Structure of Mycobacterium tuberculosis cytochrome bcc in complex with Q203 and TB47, two anti-TB drug candidates
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
The authors have determined the atomic resolution cryo-EM structures of M. tuberculosis cytochrome bcc at 2.7 Å resolution and in complex with anti-tuberculous drugs Q203 at 2.7 Å and TB47 at 2.9 Å resolution. The Q203 compound is a drug candidate otherwise known as Telacebec with promising results in phase 2 clinical trials. The complex structure could pave the way for rational-based drug design.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
Pathogenic mycobacteria pose a sustained threat to global human health. Recently, cytochrome bcc complexes have gained interest as targets for antibiotic drug development. However, there is currently no structural information for the cytochrome bcc complex from these pathogenic mycobacteria. Here, we report the structures of Mycobacterium tuberculosis cytochrome bcc alone (2.68 Å resolution) and in complex with clinical drug candidates Q203 (2.67 Å resolution) and TB47 (2.93 Å resolution) determined by single-particle cryo-electron microscopy. M. tuberculosis cytochrome bcc forms a dimeric assembly with endogenous menaquinone/menaquinol bound at the quinone/quinol-binding pockets. We observe Q203 and TB47 bound at the quinol-binding site and stabilized by hydrogen bonds with the side chains of QcrB Thr 313 and QcrB Glu 314 , residues that are conserved across pathogenic mycobacteria. These high-resolution images provide a basis for the design of new mycobacterial cytochrome bcc inhibitors that could be developed into broad-spectrum drugs to treat mycobacterial infections.
Article activity feed
-

-

Author Response:
Reviewer #3:
The authors modified a previously reported hybrid cytochrome bcc-aa3 supercomplex, consisting of bcc from M. tuberculosis and aa3 from M. smegmatis, (Kim et al 2015) by appending an affinity tag facilitating purification. The cryo-EM experiments are based on the authors' earlier work (Gong et al. 2018) on the structure of the bcc-aa3 supercomplex from M. smegmatis. The authors then determine the structure of the bcc part alone and in complex with Q203 and TB47.
The manuscript is well written and the obtained results are presented in a concise, clear-cut manner. In general, the data support the conclusions drawn.
We thank the reviewer for this evaluation.
To this reviewer, the following points are unclear:
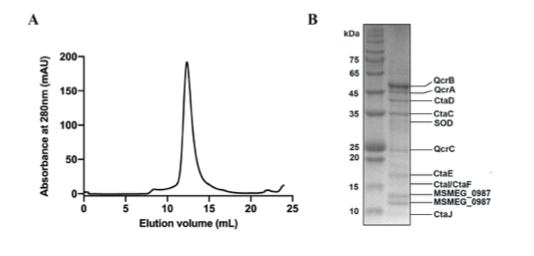
- The purified enzyme elutes from the gel filtration column as one peak, but there seems to be …
Author Response:
Reviewer #3:
The authors modified a previously reported hybrid cytochrome bcc-aa3 supercomplex, consisting of bcc from M. tuberculosis and aa3 from M. smegmatis, (Kim et al 2015) by appending an affinity tag facilitating purification. The cryo-EM experiments are based on the authors' earlier work (Gong et al. 2018) on the structure of the bcc-aa3 supercomplex from M. smegmatis. The authors then determine the structure of the bcc part alone and in complex with Q203 and TB47.
The manuscript is well written and the obtained results are presented in a concise, clear-cut manner. In general, the data support the conclusions drawn.
We thank the reviewer for this evaluation.
To this reviewer, the following points are unclear:
- The purified enzyme elutes from the gel filtration column as one peak, but there seems to be no information given on the subunit composition and the enzymatic activity of the purified hybrid cytochrome bcc-aa3 supercomplex.
See answers to Question 1 from the major Essential Revisions and Question 1 from the minor Essential Revisions.
"We have now shown that the purified chimeric supercomplex is a functional assembly with a (mean ± s.d., n = 4), in agreement with the previous study that shows M. tuberculosis CIII can functionally complement native M. smegmatis CIII and maintain the growth of M. smegmatis (Kim et al., 2015). The in vitro inhibitions of this enzyme by Q203 and TB47 was determined by means of an DMNQH2/oxygen oxidoreductase activity assay. In the assay, 500 nM Q203 or TB47 was chosen, which is close to the median inhibitory concentration (IC50) obtained from the menadiol-induced oxygen consumption in our previous study (Gong et al., 2018). After addition of Q203 and TB47, the values of turnover number of the hybrid supercomplex are reduced to 5.8 +/- 2.4 e-s-1 (Figure 4-figure supplement 4) and 5.1 +/- 2.9 e-s-1 (Figure 5-figure supplement 4) respectively, from 23.3 +/- 2.4 e-s-1. We have incorporated this new data into the text (lines 90-93, 187-189, 206-209)."
"The subunit composition of the purified enzyme has now been provided in Figure 2-figure supplement 1."
- It is unclear what is the conclusion of the structure comparison (Fig 6) is regarding the affinity of Q203 for M. smegmatis.
The structural comparison indicates that Q203 should have a similar binding mechanism and a similar effect on the activity of cytochrome bcc from M. smegmatis and M. tuberculosis. This is in good agreement with previous antimycobacterial activity data and inhibition data for the bcc complexes from M. smegmatis and M. tuberculosis (Gong et al., 2018; Lu et al., 2018a). These have now been incorporated into the revised manuscript (line 223-227).
-

