Brain-wide analysis of the supraspinal connectome reveals anatomical correlates to functional recovery after spinal injury
Curation statements for this article:-
Curated by eLife
Evaluation Summary:
This work seeks to resolve questions surrounding "unexplained variability in functional recovery" after experimental spinal cord injury in mice using virus-based retrograde tracing from cervical, thoracic and lumbar spinal cord injection sites, tissue clearing and cutting-edge imaging, to develop a supraspinal connectome or map of neurons in the brain that project to the spinal cord. They apply their methods to understand the differences in the connections between the brain and the cervical or lumbar spinal cord and to compare the connectome from intact mice to those of mice with mild, moderate and severe spinal cord injuries. This work will be of interest to neuroscientists interested in tissue clearing, viral labelling, and its applications to spinal cord injury.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their name with the authors.)
This article has been Reviewed by the following groups
Discuss this preprint
Start a discussion What are Sciety discussions?Listed in
- Evaluated articles (eLife)
Abstract
The supraspinal connectome is essential for normal behavior and homeostasis and consists of numerous sensory, motor, and autonomic projections from brain to spinal cord. Study of supraspinal control and its restoration after damage has focused mostly on a handful of major populations that carry motor commands, with only limited consideration of dozens more that provide autonomic or crucial motor modulation. Here, we assemble an experimental workflow to rapidly profile the entire supraspinal mesoconnectome in adult mice and disseminate the output in a web-based resource. Optimized viral labeling, 3D imaging, and registration to a mouse digital neuroanatomical atlas assigned tens of thousands of supraspinal neurons to 69 identified regions. We demonstrate the ability of this approach to clarify essential points of topographic mapping between spinal levels, measure population-specific sensitivity to spinal injury, and test the relationships between region-specific neuronal sparing and variability in functional recovery. This work will spur progress by broadening understanding of essential but understudied supraspinal populations.
Article activity feed
-

-

Author Response
Reviewer #2 (Public Review):
Summary: This substantial collaborative effort utilized virus-based retrograde tracing from cervical, thoracic and lumbar spinal cord injection sites, tissue clearing and cutting-edge imaging to develop a supraspinal connectome or map of neurons in the brain that project to the spinal cord. The need for such a connectome-atlas resource is nicely described, and the combination of the actual data with the means to probe that data is truly outstanding.
They then compared the connectome from intact mice to those of mice with mild, moderate and severe spinal cord injuries to reveal the neuronal populations that retain axons and synapses below the level of injury. Finally, they look for correlations between the remaining neuronal populations and functional recovery to reveal which are likely …
Author Response
Reviewer #2 (Public Review):
Summary: This substantial collaborative effort utilized virus-based retrograde tracing from cervical, thoracic and lumbar spinal cord injection sites, tissue clearing and cutting-edge imaging to develop a supraspinal connectome or map of neurons in the brain that project to the spinal cord. The need for such a connectome-atlas resource is nicely described, and the combination of the actual data with the means to probe that data is truly outstanding.
They then compared the connectome from intact mice to those of mice with mild, moderate and severe spinal cord injuries to reveal the neuronal populations that retain axons and synapses below the level of injury. Finally, they look for correlations between the remaining neuronal populations and functional recovery to reveal which are likely contributing to recovery and its variability after injury. Overall, they successfully achieve their primary goals with the following caveats: The injury model chosen is not the most widely employed in the field, and the anatomical assessment of the injuries is incomplete/not ideal.
Concerns/issues:
- I would like to see additional discussion/rationale for the chosen injury model and how it compares to other more commonly employed animal models and clinical injuries. Please relate how what is being observed with the supraspinal connectome might be different for these other models and for clinical injuries.
We have added text to the Results and Discussion to explain our rationale for selecting the crush injury model, and to acknowledge differences between this model and more clinically relevant contusion models. (Results: line 360-364, Discussion 608-615). We agree wholeheartedly that a critical future direction will be to deploy brain-wide quantification in contusion models, and we are currently seeking funding to obtain the needed equipment.
- The assessment of the thoracic injuries employed is not ideal because it provides no anatomical description of spared white matter (or numbers of spared axons) at the injury epicenter.
