is an exciting study, the presented data don't fully support the central hypothesis and the link with trained immunity is currently weak.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
The study presented by AL Seufert et al. follows the trajectory of trained immunity research in the context of sterile inflammatory diseases such as gout, cardiovascular disease and obesity. Previous studies in mice have shown that a 4 week Western-type diet is sufficient to induce systemic trained immunity, with gross reorganization of the bone marrow to support a potentiated inflammatory response [PMID: 29328911]. The current study demonstrates that mice on a Western-type diet (WD) and the more extreme Ketogenic diet (KD; where carbohydrates are essentially eliminated from the diet) for 2 weeks results in a state of increased monocyte-driven immune responsiveness when compared to standard chow diets (SC). This increased immune responsiveness after high-fat diet resulted in a deadly hyper-inflammatory in …
Reviewer #1 (Public Review):
The study presented by AL Seufert et al. follows the trajectory of trained immunity research in the context of sterile inflammatory diseases such as gout, cardiovascular disease and obesity. Previous studies in mice have shown that a 4 week Western-type diet is sufficient to induce systemic trained immunity, with gross reorganization of the bone marrow to support a potentiated inflammatory response [PMID: 29328911]. The current study demonstrates that mice on a Western-type diet (WD) and the more extreme Ketogenic diet (KD; where carbohydrates are essentially eliminated from the diet) for 2 weeks results in a state of increased monocyte-driven immune responsiveness when compared to standard chow diets (SC). This increased immune responsiveness after high-fat diet resulted in a deadly hyper-inflammatory in the mice in response to endotoxin (LPS) challenge in vivo. These initial findings as displayed in Figure 1 are made difficult to interpret because the authors use a mix of male and female mice coupled with very small sample sizes ( n = 5 - 9). Male and female mice are shown to have dimorphic responses to LPS exposure in vivo, with males having elevated cytokine levels (TNF, IL-6, IL-1β, and also interesting IL-10) increased rates severe outcomes to LPS challenge [PMID: 27631979]. As a reader it is impossible to discern from their methodological description what the proportion of the sexes were in each group, and therefore cannot determine if their data are skewed or biased due to sexual dimorphic responses to LPS rather than diet. Additionally due to the very small sample sizes, the authors can't perform a stratified analysis based on sex to determine whether the diets are having the greatest effects in accordance with LPS induce inflammation.
When comparing SC to the KD, the authors identify large changes in fatty acid distribution circulating in the blood. The majority of the fatty acids were shown to relate to saturated fatty acids (SFA). Although Lauric, Myristic, and Myristovaccenic acid where the most altered after KD, the authors focus their research on the more thoroughly studied palmitic acid (PA). PA was shown to increase the expression of inflammatory cytokines gene expression and protein production of TNF, IL-6 and IL-1β in bone marrow derived macrophages (BMDMs). The authors tie these effects to ceramide synthesis through a pharmacological blockade as well as the use of oleic acid, which allegedly sequesters ceramide synthesis. The author's claim that oleic acid supplementation reverses the inflammatory signaling induced by PA is invalid, as oleic acid was shown to induce a high level of cytokines in their model. When PA was added along with oleic acid, the cytokine levels returned to the levels produced by BMDM's stimulated with PA alone (see Figure 4 panels D- F).
Finally the authors test whether injection of PA into mice can recapitulate the systemic inflammatory response seen by WD and KD feeding followed by LPS exposure. They were able to demonstrate that injecting 1 mM of PA, waiting for 12h, and then exposing the mice to LPS for 24h could similarly result in a hyper-inflammatory state resulting in greater mortality. The reviewer is skeptical that 1 mM of PA truly represents post-prandial PA levels as one would expect to see after a single fatty meal, and whether this injection is generally well tolerated by mice. Looking into the paper cited by Eguchi et al. to inform their methods, it's shown that the earlier study continuously infused an emulsified ethyl palmitate solution (which contained 600 mM) at a rate of 0.2 uL/min. As far as I can read by Eguchi, they only managed to reach a serum PA concentration of 0.5 mM. This is hardly the same thing as a single i.p. injection of 1 mM PA. and reflects a single bolus injection of double the serum concentration of PA achieved by Eguchi et al.
PA is known to induce inflammation in monocytes and macrophages, therefore the findings certainly make sense in the context of previously published literature. However the authors have made some poor methodological decisions in their mouse studies, namely haphazardly switching between groups of young and old mice (4-6 weeks, 8-9 weeks, and 14-23 weeks), using different LPS injection protocols (6, 10, and 50 mg/ml of LPS), and including multiple sexes of mice. All of which are drastically alter the interpretation of the data, and preventing solid conclusions from being drawn.
-

Reviewer #2 (Public Review):
In this manuscript entitled "Dietary palmitic acid induces innate immune memory via ceramide production that enhances severity of acute septic shock and clearance of infection" Seufert and colleagues have investigated how saturated fatty acids increase susceptibility of the host in a murine model of LPS-mediated septic shock. They have shown that pretreatment of macrophages with palmitic acid (PA) reprograms macrophages towards hyper-inflammatory phenotype, which was dependent on ceremide. Importantly, depletion of macrophages intracellular ceremide with oleic acid reverse their hyper-inflammatory phenotype. Interestingly, while PA was harmful in the LPS-acute septic shock model, it was beneficial in clearance of C. albicans in Rag deficient mice lacking both B and T cells. While this is an exciting study, …
Reviewer #2 (Public Review):
In this manuscript entitled "Dietary palmitic acid induces innate immune memory via ceramide production that enhances severity of acute septic shock and clearance of infection" Seufert and colleagues have investigated how saturated fatty acids increase susceptibility of the host in a murine model of LPS-mediated septic shock. They have shown that pretreatment of macrophages with palmitic acid (PA) reprograms macrophages towards hyper-inflammatory phenotype, which was dependent on ceremide. Importantly, depletion of macrophages intracellular ceremide with oleic acid reverse their hyper-inflammatory phenotype. Interestingly, while PA was harmful in the LPS-acute septic shock model, it was beneficial in clearance of C. albicans in Rag deficient mice lacking both B and T cells. While this is an exciting study, the presented data don't fully support the central hypothesis and the link with trained immunity is currently weak.
-