Evaluation Summary:
The authors have determined the atomic resolution cryo-EM structures of M. tuberculosis cytochrome bcc at 2.7 Å resolution and in complex with anti-tuberculous drugs Q203 at 2.7 Å and TB47 at 2.9 Å resolution. The Q203 compound is a drug candidate otherwise known as Telacebec with promising results in phase 2 clinical trials. The complex structure could pave the way for rational-based drug design.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
The authors have determined the atomic resolution cryo-EM structures of M. tuberculosis cytochrome bcc at 2.7 Å resolution and in complex with anti-tuberculous drugs Q203 at 2.7 Å and TB47 at 2.9 Å resolution. The Q203 compound is a drug candidate otherwise known as Telacebec with promising results in phase 2 clinical trials. The complex structure could pave the way for rational-based drug design.
Strengths:
The study builds upon the previously determined supercomplex cryo EM structures of cytochrome bcc complex from a related bacterial host Mycobacterium smegmatis. The cryo EM structures are of excellent quality and it is possible to map the detailed interactions of the drug compounds with the bcc complex. The Q203 and TB47 drugs bind to the Qp site, which is responsible for menaquinol oxidation. Some of …
Reviewer #1 (Public Review):
The authors have determined the atomic resolution cryo-EM structures of M. tuberculosis cytochrome bcc at 2.7 Å resolution and in complex with anti-tuberculous drugs Q203 at 2.7 Å and TB47 at 2.9 Å resolution. The Q203 compound is a drug candidate otherwise known as Telacebec with promising results in phase 2 clinical trials. The complex structure could pave the way for rational-based drug design.
Strengths:
The study builds upon the previously determined supercomplex cryo EM structures of cytochrome bcc complex from a related bacterial host Mycobacterium smegmatis. The cryo EM structures are of excellent quality and it is possible to map the detailed interactions of the drug compounds with the bcc complex. The Q203 and TB47 drugs bind to the Qp site, which is responsible for menaquinol oxidation. Some of the residues around the Q203 sites are different to other determined structures and match known acquired mutations shown to show resistance to these compounds.
Weaknesses:
While the structure of the Q203 is of high-resolution the authors have not robustly demonstrated what determines specificity. Structures of other cytochrome bcc complexes show similar residues to M. tuberculosis. The main differences between the M. tuberculosis bcc complex and previously determined structures is that the pocket in M. tuberculosis is more open, However, in the apo structure of M. tuberculosis the pocket is also more open. Basically, since M. tuberculosis bcc shows accommodation of the drugs, it is unclear if specificity is achieved by specific interactions and/or a preferred shape to the binding pocket.
-

Reviewer #2 (Public Review):
Zhou et al. have resolved cryoEM structures (at 2.7-2.9 Å resolution) of cytochrome bcc from mycobacteria with two potential drug molecules (Q203 and TB47) used in the treatment of tuberculosis. This was achieved by expressing a hybrid supercomplex with the bcc part from M. tuberculosis and the cytochrome oxidase part from M. smegmatis. The structures show how the inhibitors bind and block the Qo site, with important implications for developing new drug molecules against, e.g., tuberculosis. The work is interesting from a structural biology and bioenergetic perspective, and it opens up new possibilities to understand inhibition mechanisms of respiratory enzymes.
-

Reviewer #3 (Public Review):
The authors modified a previously reported hybrid cytochrome bcc-aa3 supercomplex, consisting of bcc from M. tuberculosis and aa3 from M. smegmatis, (Kim et al 2015) by appending an affinity tag facilitating purification. The cryo-EM experiments are based on the authors' earlier work (Gong et al. 2018) on the structure of the bcc-aa3 supercomplex from M. smegmatis. The authors then determine the structure of the bcc part alone and in complex with Q203 and TB47.
The manuscript is well written and the obtained results are presented in a concise, clear-cut manner. In general, the data support the conclusions drawn.
To this reviewer, the following points are unclear:
1. The purified enzyme elutes from the gel filtration column as one peak, but there seems to be no information given on the subunit composition and …
Reviewer #3 (Public Review):
The authors modified a previously reported hybrid cytochrome bcc-aa3 supercomplex, consisting of bcc from M. tuberculosis and aa3 from M. smegmatis, (Kim et al 2015) by appending an affinity tag facilitating purification. The cryo-EM experiments are based on the authors' earlier work (Gong et al. 2018) on the structure of the bcc-aa3 supercomplex from M. smegmatis. The authors then determine the structure of the bcc part alone and in complex with Q203 and TB47.
The manuscript is well written and the obtained results are presented in a concise, clear-cut manner. In general, the data support the conclusions drawn.
To this reviewer, the following points are unclear:
1. The purified enzyme elutes from the gel filtration column as one peak, but there seems to be no information given on the subunit composition and the enzymatic activity of the purified hybrid cytochrome bcc-aa3 supercomplex.
2. It is unclear what is the conclusion of the structure comparison (Fig 6) is regarding the affinity of Q203 for M. smegmatis.
-