We address this more fully in the related point below. Briefly, we agree with a need to improve the assessment of the lesion but are hampered by tissue availability. We are unable to assess white matter sparing but can offer quantification of the width of residual astrocyte tissue bridges in four spinal sections from each animal (new Figure 5 – figure supplement 3). As discussed below, however, we recognize the limitations of the lesion assessment and agree with the larger point that the current quantification methods do not position us to make claims about the relative efficacy of spinal injury analyses versus whole-brain sparing analyses to stratify severity or predict outcomes. Our approach should be seen as a complement, not a substitute, for existing lesion-based analyses. We have edited language throughout the manuscript to make this position clearer.
- Related to this, but an issue that requires separate attention is the highly variable appearance of the injury and tracer/virus injection sites, the variability in the spatial relationship with labeled neurons (lumbar) and how these differences could influence labeling, sprouting of axons of passage and interpretation of the data. In particular this is referring to the data shown in Figure 6 (and related data).
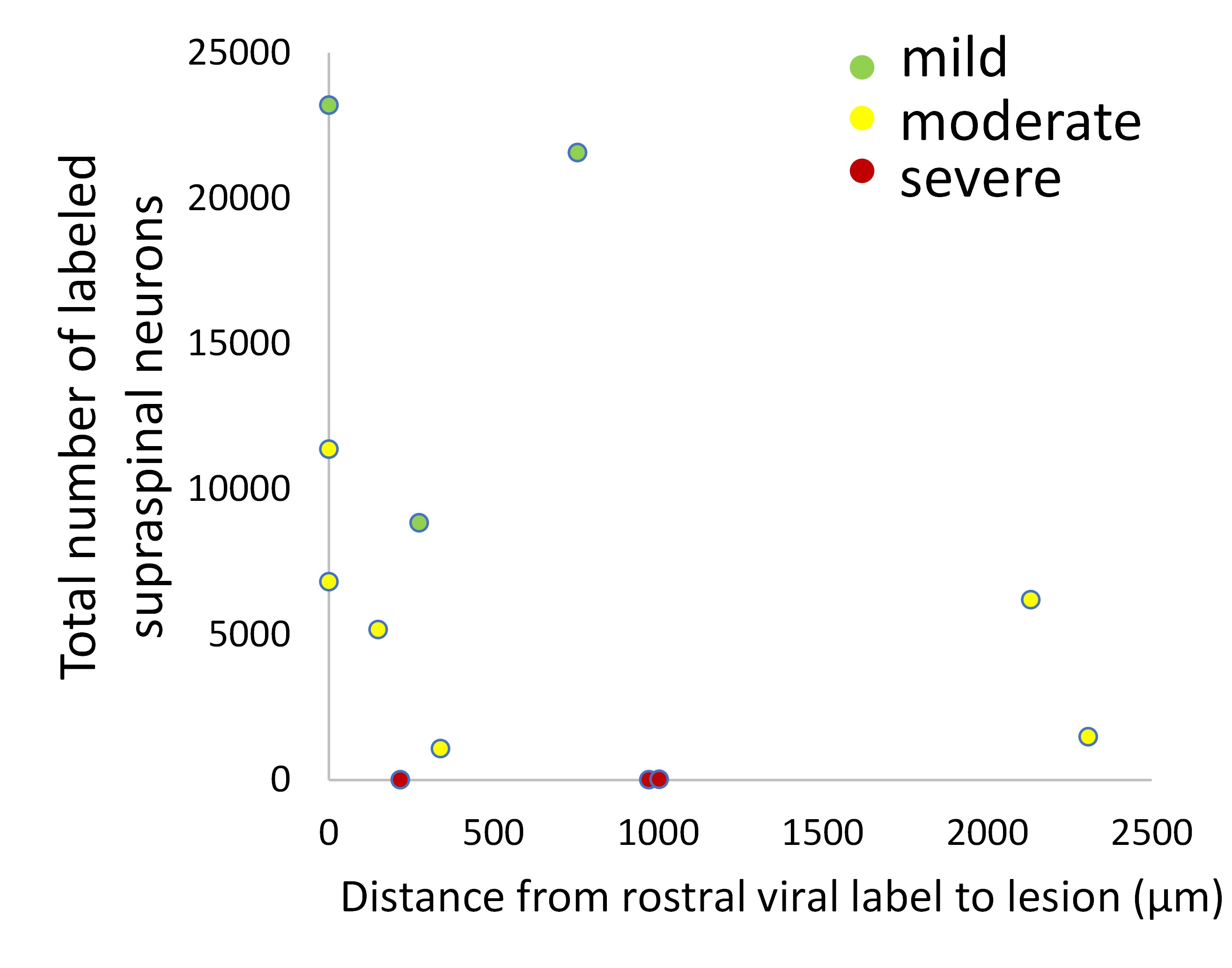
It is true that there is some variability in the relative position of the injury and injection, a surgical reality. The degree of variability was perhaps exaggerated in the original Figure 6 (Now Figure 5), in which one image came from one of two animals in the cohort with a notably larger gap between the injury and injection. Nevertheless, this comment raises the important question of how variability in injection-to-injury distance might affect supraspinal label. First, we would emphasize the data in Figure 1 – Figure Supplement 6, in which we showed that the number of retrogradely labeled supraspinal neurons is relatively stable as injection sites are deliberately varied across the lower thoracic and lumbar cord. Indeed, the question raised here is precisely the reason we performed this early test to determine how sensitive the results might be to shifts in segmental targeting. The results indicate that retrograde labeling is fairly insensitive to L1 versus L4 targeting. As an additional check for this specific experiment we also measured the distance between the rostral spread of viral label and the caudal edge of the lesion and plotted it against the total number of retrogradely labeled neurons in the brain. If a smaller injury/injection gap favored more labeling we might expect negative correlation, but none is apparent. We conclude that although the injury/injection distance did vary in the experiment, it likely did not exert a strong influence on retrograde labeling.
Reviewer #3 (Public Review):
In this manuscript, Wang et al describe a series of experiments aimed at optimizing the experimental and computational approach to the detection of projection-specific neurons across the entire mouse brain. This work builds on a large body of work that has developed nuclear-fused viral labelling, next-generation fluorophores, tissue clearing, image registration, and automated cell segmentation. They apply their techniques to understand projection-specific patterns of supraspinal neurons to the cervical and lumbar spinal cord, and to reveal brain and brainstem connections that are preferentially spared or lost after spinal cord injury.
Strengths:
Although this work does not put forward any fundamentally new methodologies, their careful optimization of the experimental and quantification process will be appreciated by other laboratories attempting to use these types of methods. Moreover, the observations of topological arrangement of various supraspinal centres are important and I believe will be interesting to others in the field.
The web app provided by the authors provides a nice interface for users to explore these data. I think this will be appreciated by people in the field interested in what happens to their brain or brainstem region of interest.
Weaknesses:
Overall the work is well done; however, some of the novelty claims should be better aligned with the experimental findings. Moreover, the statistical approaches put forward to understand the relationship between spinal cord injury severity and cell counts across the mouse brain needs to be more carefully considered.
The authors state that they provide an experimental platform for these types of analysis to be done. My apologies if I missed it but I could not find anywhere the information on viral construct availability or code availability to reproduce the results. Certainly both of these aspects would be required for people to replicate the pipeline. Moreover, the described methodology for imaging and processing is quite sparse. While I appreciate that this information is widely provided in papers that have developed these methods, I do not think it is appropriate to claim to have provided a platform for people to enable these types of analyses without a more in-depth description of the methods. Alternatively, the authors could instead focus on how they optimized current methodologies and avoid the overstatement that this work provides a tool for users. The exception to this is of course the viral constructs, the plasmids of which should be deposited.
We agree that we have not provided a tool per se, more of an example that could be followed. We have revised language in the abstract, introduction, and discussion to make it clear that we optimized existing methods and provide an example of how this can be done, but are not offering a “plug and play” solution to the problem of registration that would, for example, allow upload of external data. For example, in the abstract we replaced “We now provide an experimental platform” with “Here we assemble an experimental workflow.” (Line 28). The term “platform” no longer appears in the manuscript and has been replaced throughout by “example.” We how this matches the intention of the comment and are happy to revise further as needed. Note that the plasmids have been deposited to Addgene.
It was not completely to me clear why or when the authors switch back and forth between different resolutions throughout the manuscript. In the abstract it states that 60 regions were examined, but elsewhere the number is as many as 500. My understanding is that current versions of the Allen Brain Annotation include more than 2000 regions. I think it would make things clear for the readers if a single resolution was used throughout, or at least justified narratively throughout the text to avoid confusion.
Thank you for pointing this out. The Cellfinder application recognizes 645 discrete regions in the brain, and across all experiments we detected supraspinal nuclei in 69 of these. This number, however, includes some very fine distinctions, for example three separate subregions of vestibular nuclei, three subregions of the superior olivary complex, etc. True experts may desire this level of information, but with the goal of accessibility we find it useful to collapse closely related / adjacent regions to an umbrella term. Doing so generates a list of 25 grouped or summary regions. In the revised version we move the 69-region data completely to the supplemental data (there for the experts who wish to parse), and use the consistent 25-region system (plus cervical spinal cord in later sections) to present data in the main figures. We have added text to the Results section (lines 157-162) to clarify this grouping system.
The others provide an interesting analysis of the difference between cervical and lumbar projections. I think this might be one of the more interesting aspects of the paper - yet I found myself a bit confused by the analysis, and whether any of the differences observed were robust. Just prior to this experiment the authors provide a comparison of the mScarlet vs. the mGL, and demonstrate that mGL may label more cells. Yet, in the cervical vs. lumbar analysis it appears they are being treated 1 to 1. Moreover, I could not find any actual statistical analysis of this data? My impression would be that given the potential difference in labelling efficiency between the mScarlet and mGL this should be done using some kind of count analysis that takes into account the overall number of neurons labelled, such as a Chi-sq test or perhaps something more sophisticated. Then, with this kind of statistical analysis in place, do any of the discussed differences hold up? If not, I do not think this would detract from the interesting topological observations - but would call on the authors to be a bit more conservative about their statements and discussion regarding differences in the proportions of neurons projecting to certain supraspinal centers.
This is an important point. In response to this input and related comments from other reviewers we performed new experiments to assess co-localization. The new data address the point above by including quantification of the degree of colocalization that results from titer-matched co-injection of the two fluorophores, providing baseline data. The results of this can be found in Figure 3 – figure supplement 3 and form the basis for statistical comparisons to experimental animals shown in Figure 3.
Finally, I do have some concerns about the author's use of linear regression in their analysis of brain regions after varying severities of SCI. First of all, the BMS score is notoriously non-linear. Despite wide use of linear regressions in the field to attempt to associate various outcomes to these kinds of ordinal measures, this is not appropriate. Some have suggested a rank conversion of the BMS prior to linear analyses, but even this comes with its own problems. Ultimately, the authors have here 2-3 clear cohorts of behavioral scores and drawing a linear regression between these is unlikely to be robustly informative. Moreover, it is unclear whether the authors properly adjusted their p-values from running these regressions on 60 (600?) regions. Finally, the statement in the abstract and discussion that the authors "explain more variability" compared to typical lesion severity analysis is also unsupported. My suggestion would be the following:
Remove the linear regression analyses associated with BMS. I do not think these add value to the paper, and if anything provide a large window of false interpretation due to a violation of the assumptions of this test.
Consider adding a more appropriate statistical analysis of the brain regions, such as a non-parametric group analysis. Knowing which brain regions are severity dependent, and which ones are not, would already be an interesting finding. This finding would not be confounded by any attempt to link it to crude measures of behavior.
We agree that the linear regression approach was flawed and appreciate the opportunity to correct it. After consultation with two groups of statisticians we were forced to conclude that the data are simply underpowered for mixed model and ranking approaches. We therefore adopted a much simpler strategy. As you point out (and as noted by the statisticians), the behavioral data are bimodal; one group of animals regained plantar stepping ability, albeit with varying degrees of coordination (BMS 6-8), while the others showed at most rare plantar steps (BMS 0-3.5). We therefore asked whether the number of spared neurons in each brain region differed between the two groups and also examined the degree of “overlap” in the sparing values between the two groups. The data are now presented in Figure 6.
If the authors would like to state anything about 'explaining more variability' then the proper statistical analysis should be used, which in this case would be to compare the models using a LRT or equivalent. However, as I mentioned it does not seem to be appropriate to be doing this with linear models so the authors should consider a non-linear equivalent if they choose to proceed with this.
We thank the reviewer for the excellent suggestion. However as we explained above after consultation with two groups of statisticians we were forced to conclude that the data are underpowered and could not apply some of the methods suggested. Especially in light of our simplified analysis, we think it is better to remove any claims of the relative success of the sparing in different regions to explain more or less variability. Instead we can simply report that sparing in some regions, but not others, is significantly different between “low-performing” and “high-performing” groups.
-

Evaluation Summary:
This work seeks to resolve questions surrounding "unexplained variability in functional recovery" after experimental spinal cord injury in mice using virus-based retrograde tracing from cervical, thoracic and lumbar spinal cord injection sites, tissue clearing and cutting-edge imaging, to develop a supraspinal connectome or map of neurons in the brain that project to the spinal cord. They apply their methods to understand the differences in the connections between the brain and the cervical or lumbar spinal cord and to compare the connectome from intact mice to those of mice with mild, moderate and severe spinal cord injuries. This work will be of interest to neuroscientists interested in tissue clearing, viral labelling, and its applications to spinal cord injury.
(This preprint has been reviewed by eLife. We …
Evaluation Summary:
This work seeks to resolve questions surrounding "unexplained variability in functional recovery" after experimental spinal cord injury in mice using virus-based retrograde tracing from cervical, thoracic and lumbar spinal cord injection sites, tissue clearing and cutting-edge imaging, to develop a supraspinal connectome or map of neurons in the brain that project to the spinal cord. They apply their methods to understand the differences in the connections between the brain and the cervical or lumbar spinal cord and to compare the connectome from intact mice to those of mice with mild, moderate and severe spinal cord injuries. This work will be of interest to neuroscientists interested in tissue clearing, viral labelling, and its applications to spinal cord injury.
(This preprint has been reviewed by eLife. We include the public reviews from the reviewers here; the authors also receive private feedback with suggested changes to the manuscript. Reviewer #1 and Reviewer #2 agreed to share their name with the authors.)
-

Reviewer #1 (Public Review):
This is a very interesting paper on the connectivity between the brain and the spinal cord, that touches upon, in an elegant and complementary way, several important aspects in the field: first, a methodological advance which is then applied for studying the organization of descending pathways from a fundamental perspective and then in the context of spinal cord injury. The associated web-based interface is also excellent and well-made.
The authors convincingly show the potency of nuclear fluorescent tagging of supra-spinal neurons (using new retrograde vectors they engineered) combined with iDISCO-based clearing and reconstruction tools derived from the Brainglobe initiative. The method is potent, apparently rapid, and allows quantitative measurement in virtually all brain areas on the same sample, …
Reviewer #1 (Public Review):
This is a very interesting paper on the connectivity between the brain and the spinal cord, that touches upon, in an elegant and complementary way, several important aspects in the field: first, a methodological advance which is then applied for studying the organization of descending pathways from a fundamental perspective and then in the context of spinal cord injury. The associated web-based interface is also excellent and well-made.
The authors convincingly show the potency of nuclear fluorescent tagging of supra-spinal neurons (using new retrograde vectors they engineered) combined with iDISCO-based clearing and reconstruction tools derived from the Brainglobe initiative. The method is potent, apparently rapid, and allows quantitative measurement in virtually all brain areas on the same sample, including in regions located deeply (cerebellum, brainstem).
In a second part, authors investigate, using retrograde viruses, whether different supra-spinal brain areas control distinct spinal segments through abundant collaterals, or by specific subsets that differ by their projection targets. This matter is becoming the center of attention in many studies on descending tracts. The strength here is to consider all supra-spinal brain areas in a unique study, which is extremely informative. Authors conclude that in all brain areas contacting the spinal cord, cells are uniquely labeled either from the cervical or the lumbar cord. However this part cannot be interpreted meaningfully without the quantification of the double-labeled cells, presently missing for all regions except the cortex. Are there strictly no cells with collaterals to both segments in the whole brain, or are these dual-projecting neurons not detected by the method? Another weakness is the possibility that one virus being brighter may occult or reduce the expression of the dimmer one. Quantifications of such controls are presently missing.
Finally, in the third part, the authors apply their labeling and detecting pipeline in the context of contusive injuries. They reveal that some brain areas show significantly more sparing (or plasticity potentially?) than others, and that this sparing might correlate with the extent of the locomotor recovery. This is convincing and should help draw a better picture of the relevance of different descending tracts in locomotor recovery following SCI.
Another strength is the web-based interface. While a few bugs are still there (notably on the indexation), this is an extremely timely and useful initiative.
-

Reviewer #2 (Public Review):
Summary: This substantial collaborative effort utilized virus-based retrograde tracing from cervical, thoracic and lumbar spinal cord injection sites, tissue clearing and cutting-edge imaging to develop a supraspinal connectome or map of neurons in the brain that project to the spinal cord. The need for such a connectome-atlas resource is nicely described, and the combination of the actual data with the means to probe that data is truly outstanding.
They then compared the connectome from intact mice to those of mice with mild, moderate and severe spinal cord injuries to reveal the neuronal populations that retain axons and synapses below the level of injury. Finally, they look for correlations between the remaining neuronal populations and functional recovery to reveal which are likely contributing to …
Reviewer #2 (Public Review):
Summary: This substantial collaborative effort utilized virus-based retrograde tracing from cervical, thoracic and lumbar spinal cord injection sites, tissue clearing and cutting-edge imaging to develop a supraspinal connectome or map of neurons in the brain that project to the spinal cord. The need for such a connectome-atlas resource is nicely described, and the combination of the actual data with the means to probe that data is truly outstanding.
They then compared the connectome from intact mice to those of mice with mild, moderate and severe spinal cord injuries to reveal the neuronal populations that retain axons and synapses below the level of injury. Finally, they look for correlations between the remaining neuronal populations and functional recovery to reveal which are likely contributing to recovery and its variability after injury. Overall, they successfully achieve their primary goals with the following caveats: The injury model chosen is not the most widely employed in the field, and the anatomical assessment of the injuries is incomplete/not ideal.
Concerns/issues:
1. I would like to see additional discussion/rationale for the chosen injury model and how it compares to other more commonly employed animal models and clinical injuries. Please relate how what is being observed with the supraspinal connectome might be different for these other models and for clinical injuries.
2. The assessment of the thoracic injuries employed is not ideal because it provides no anatomical description of spared white matter (or numbers of spared axons) at the injury epicenter.
3. Related to this, but an issue that requires separate attention is the highly variable appearance of the injury and tracer/virus injection sites, the variability in the spatial relationship with labeled neurons (lumbar) and how these differences could influence labeling, sprouting of axons of passage and interpretation of the data. In particular this is referring to the data shown in Figure 6 (and related data).
-

Reviewer #3 (Public Review):
In this manuscript, Wang et al describe a series of experiments aimed at optimizing the experimental and computational approach to the detection of projection-specific neurons across the entire mouse brain. This work builds on a large body of work that has developed nuclear-fused viral labelling, next-generation fluorophores, tissue clearing, image registration, and automated cell segmentation. They apply their techniques to understand projection-specific patterns of supraspinal neurons to the cervical and lumbar spinal cord, and to reveal brain and brainstem connections that are preferentially spared or lost after spinal cord injury.
Strengths:
Although this work does not put forward any fundamentally new methodologies, their careful optimization of the experimental and quantification process will be …
Reviewer #3 (Public Review):
In this manuscript, Wang et al describe a series of experiments aimed at optimizing the experimental and computational approach to the detection of projection-specific neurons across the entire mouse brain. This work builds on a large body of work that has developed nuclear-fused viral labelling, next-generation fluorophores, tissue clearing, image registration, and automated cell segmentation. They apply their techniques to understand projection-specific patterns of supraspinal neurons to the cervical and lumbar spinal cord, and to reveal brain and brainstem connections that are preferentially spared or lost after spinal cord injury.
Strengths:
Although this work does not put forward any fundamentally new methodologies, their careful optimization of the experimental and quantification process will be appreciated by other laboratories attempting to use these types of methods. Moreover, the observations of topological arrangement of various supraspinal centres are important and I believe will be interesting to others in the field.
The web app provided by the authors provides a nice interface for users to explore these data. I think this will be appreciated by people in the field interested in what happens to their brain or brainstem region of interest.
Weaknesses:
Overall the work is well done; however, some of the novelty claims should be better aligned with the experimental findings. Moreover, the statistical approaches put forward to understand the relationship between spinal cord injury severity and cell counts across the mouse brain needs to be more carefully considered.
The authors state that they provide an experimental platform for these types of analysis to be done. My apologies if I missed it but I could not find anywhere the information on viral construct availability or code availability to reproduce the results. Certainly both of these aspects would be required for people to replicate the pipeline. Moreover, the described methodology for imaging and processing is quite sparse. While I appreciate that this information is widely provided in papers that have developed these methods, I do not think it is appropriate to claim to have provided a platform for people to enable these types of analyses without a more in-depth description of the methods. Alternatively, the authors could instead focus on how they optimized current methodologies and avoid the overstatement that this work provides a tool for users. The exception to this is of course the viral constructs, the plasmids of which should be deposited.
It was not completely to me clear why or when the authors switch back and forth between different resolutions throughout the manuscript. In the abstract it states that 60 regions were examined, but elsewhere the number is as many as 500. My understanding is that current versions of the Allen Brain Annotation include more than 2000 regions. I think it would make things clear for the readers if a single resolution was used throughout, or at least justified narratively throughout the text to avoid confusion.
The others provide an interesting analysis of the difference between cervical and lumbar projections. I think this might be one of the more interesting aspects of the paper - yet I found myself a bit confused by the analysis, and whether any of the differences observed were robust. Just prior to this experiment the authors provide a comparison of the mScarlet vs. the mGL, and demonstrate that mGL may label more cells. Yet, in the cervical vs. lumbar analysis it appears they are being treated 1 to 1. Moreover, I could not find any actual statistical analysis of this data? My impression would be that given the potential difference in labelling efficiency between the mScarlet and mGL this should be done using some kind of count analysis that takes into account the overall number of neurons labelled, such as a Chi-sq test or perhaps something more sophisticated. Then, with this kind of statistical analysis in place, do any of the discussed differences hold up? If not, I do not think this would detract from the interesting topological observations - but would call on the authors to be a bit more conservative about their statements and discussion regarding differences in the proportions of neurons projecting to certain supraspinal centres.
Finally, I do have some concerns about the author's use of linear regression in their analysis of brain regions after varying severities of SCI. First of all, the BMS score is notoriously non-linear. Despite wide use of linear regressions in the field to attempt to associate various outcomes to these kinds of ordinal measures, this is not appropriate. Some have suggested a rank conversion of the BMS prior to linear analyses, but even this comes with its own problems. Ultimately, the authors have here 2-3 clear cohorts of behavioural scores and drawing a linear regression between these is unlikely to be robustly informative. Moreover, it is unclear whether the authors properly adjusted their p-values from running these regressions on 60 (600?) regions. Finally, the statement in the abstract and discussion that the authors "explain more variability" compared to typical lesion severity analysis is also unsupported. My suggestion would be the following:
Remove the linear regression analyses associated with BMS. I do not think these add value to the paper, and if anything provide a large window of false interpretation due to a violation of the assumptions of this test.
Consider adding a more appropriate statistical analysis of the brain regions, such as a non-parametric group analysis. Knowing which brain regions are severity dependent, and which ones are not, would already be an interesting finding. This finding would not be confounded by any attempt to link it to crude measures of behaviour.
If the authors would like to state anything about 'explaining more variability' then the proper statistical analysis should be used, which in this case would be to compare the models using a LRT or equivalent. However, as I mentioned it does not seem to be appropriate to be doing this with linear models so the authors should consider a non-linear equivalent if they choose to proceed with this.
-